Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels
Abstract
:1. Introduction
2. Results
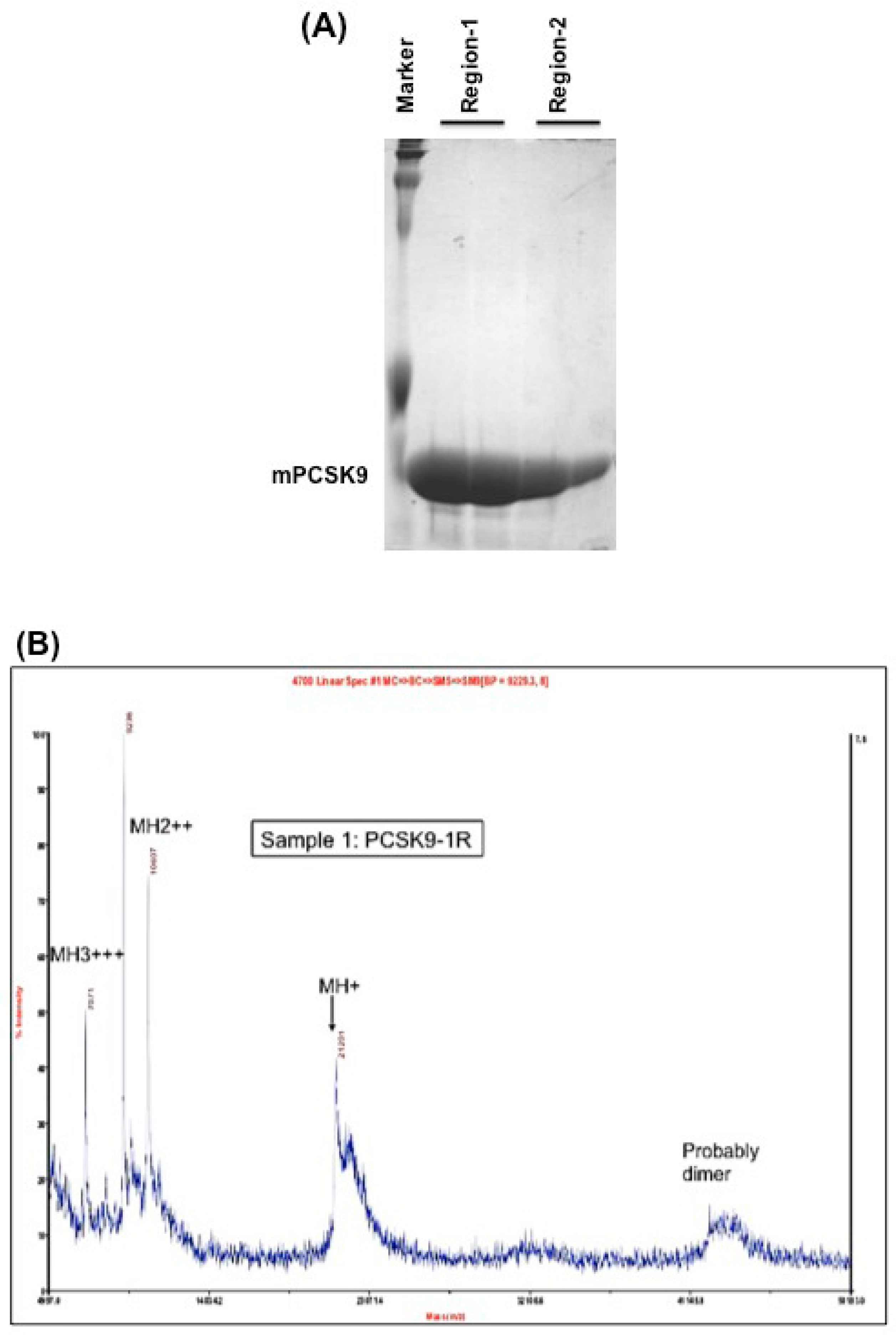
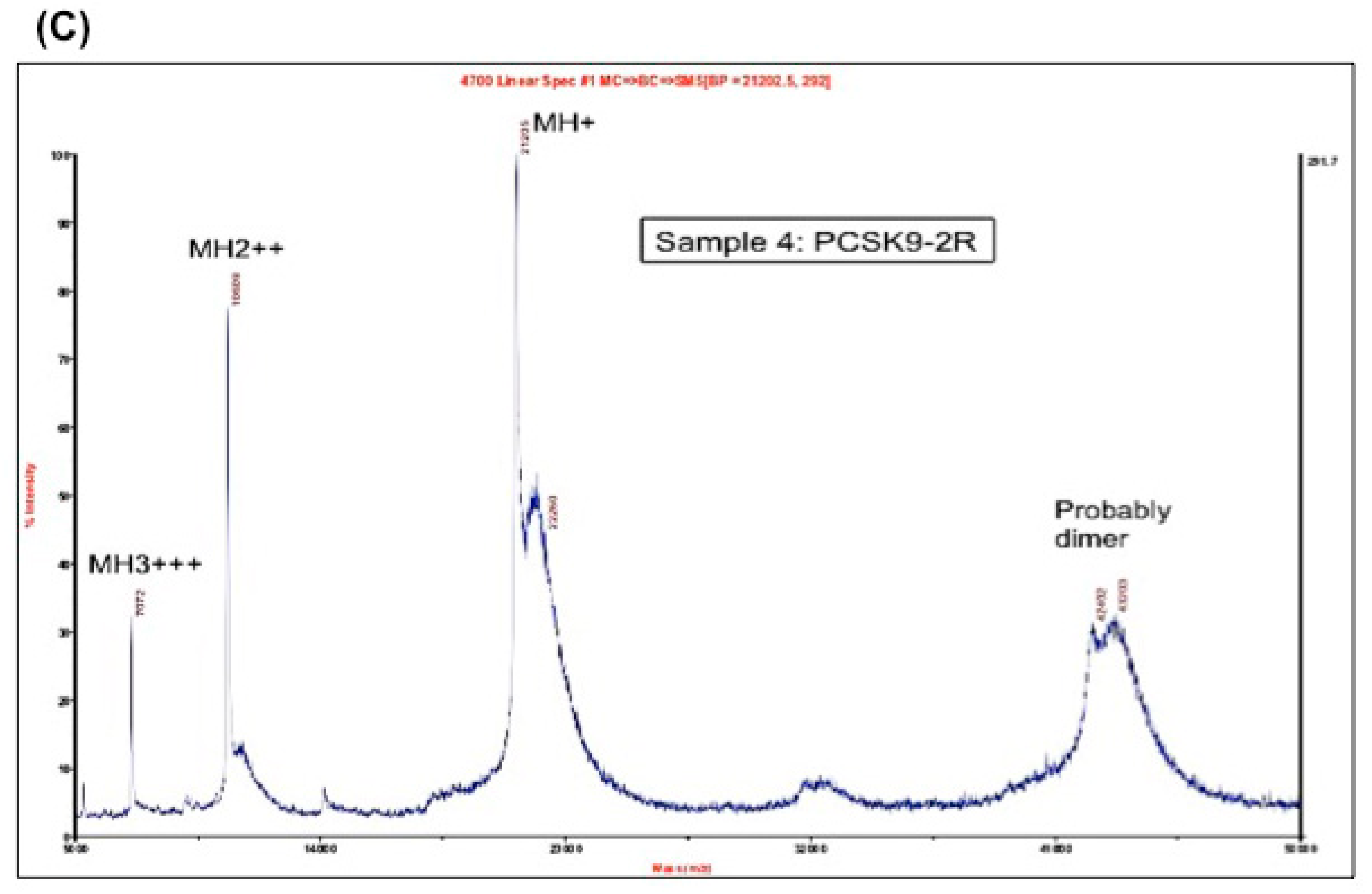
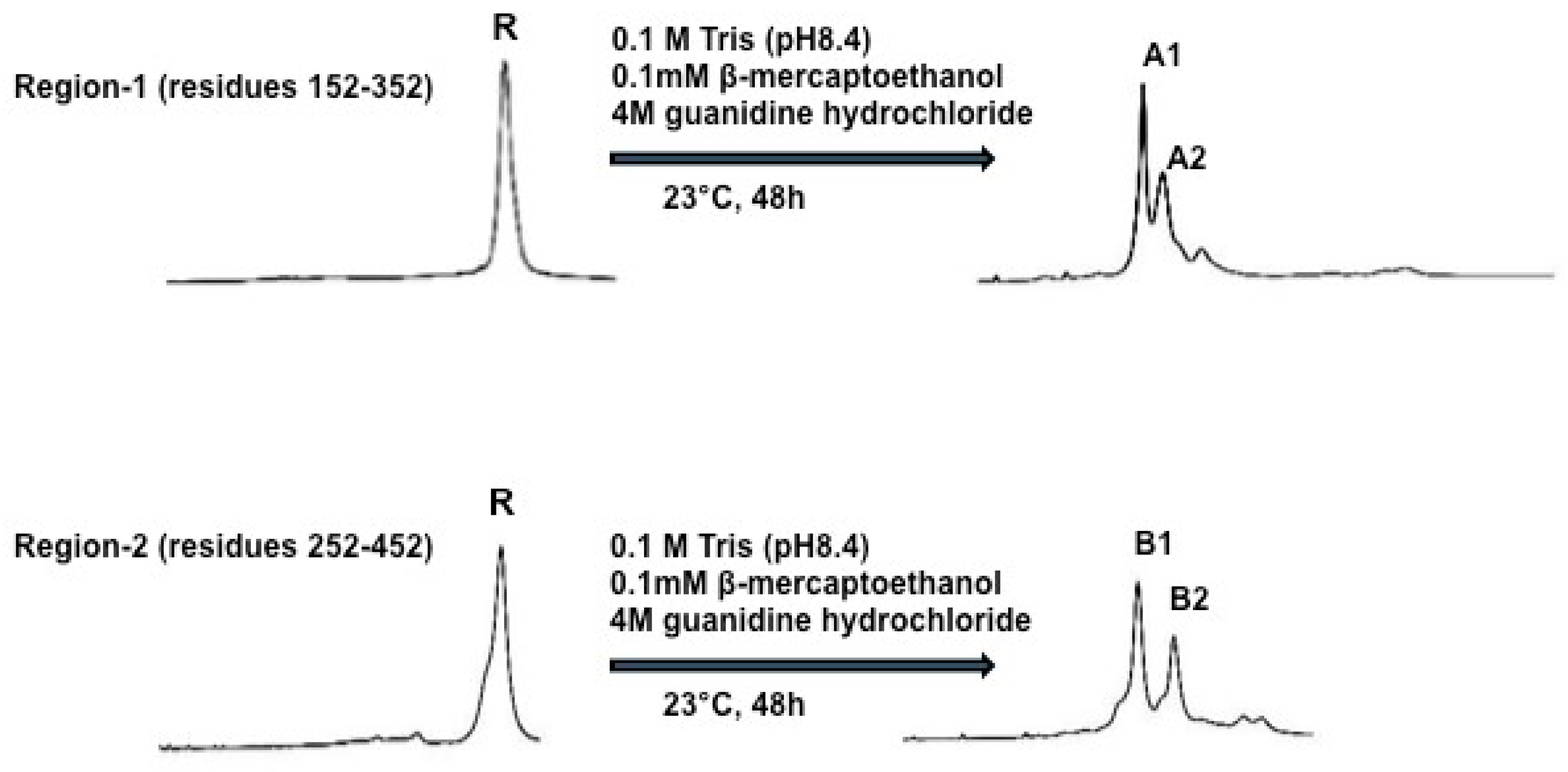
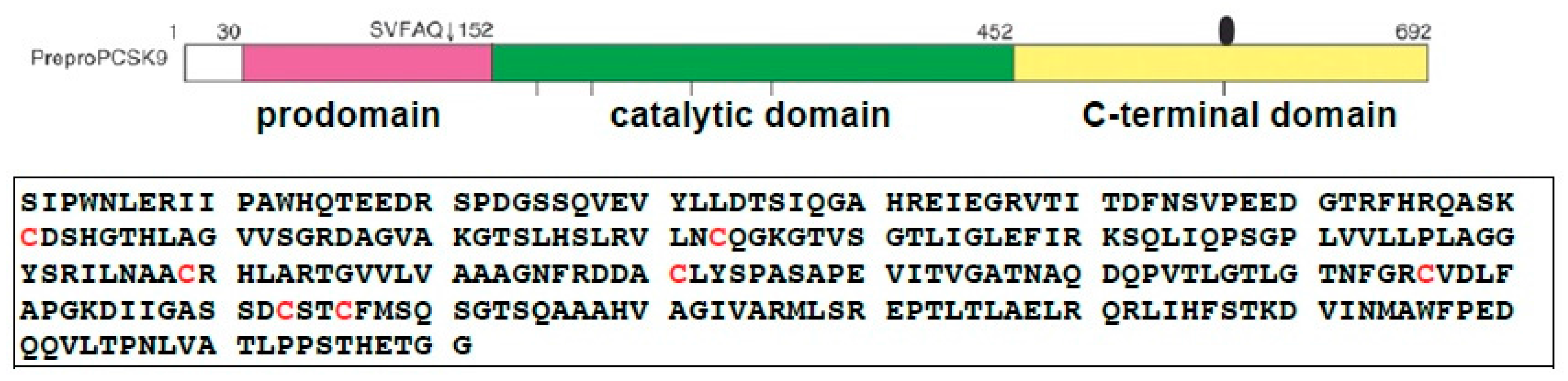
2.1. Expression, Purification and Disulfide Scrambling of Region-1 And -2 of the Catalytic Domain of PCSK9
2.1.1. Expression and Purification
2.1.2. Disulfide Scrambling
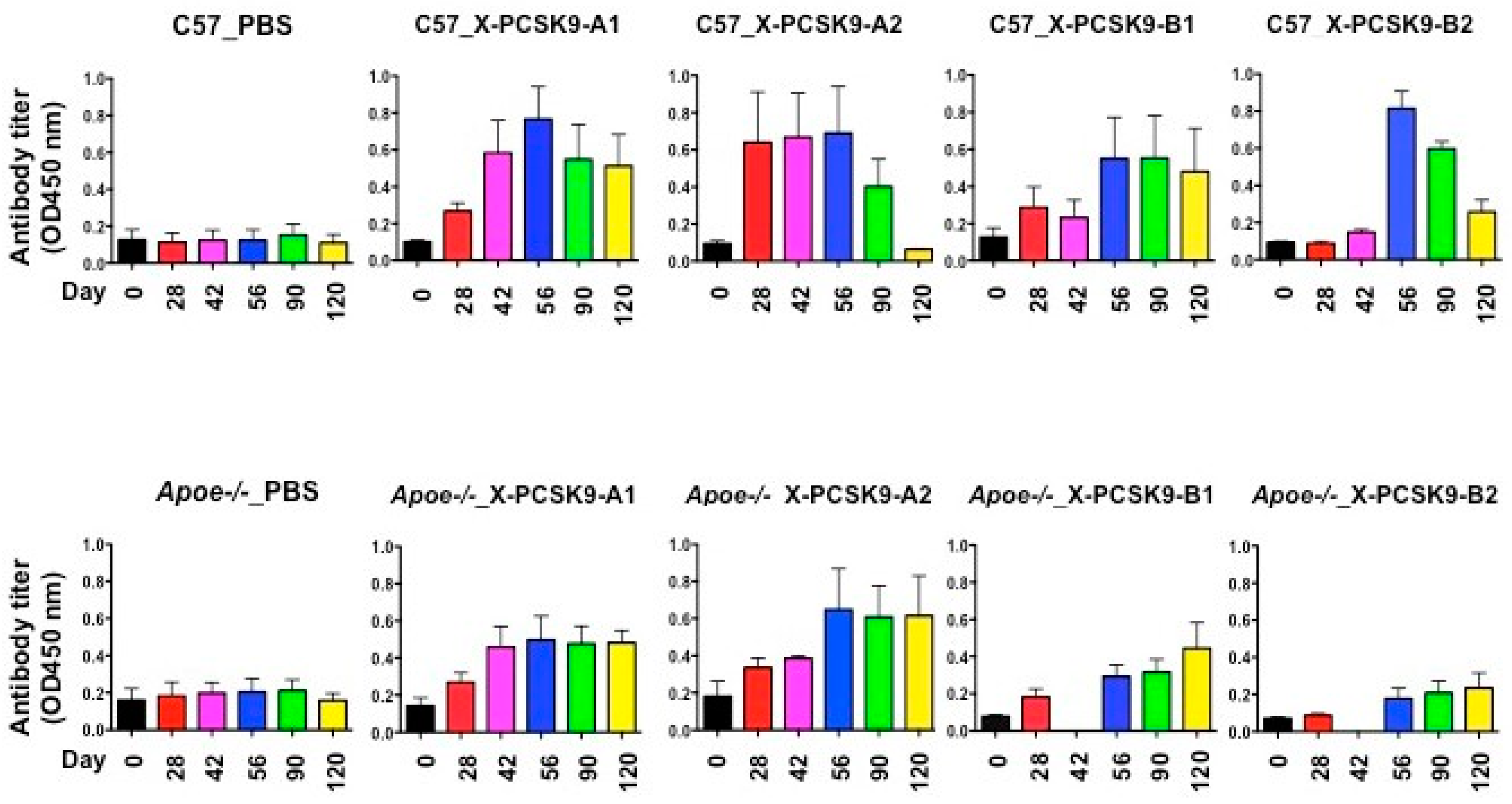
2.2. X-Pcsk9 Isomers Produced Antibody Against Native Pcsk9 in Mice
2.3. X-PCSK9 Immunogens Decreased Plasma Cholesterol Levels in C57BL/6J and Apoe−/− Mice
2.4. X-PCSK9 Immunogens Decreased Plasma Triglyceride Levels in C57BL/6J and Apoe−/− Mice
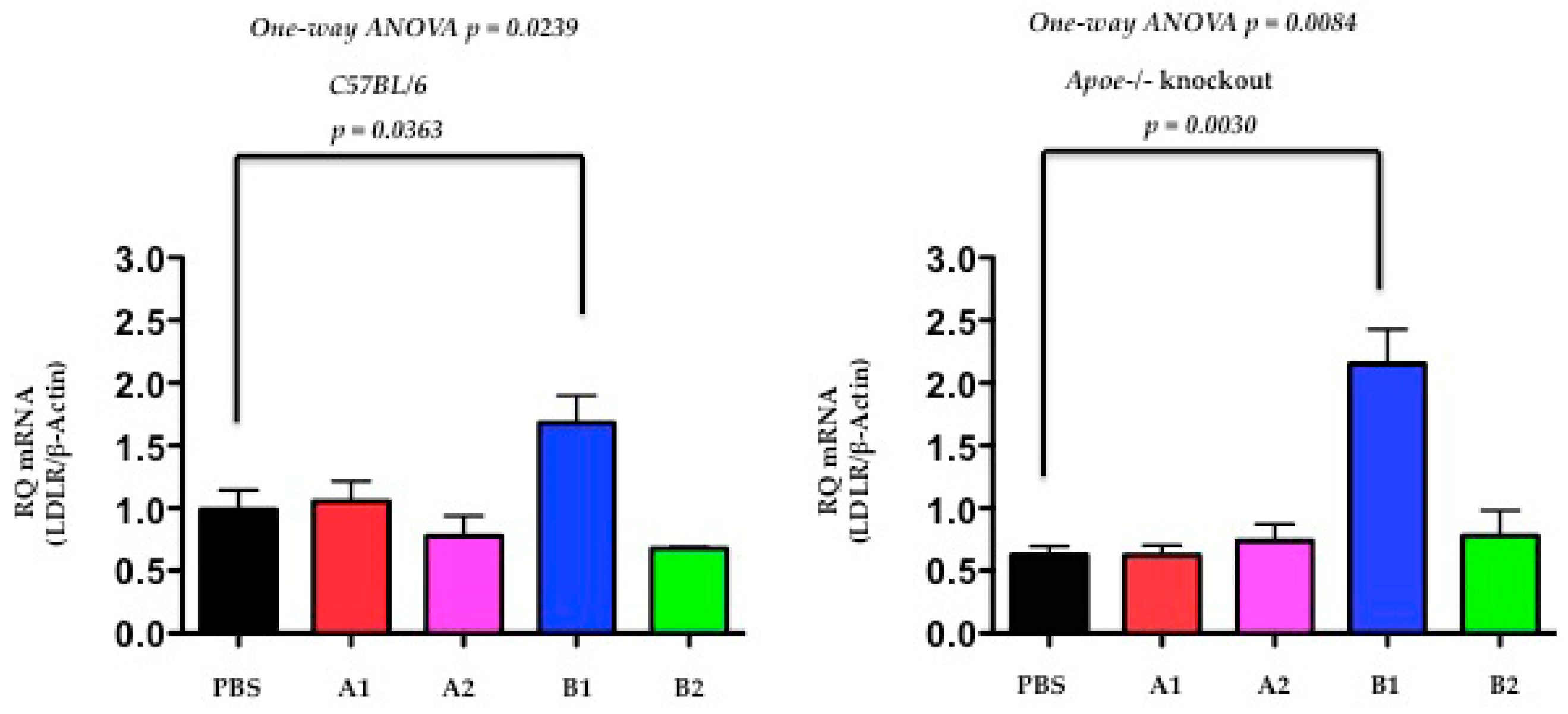
2.5. The Functional Effect of X-PCSK9 in LDL Receptor mRNA: X-PCSK9-B1 Treatment Increased Hepatic LDL Receptor mRNA Level in Both C57BL/6J and Apoe−/− Mice
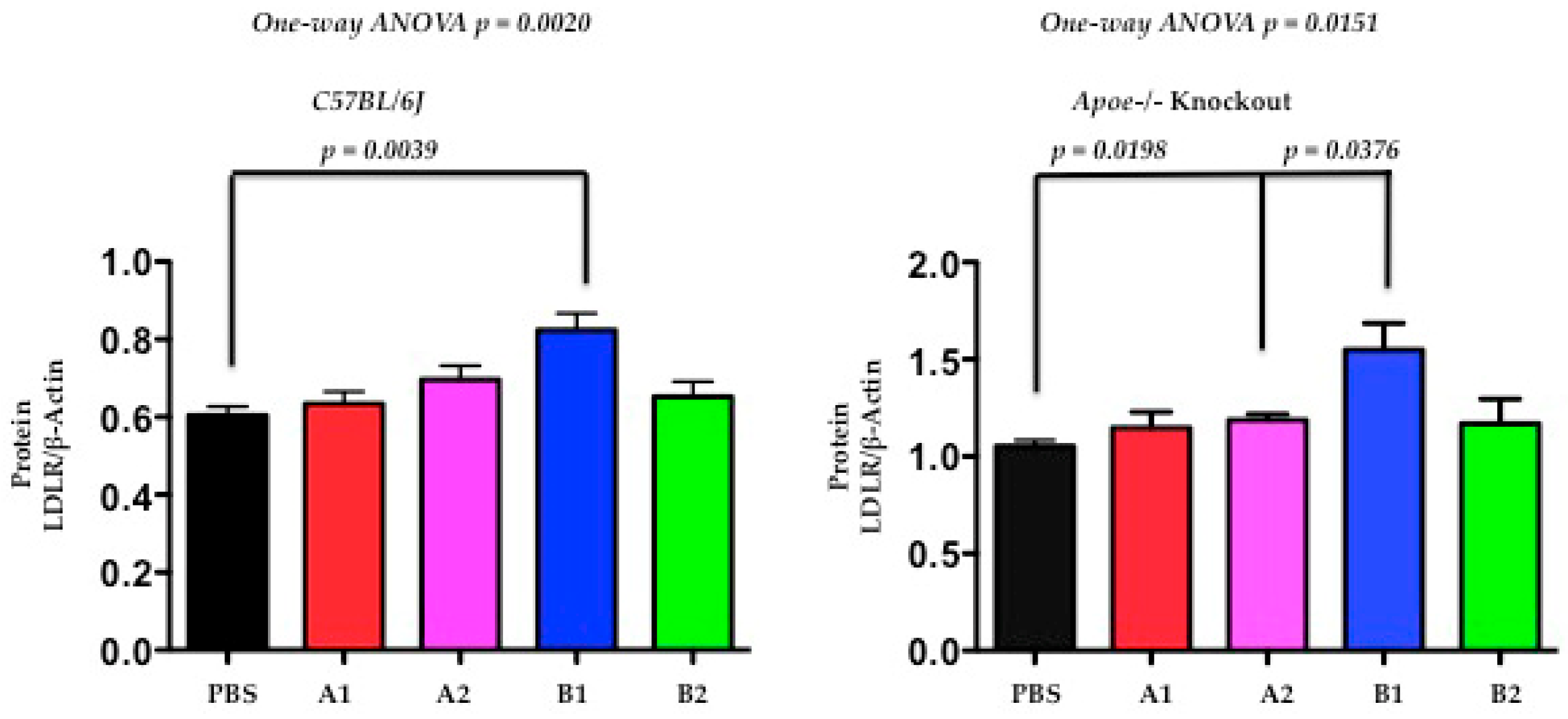
2.6. The Functional Effect of X-PCSK9 in LDL Receptor Protein: X-PCSK9-B1 Treatment Increased the Levels of Hepatic LDL Receptor Proteins in Both C57BL/6J and Apoe−/− Mice
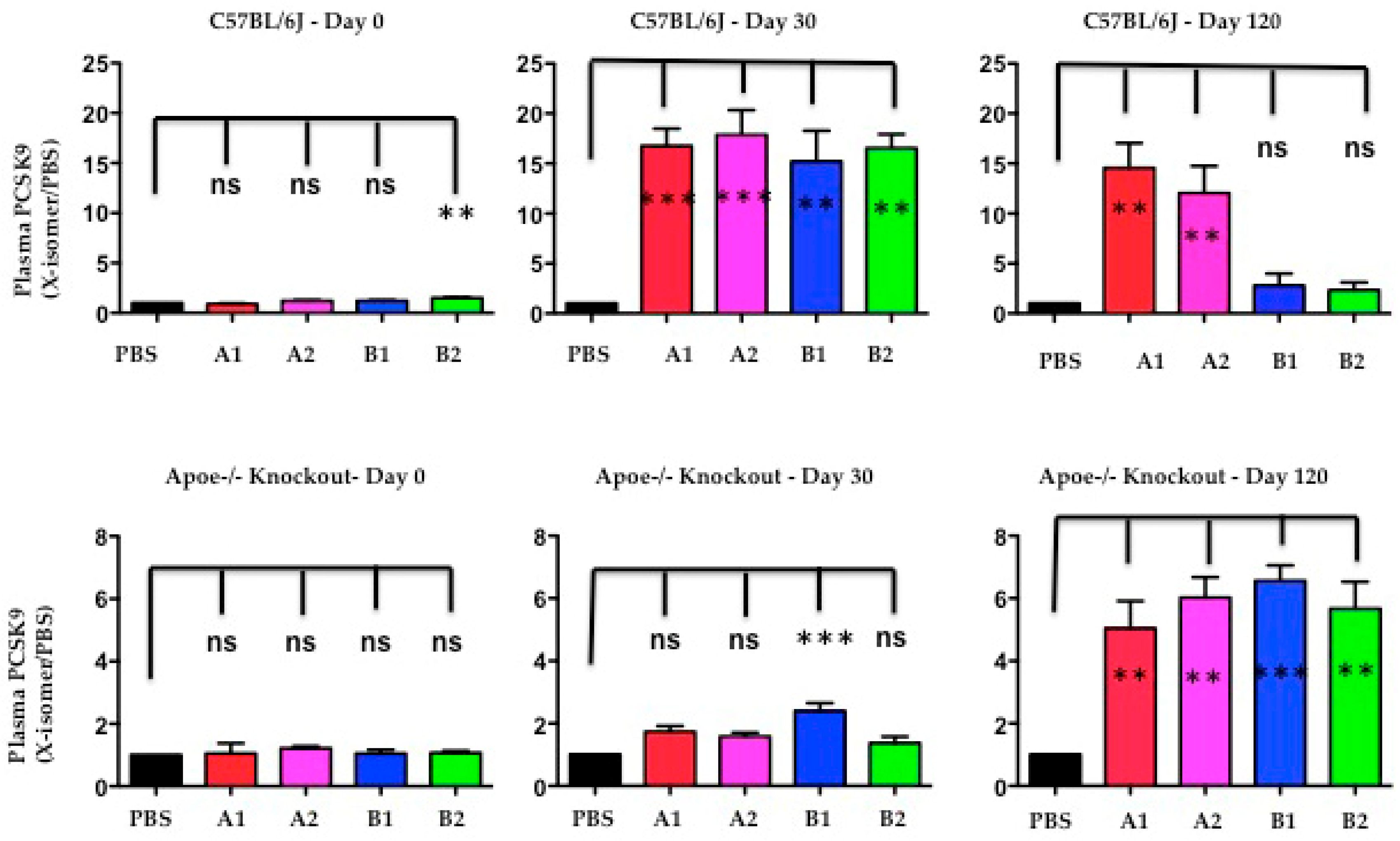
2.7. The Functional Effect of X-PCSK9 in Plasma PCSK9 Concentration: X-PCSK9-Isomers Treatment Increased the Levels of Plasma PCSK9 in Both C57BL/6J and Apoe−/− Mice
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of the PCSK9 Catalytic Domain for Isomer Formation
4.2. Preparation of X-Isomers (X-PCSK9) from the Fully Reduced Proteins Using Oxidative Folding and Disulfide Scrambling
4.3. Q-TOF LC/MS
4.4. Calculations
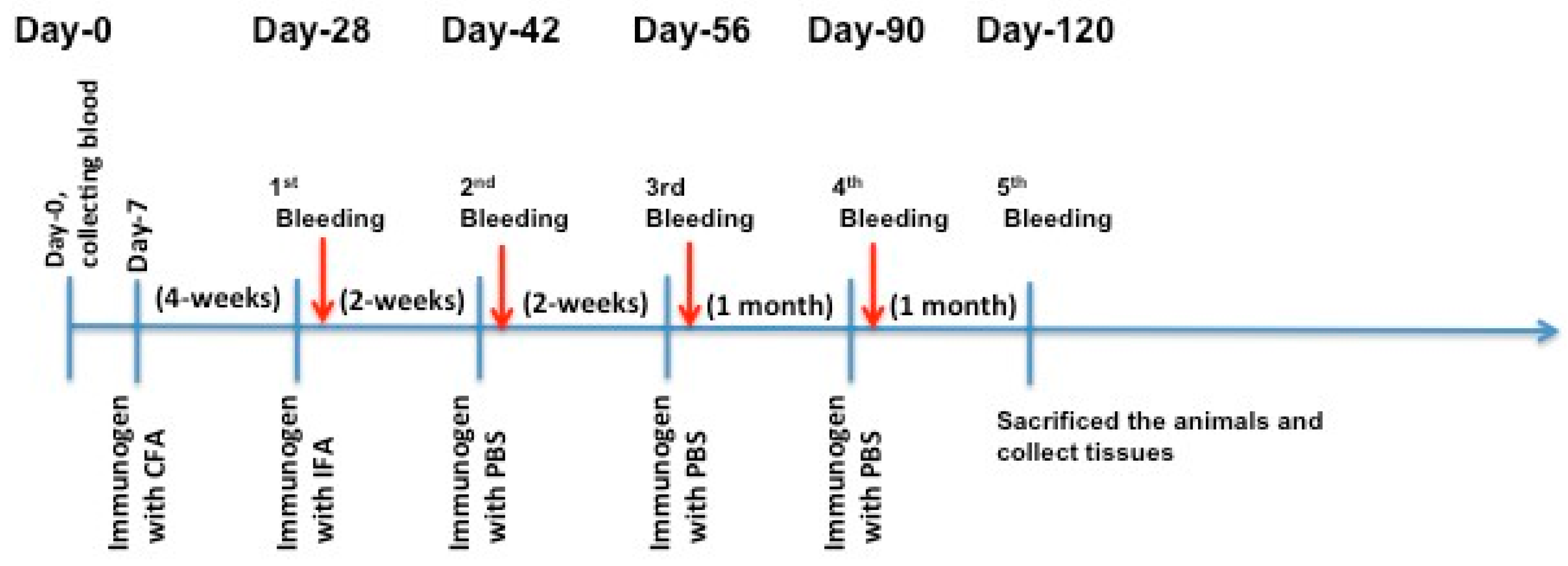
4.5. Immunization of Male C57BL/6J and Apoe−/− Knockout Mice
4.6. Immunogenicity Determination Using ELISA
4.7. Analysis of Plasma Cholesterol and Triglyceride Levels
4.8. Analysis of Hepatic LDL Receptor mRNA and Protein Levels
4.8.1. Real-Time qPCR
4.8.2. Western Blot Analysis
4.8.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Molecular biology of PCSK9: Its role in LDL metabolism. Trends Biochem. Sci. 2007, 32, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. PCSK9: A convertase that coordinates LDL catabolism. J. Lipid Res. 2009, 50, S172–S177. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Rabes, J.P.; Devillers, M.; Munnich, A.; Erlich, D.; Junien, C.; Varret, M.; Boileau, C. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 2009, 30, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Grande, P.; Schnohr, P.; Tybjaerg-Hansen, A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 2010, 55, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tuakli-Wosornu, Y.; Lagace, T.A.; Kinch, L.; Grishin, N.V.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 2006, 79, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Mach, F.; Zavitz, K.; Kurtz, C.; Im, K.; Kanevsky, E.; Schneider, J.; Wang, H.; Keech, A.; Pedersen, T.R.; et al. Cognitive Function in a Randomized Trial of Evolocumab. N. Engl. J. Med. 2017, 377, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Investigators, Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Revkin, J.; Amarenco, P.; Brunell, R.; Curto, M.; Civeira, F.; Flather, M.; Glynn, R.J.; Gregoire, J.; Jukema, J.W.; et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N. Engl. J. Med. 2017, 376, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Tardif, J.C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.P.; Kim, A.M.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N. Engl. J. Med. 2017, 376, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.; Ginsberg, H.N.; Langslet, G.; Hovingh, G.K.; Ceska, R.; Dufour, R.; Blom, D.; Civeira, F.; Krempf, M.; Lorenzato, C.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Somaratne, R.; Nissen, S.E. Evolocumab Added to Statins to Reduce Progression of Coronary Atherosclerosis-Reply. JAMA 2017, 317, 1691. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Kazi, D.S. PCSK9 Inhibitors: Economics and Policy. J. Am. Coll. Cardiol. 2017, 70, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y. The folding pathway of α-lactalbumin elucidated by the technique of disulfide scrambling. Isolation of on-pathway and off-pathway intermediates. J. Biol. Chem. 2002, 277, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y. Conformational isomers of denatured and unfolded proteins: Methods of production and applications. Protein J. 2009, 28, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xiong, W.; Lu, B.Y.; Gonda, M.A.; Chang, J.Y. Synthesis and immune response of non-native isomers of vascular endothelial growth factor. Biochemistry 2010, 49, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.D.; Miles, J.; Tavori, H.; Fazio, S. Diagnosing Resistance to a Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor. Ann. Intern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Penko, J.; Coxson, P.G.; Moran, A.E.; Ollendorf, D.A.; Tice, J.A.; Bibbins-Domingo, K. Updated Cost-effectiveness Analysis of PCSK9 Inhibitors Based on the Results of the FOURIER Trial. JAMA 2017, 318, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Piper, D.E.; Cao, Q.; Liu, D.; King, C.; Wang, W.; Tang, J.; Liu, Q.; Higbee, J.; Xia, Z.; et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA 2009, 106, 9820–9825. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Herz, J.; Maeda, N.; Goldstein, J.L.; Brown, M.S. The two-receptor model of lipoprotein clearance: Tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 4431–4435. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Marcinkiewicz, J.; Zaid, A.; Gauthier, D.; Poirier, S.; Lazure, C.; Seidah, N.G.; Prat, A. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation 2012, 125, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Roche-Molina, M.; Sanz-Rosa, D.; Cruz, F.M.; Garcia-Prieto, J.; Lopez, S.; Abia, R.; Muriana, F.J.; Fuster, V.; Ibanez, B.; Bernal, J.A. Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tavori, H.; Giunzioni, I.; Predazzi, I.M.; Plubell, D.; Miles, J.; DeVay, R.M.; Liang, H.; Rashid, S.; Linton, M.F.; Fazio, S. Human PCSK9 promotes hepatic lipogenesis and atherosclerosis development via apoE- and LDLR-mediated mechanisms. Cardiovasc. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ason, B.; van der Hoorn, J.W.; Chan, J.; Lee, E.; Pieterman, E.J.; Nguyen, K.K.; Di, M.; Shetterly, S.; Tang, J.; Yeh, W.C.; et al. PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE. J. Lipid Res. 2014, 55, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Ambuhl, P.M.; Tissot, A.C.; Fulurija, A.; Maurer, P.; Nussberger, J.; Sabat, R.; Nief, V.; Schellekens, C.; Sladko, K.; Roubicek, K.; et al. A vaccine for hypertension based on virus-like particles: Preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 2007, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Quan, W.Y.; Pandey, R.; Yi, S.; Chi, L.; Xia, L.Z.; Yuan, M.; Ming, L. A vaccine for hypertension based on peptide AngI-R: A pilot study. Int. J. Cardiol. 2011, 148, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Quan, W.Y.; Hong, F.; Jie, S.L. Vaccine for hypertension: Modulating the renin-angiotensin system. Int. J. Cardiol. 2009, 134, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kosloski, L.M.; Ha, D.M.; Hutter, J.A.; Stone, D.K.; Pichler, M.R.; Reynolds, A.D.; Gendelman, H.E.; Mosley, R.L. Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. J. Neurochem. 2010, 114, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.D.; Stone, D.K.; Hutter, J.A.; Benner, E.J.; Mosley, R.L.; Gendelman, H.E. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J. Immunol. 2010, 184, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Redman, J.M.; Collins, J.M.; Bilusic, M. Cancer Vaccines: Enhanced Immunogenic Modulation through Therapeutic Combinations. Hum. Vaccin Immunother. 2017, 13, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Hamouda, H.A.; Villeneuve, L.; Demers, A.; Mayer, G. Trafficking Dynamics of PCSK9-Induced LDLR Degradation: Focus on Human PCSK9 Mutations and C-Terminal Domain. PLoS ONE 2016, 11, e0157230. [Google Scholar] [CrossRef] [PubMed]
- Schiele, F.; Park, J.; Redemann, N.; Luippold, G.; Nar, H. An antibody against the C-terminal domain of PCSK9 lowers LDL cholesterol levels in vivo. J. Mol. Biol. 2014, 426, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y. The properties of scrambled hirudins. J. Biol. Chem. 1995, 270, 25661–25666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chang, J.Y. Isomers of human α-synuclein stabilized by disulfide bonds exhibit distinct structural and aggregative properties. Biochemistry 2007, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]


| C57BL/6J Mice | |||||
| Treatment | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.9025 | 0.0002 | <0.0001 | 0.0061 | 0.0006 |
| Apoe−/− Mice | |||||
| Treatment | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.0007 | 0.0002 | 0.001 | <0.0001 | 0.0007 |
| C57BL/6J Cholesterol (mg/dL) | Day-0 | Day-28 | Day-42 | Day-56 | Day-90 | Day-120 |
| PBS | 58 ± 13 | 63 ± 17 | 66 ± 12 | 56 ± 5.1 | 60 ± 6.1 | 63 ± 9.3 |
| X-PCSK9-A1 | 60 ± 9.4 | 56 ± 7.6 | 50 ± 7.2 | 46 ± 4.5 | 50 ± 9.1 | 56 ± 12 |
| X-PCSK9-A2 | 68 ± 5.1 | 59 ± 5.0 | 62 ± 6.0 | 58 ± 7.2 | 59 ± 7.6 | 61 ± 4.2 |
| X-PCSK9-B1 | 66 ± 9.6 | 64 ± 6.3 | 71 ± 6.9 | 61 ± 3.8 | 59 ± 14 | 57 ± 12 |
| X-PCSK9-B2 | 66 ± 3.8 | 61 ± 4.5 | 61 ± 1.8 | 62 ± 2.1 | 62 ± 3.4 | 65 ± 2.6 |
| Apoe−/− Cholesterol (mg/dL) | Day-0 | Day-28 | Day-42 | Day-56 | Day-90 | Day-120 |
| PBS | 270 ± 39 | 391 ± 46 | 403 ± 48 | 410 ± 98 | 467 ± 128 | 471 ± 128 |
| X-PCSK9-A1 | 342 ± 50 | 384 ± 36 | 365 ± 63 | 348 ± 61 | 361 ± 69 | 342 ± 66 |
| X-PCSK9-A2 | 358 ± 60 | 393 ± 58 | 362 ± 79 | 312 ± 50 | 304 ± 55 | 303 ± 62 |
| X-PCSK9-B1 | 406 ± 64 | 357 ± 36 | ND | 335 ± 42 | 350 ± 58 | 340 ± 70 |
| X-PCSK9-B2 | 357 ± 80 | 358 ± 43 | ND | 412 ± 38 | 333 ± 53 | 321 ± 57 |
| C57BL/6J Mice (Cholesterol) | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.6978 | 0.0003 | 0.0044 | 0.0014 | 0.0114 |
| A1 vs. PBS | A2 vs. PBS | B1 vs. PBS | B2 vs. PBS | ||
| Treatment effect (p-value) | 0.0612 | 0.9665 | 0.0325 | 0.7035 | |
| Apoe−/− Mice (Cholesterol) | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.0006 | 0.5566 | 0.1701 | 0.0069 | 0.7087 |
| A1 vs. PBS | A2 vs. PBS | B1 vs. PBS | B2 vs. PBS | ||
| Treatment effect (p-value) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C57BL/6J Triglyceride (mg/dL) | Day-0 | Day-28 | Day-42 | Day-56 | Day-90 | Day-120 |
| PBS | 12 ± 5.2 | 14 ± 5.5 | 13 ± 2.4 | 16 ± 2.7 | 13 ± 10 | 9.5 ± 3.2 |
| X-PCSK9-A1 | 51 ± 33 | 25 ± 4.9 | 24 ± 9.5 | 28 ± 7.1 | 25 ± 11 | 19 ± 4.7 |
| X-PCSK9-A2 | 30 ± 8.6 | 26 ± 2.5 | 53 ± 15 | 24 ± 5.6 | 24 ± 6.5 | 17 ± 2.0 |
| X-PCSK9-B1 | 28 ± 11 | 43 ± 13 | 22 ± 7.0 | 39 ± 14 | 30 ± 10 | 24 ± 8.0 |
| X-PCSK9-B2 | 26 ± 10 | 39 ± 11 | 17 ± 3.4 | 26 ± 2.3 | 29 ± 6.5 | 22 ± 5.9 |
| Apoe−/− Triglyceride (mg/dL) | Day-0 | Day-28 | Day-42 | Day-56 | Day-90 | Day-120 |
| PBS | 55 ± 13 | 59 ± 18 | 54 ± 19 | 40 ± 9.4 | 49 ± 3.4 | 37 ± 5.5 |
| X-PCSK9-A1 | 64 ± 19 | 90 ± 33 | 75 ± 30 | 45 ± 17 | 50 ± 18 | 45 ± 19 |
| X-PCSK9-A2 | 85 ± 30 | 68 ± 18 | 69 ± 28 | 40 ± 19 | 41 ± 27 | 43 ± 19 |
| X-PCSK9-B1 | 82 ± 13 | 73 ± 21 | ND | 41 ± 5.5 | 48 ± 8.1 | 54 ± 9.9 |
| X-PCSK9-B2 | 71 ± 25 | 55 ± 17 | ND | 67 ± 13 | 61 ± 9.2 | 40 ± 13 |
| C57BL/6J Mice (Triglyceride) | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.3284 | 0.0045 | 0.1516 | 0.0434 | 0.4195 |
| A1 vs. PBS | A2 vs. PBS | B1 vs. PBS | B2 vs. PBS | ||
| Treatment effect (p-value) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Apoe−/− Mice (Triglyceride) | PBS | X-PCSK9-A1 | X-PCSK9-A2 | X-PCSK9-B1 | X-PCSK9-B2 |
| Time effect (p-value) | 0.0756 | 0.0038 | 0.0029 | 0.0033 | 0.1784 |
| A1 vs. PBS | A2 vs. PBS | B1 vs. PBS | B2 vs. PBS | ||
| Treatment effect (p-value) | 0.5777 | 0.7993 | 0.5533 | 0.5051 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Nischal, H.; Sun, H.; Li, L.; Cao, Y.; Wei, P.; Chang, J.-Y.; Teng, B.-B. Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels. Int. J. Mol. Sci. 2018, 19, 640. https://doi.org/10.3390/ijms19020640
Jiang C, Nischal H, Sun H, Li L, Cao Y, Wei P, Chang J-Y, Teng B-B. Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels. International Journal of Molecular Sciences. 2018; 19(2):640. https://doi.org/10.3390/ijms19020640
Chicago/Turabian StyleJiang, Chuantao, Hersharan Nischal, Hua Sun, Li Li, Ying Cao, Peng Wei, Jui-Yoa Chang, and Ba-Bie Teng. 2018. "Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels" International Journal of Molecular Sciences 19, no. 2: 640. https://doi.org/10.3390/ijms19020640
APA StyleJiang, C., Nischal, H., Sun, H., Li, L., Cao, Y., Wei, P., Chang, J.-Y., & Teng, B.-B. (2018). Non-Native Conformational Isomers of the Catalytic Domain of PCSK9 Induce an Immune Response, Reduce Lipids and Increase LDL Receptor Levels. International Journal of Molecular Sciences, 19(2), 640. https://doi.org/10.3390/ijms19020640




