Contributions of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration
Abstract
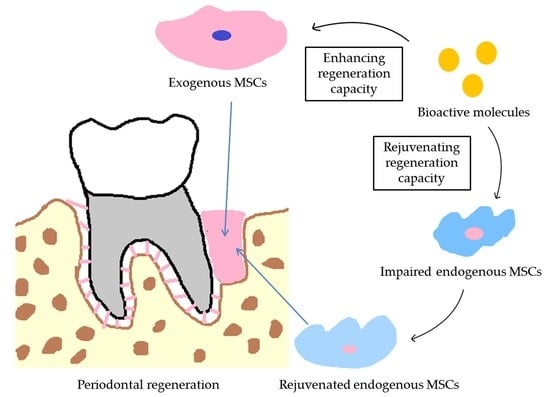
:1. Introduction
2. The Vital Role of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration
2.1. The Extracellular Microenvironment Releases Molecular Signals to Modulate MSCs
2.2. MSCs Release Molecular Signals to Modulate the Microenvironment
2.3. Bioactive Molecules Act as Molecular Signals That Modulate MSCs and Recreate the Microenvironment
3. Bioactive Molecules Associated with Periodontal Regeneration
3.1. Growth Factors
3.2. Pharmaceuticals
3.3. Plant Extracts
4. Bioactive Molecules Enhance the Effects of Cell Aggregates/Cell Sheets in Periodontal Regeneration
4.1. Cell Aggregates/Cell Sheets as a 3D Scaffolding Material in Periodontal Regeneration
4.2. A Combination of Bioactive Molecules and Cell Aggregates/Cell Sheets to Strengthen Periodontal Regeneration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| PRP | Platelet-rich plasma |
| PRF | Platelet-rich fibrin |
| LPS | Lipopolysaccharide |
| PDLSCs | Periodontal ligament stem cells |
| I-PDLSCs | Inflamed periodontal ligament stem cells |
| H-PDLSCs | Healthy periodontal ligament stem cells |
| OVX | Ovariectomy |
| BMMSCs | Bone marrow mesenchymal stem cells |
| ECM | Extracellular matrix |
| EMD | Enamel matrix derivative |
| PDGF | Platelet-derived growth factor |
References
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.; Michalowicz, B.; Johnson, N. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Amano, A. Periodontal diseases and systemic diseases. Clin. Calcium 2017, 27, 1383–1391. [Google Scholar] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Nugala, B.; Kumar, B.S.; Sahitya, S.; Krishna, P.M. Biologic width and its importance in periodontal and restorative dentistry. J. Conserv. Dent. 2012, 15, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef] [PubMed]
- Slavin, S.; Nagler, A.; Naparstek, E.; Kapelushnik, Y.; Aker, M.; Cividalli, G.; Varadi, G.; Kirschbaum, M.; Ackerstein, A.; Samuel, S.; et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998, 91, 756–763. [Google Scholar] [PubMed]
- Rohban, R.; Pieber, T.R. Mesenchymal Stem and Progenitor Cells in Regeneration, Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.M.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015, 14, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv. Mater. 2014, 26, 4961–4966. [Google Scholar] [CrossRef] [PubMed]
- Subramony, S.D.; Dargis, B.R.; Castillo, M.; Azeloglu, E.U.; Tracey, M.S.; Su, A.; Lu, H.H. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 2013, 34, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Tan, Y.; Wang, J.; Liu, H.; Wang, Y.; Yang, S.; Shi, M.; Zhao, S.; Zhang, Y.; et al. A Difunctional Regeneration Scaffold for Knee Repair based on Aptamer-Directed Cell Recruitment. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, F.; Zhang, X.; Liu, X.; Zhang, Y.; Liu, J.; Duan, Y.; Jin, Y. A Novel Possible Strategy Based on Self-Assembly Approach to Achieve Complete Periodontal Regeneration. Artif. Organs 2010, 34, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dong, Z.; Zhang, Y.; He, X.; Fei, D.; Jin, F.; Yuan, L.; Li, B.; Jin, Y. Osthole improves function of periodontitis periodontal ligament stem cells via epigenetic modification in cell sheets engineering. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Mei, S.; Guo, L.; Su, Y.; Wang, H.; Liu, Y.; Zhao, Z.; Wang, S.; Liu, Y. Platelet-rich fibrin/aspirin complex promotes alveolar bone regeneration in periodontal defect in rats. J. Periodontal Res. 2018, 53, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.; Lee, J. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xia, Y.; Yu, Y.; Wu, R.; Gao, L.; Chen, F. Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities, a patient-matched comparison. J. Clin. Periodontol. 2016, 43, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.D.; Hu, C.H.; Liu, A.Q.; Zheng, C.X.; Xuan, K.; Jin, Y. Stem cell-based bone regeneration in diseased microenvironments, Challenges and solutions. Biomaterials 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, J.; Liu, O.; Fan, Z.; Liu, Y.; Wang, F.; Ding, G.; Wei, F.; Zhang, C.; Wang, S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol. 2012, 39, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Liu, W.; Li, Q.; Jin, Z.; Jin, Y. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS ONE 2014, 9, e108752. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.; Ma, D.; Tang, L.; Duan, Y.; Jin, Y. Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng. 2009, 15, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, N.; Zhou, J.; Tang, L.; Ding, B.; Duan, Y.; Jin, Y. Inflammatory environment induces gingival tissue-specific mesenchymal stem cells to differentiate towards a pro-fibrotic phenotype. Biol. Cell 2013, 105, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling, Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review, MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Trubiani, O.; Diomede, F.; Piattelli, A.; Bramanti, P.; Mazzon, E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp. Cell Res. 2016, 349, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Umezu, T.; Ohyashiki, K. Extracellular vesicle-mediated cell-cell communication in haematological neoplasms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Chandad, F.; Buser, D.; Sculean, A.; Cochran, D.L.; Zhang, Y. Effect of Enamel Matrix Derivative Liquid on Osteoblast and Periodontal Ligament Cell Proliferation and Differentiation. J. Periodontol. 2016, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Zhang, Y.; Caballe-Serrano, J.; Shirakata, Y.; Bosshardt, D.D.; Buser, D.; Sculean, A. Osteogain improves osteoblast adhesion, proliferation and differentiation on a bovine-derived natural bone mineral. Clin. Oral Implants Res. 2017, 28, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative, the past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Z.; Xia, Y.; Shu, R. Extracellular matrix derived from periodontal ligament cells maintains their stemness and enhances redifferentiation via the wnt pathway. J. Biomed. Mater. Res. Part A 2017, 106, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Miron, R.J.; Nakamura, T.; Sena, K.; Shinohara, Y.; Horai, N.; Bosshardt, D.D.; Noguchi, K.; Sculean, A. Effects of EMD liquid (Osteogain) on periodontal healing in class III furcation defects in monkeys. J. Clin. Periodontol. 2017, 44, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.J.; Jhingran, R.; Gupta, V.; Bains, V.K.; Madan, R.; Rizvi, I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects, a clinical and cone beam computed tomography study. J. Int. Acad. Periodontol. 2014, 16, 86–96. [Google Scholar] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.; Fratini, A.; Giraudi, M.; Romano, F. Enamel Matrix Derivative Proteins in Combination with a Flapless Approach for Periodontal Regeneration of Intrabony Defects, A 2-Year Prospective Case Series. Int. J. Periodontics Restor. Dent. 2016, 36, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Dovban, A.S.; Lim, L.P.; Chong, L.Y.; Kuo, M.Y.; Wang, C. Dual delivery of PDGF and simvastatin to accelerate periodontal regeneration in vivo. Biomaterials 2013, 34, 9990–9997. [Google Scholar] [CrossRef] [PubMed]
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2016, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Avila, G.; Wisner-Lynch, L.; Nevins, M.L.; Nevins, M.; Rasperini, G.; Lynch, S.E.; Giannobile, W.V. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin. Biol. Ther. 2011, 11, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part, A.; intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Aydemir Turkal, H.; Demirer, S.; Dolgun, A.; Keceli, H.G. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. J. Clin. Periodontol. 2016, 43, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; An, Y.; Lei, M.; Li, B.; Yang, H.; Lu, H.; Chen, F.; Jin, Y. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials 2013, 34, 9937–9951. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Cristina, O.R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration, effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Monjo, M.; Ramis, J.M. Identification of quercitrin as a potential therapeutic agent for periodontal applications. J. Periodontol. 2014, 85, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Baylink, D.J.; Brier-Jones, J.; Neises, A.; Kiroyan, J.B.; Rundle, C.H.; Lau, K.H.; Zhang, X.B. PDGFB-based stem cell gene therapy increases bone strength in the mouse. Proc. Natl. Acad. Sci. USA 2015, 112, E3893–E3900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, Z.; Wu, G.; Bai, S.; Dong, Y.; Chen, F.; Zhao, Y. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Tabata, Y.; Sato, S. Periodontal tissue regeneration with PRP incorporated gelatin hydrogel sponges. Biomed. Mater. 2015, 10, 55016. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Mehrzadi, S.; Reiter, R.J.; Seidi, K.; Majidinia, M.; Baghi, H.B.; Khatami, N.; Yousefi, B.; Sadeghpour, A. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis, involvement of circadian clock genes. Br. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Y.; Liao, L.; Su, X.; Yu, Y.; Shao, B.; Jing, H.; Zhang, X.; Deng, Z.; Jin, Y. Melatonin Treatment Improves Mesenchymal Stem Cells Therapy by Preserving Stemness during Long-term In Vitro Expansion. Theranostics 2016, 6, 1899–1917. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, A.R.; Peralta Lopez, M.E.; Aguilar, J.; Solá, V.M. Melatonin and periodontal tissues, Molecular and clinical perspectives. Pharmacol. Res. 2017, 125, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.C.; Thomas, M.V.; Peyyala, R.; Dziubla, T.D.; Puleo, D.A. Design of a multiple drug delivery system directed at periodontitis. Biomaterials 2013, 34, 8835–8842. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole, A Review on Its Bioactivities, Pharmacological Properties, and Potential as Alternative Medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leung, W.N.; Li, G.; Lai, Y.M.; Chan, C.W. Osthole Promotes Endochondral Ossification and Accelerates Fracture Healing in Mice. Calcif. Tissue Int. 2016, 99, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol, review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Galvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ni, H.; Wang, Y. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. 2017, 89, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering, recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [PubMed]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Washio, K.; Iwata, T.; Mizutani, M.; Ando, T.; Yamato, M.; Okano, T.; Ishikawa, I. Assessment of cell sheets derived from human periodontal ligament cells, a pre-clinical study. Cell Tissue Res. 2010, 341, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, F.; Zhang, X.; Ma, D.; Han, C.; Huo, N.; Wang, Y.; Zhang, Y.; Lin, Z.; Jin, Y. Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for human periodontal ligament stem cells. Tissue Eng. Part C Methods 2009, 15, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration, crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Ming, L.; Zhou, Z.; Yu, Y.; Sun, J.; Ding, Y.; Jin, Y. The effect of licochalcone A on cell-aggregates ECM secretion and osteogenic differentiation during bone formation in metaphyseal defects in ovariectomized rats. Biomaterials 2014, 35, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Jin, F.; Huang, P.; Luo, H.; Liu, W.; Zhang, L.; Yuan, W.; Zhang, Y.; Jin, Y. Licochalcone A up-regulates of FasL in mesenchymal stem cells to strengthen bone formation and increase bone mass. Sci. Rep. 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, B.; Yuan, L.; Dong, Z.; Zhang, H.; Wang, H.; Sun, J.; Ge, S.; Jin, Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J. Tissue Eng. Regen. Med. 2017, 11, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sui, B.D.; Liu, N.; Lv, Y.J.; Zheng, C.X.; Lu, Y.B.; Huang, W.T.; Zhou, C.H.; Chen, J.; Pang, D.L.; et al. Anti-aging pharmacology in cutaneous wound healing effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef] [PubMed]


| Category | Bioactive Molecules | Effect | Experimental Model | Studies |
|---|---|---|---|---|
| Growth factors | EMD | Facilitate osteoblasts and PDLSCs adhesion, proliferation | Osteoblasts | [34,35,36] |
| PDLSCs | [34,37] | |||
| Promote periodontal regeneration | Class III furcation defects in monkeys | [38] | ||
| Human with periodontitis | [39,40] | |||
| PDGF | Accelerates the regeneration of the periodontal apparatus | Periodontal defect in rat | [41,42] | |
| Periodontal ligament interposed between newly formed cementum and alveolar bone | Human with class II furcation lesions | [43] | ||
| PRP/PRF | Strong angiogenic capacity | Human with periodontitis | [44,45,46] | |
| Provide a nature scaffold | ||||
| Slowly release bioactive factors | ||||
| Pharmaceuticals | Aspirin | Control inflammation | Periodontal defect in rat | [18] |
| Melatonin | Anti-inflammation and anti-oxidative | Experimental periodontitis in rat | [19] | |
| Plant extracts | Osthole | Improve the capacity of osteogenic differentiation | PDLSCs | [17,47] |
| Promote periodontal regeneration | Periodontal defect in rat | [17] | ||
| Resveratrol | Prevent bone loss and promote osteogenesis | Periodontitis model in rat | [20,48] | |
| Quercitrin | Anti-inflammation | Human gingival fibroblasts | [49,50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.-Q.; Hu, C.-H.; Jin, F.; Zhang, L.-S.; Xuan, K. Contributions of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 1016. https://doi.org/10.3390/ijms19041016
Liu A-Q, Hu C-H, Jin F, Zhang L-S, Xuan K. Contributions of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration. International Journal of Molecular Sciences. 2018; 19(4):1016. https://doi.org/10.3390/ijms19041016
Chicago/Turabian StyleLiu, An-Qi, Cheng-Hu Hu, Fang Jin, Li-Shu Zhang, and Kun Xuan. 2018. "Contributions of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration" International Journal of Molecular Sciences 19, no. 4: 1016. https://doi.org/10.3390/ijms19041016
APA StyleLiu, A.-Q., Hu, C.-H., Jin, F., Zhang, L.-S., & Xuan, K. (2018). Contributions of Bioactive Molecules in Stem Cell-Based Periodontal Regeneration. International Journal of Molecular Sciences, 19(4), 1016. https://doi.org/10.3390/ijms19041016