Articular Cartilage Aging-Potential Regenerative Capacities of Cell Manipulation and Stem Cell Therapy
Abstract
1. Introduction
2. Cellular Senescence
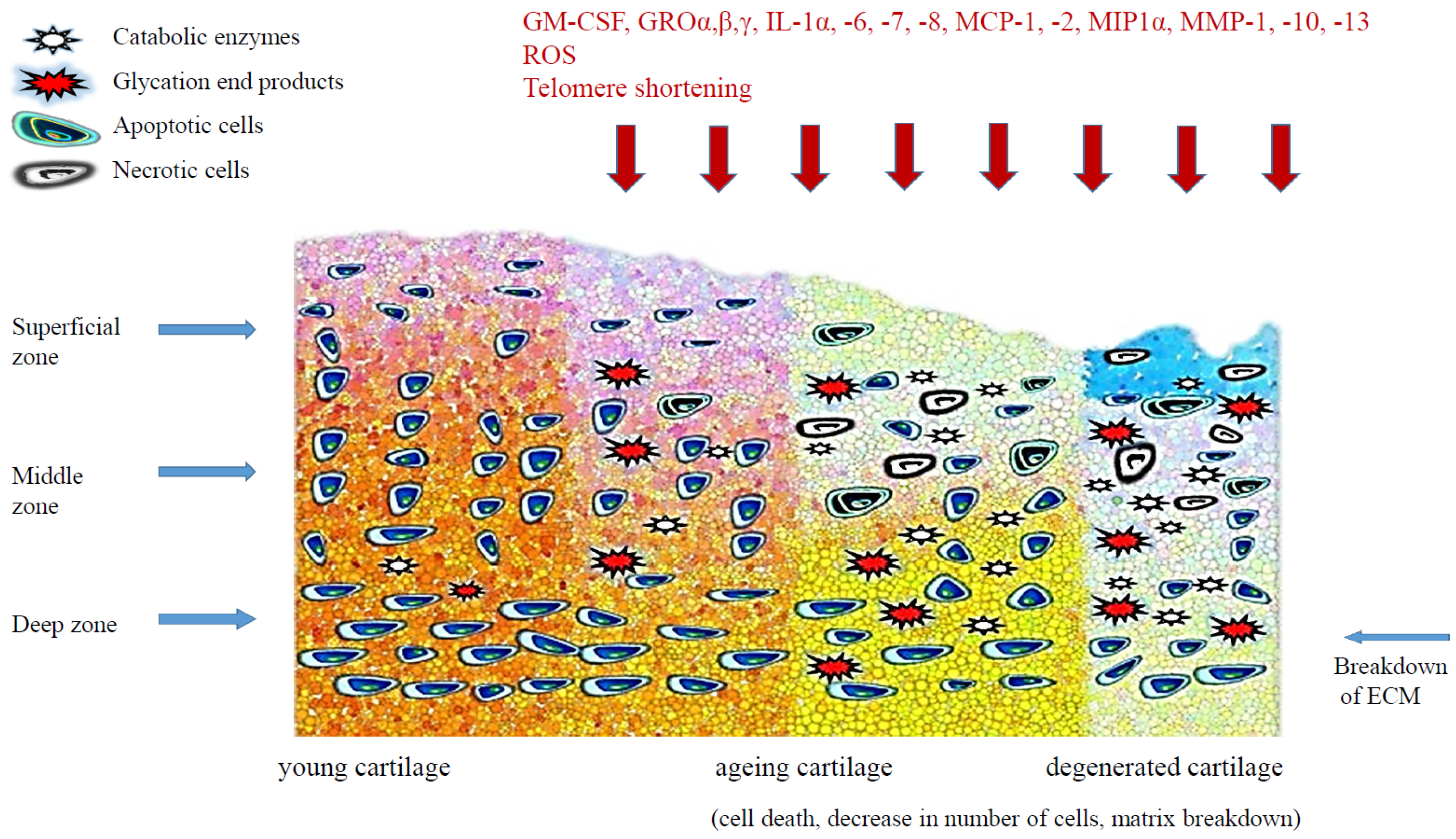
3. Senescence-Related Changes of Articular Cartilage
3.1. Telomere Shortening
3.2. Oxidative Stress
3.3. Inflammatory Cytokines
3.4. Altered Responsiveness to Growth Factors
3.5. Advanced Glycation End-Products
3.6. Autophagy
4. Possible Anti-Aging Strategies
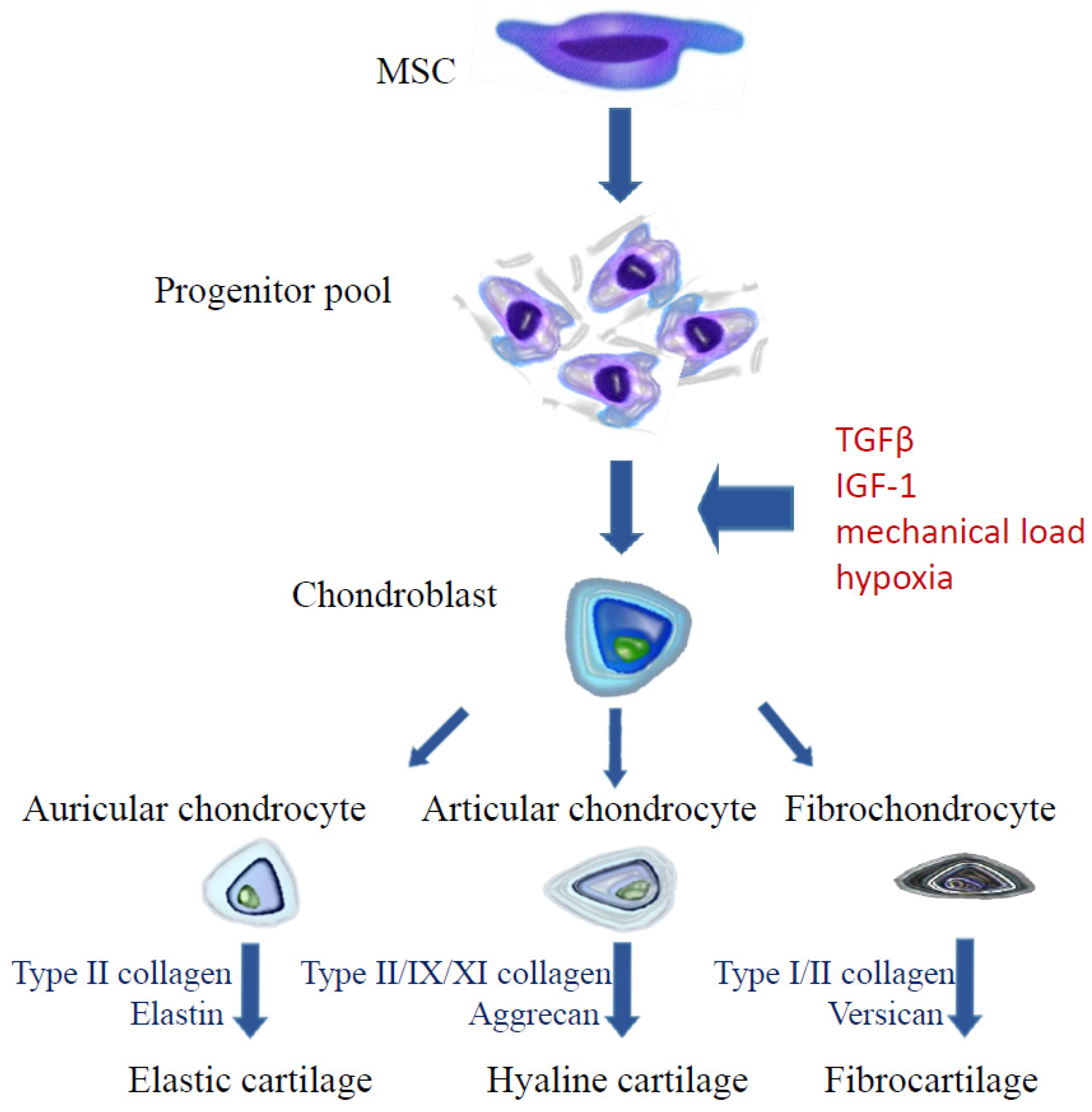
4.1. Cell Manipulation
4.1.1. Telomerase Activators
4.1.2. Antioxidants and Hypoxia
4.1.3. Mechanical Load
4.2. Cell-Based Therapy
4.2.1. Autologous Chondrocyte Implantation
4.2.2. Mesenchymal Stem Cells
4.3. Cell-Free Procedures
5. Summary
Acknowledgments
Conflicts of Interest
References
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Aigner, T.; Rose, J.; Martin, J.; Buckwalter, J. Aging theories of primary osteoarthritis: From epidemiology to molecular biology. Rejuvenation Res. 2004, 7, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Buckwalter, J.A. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J. Bone Jt. Surg. Am. 2003, 85, 106–110. [Google Scholar] [CrossRef]
- Messai, H.; Duchossoy, Y.; Khatib, A.M.; Mitrovic, D.R. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: An altered signalling pathway. Mech. Ageing Dev. 2000, 115, 21–37. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lai, K.Y.; Hung, L.F.; Wu, W.L.; Liu, F.C.; Ho, L.J. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology (Oxford) 2011, 50, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Bader, A.; Hepp, P.; Grill, W.; Fuchs, B.; Käs, J.A.; Krinner, A.; Marquass, B.; Müller, K.; Schiller, J.; et al. Mesenchymal Stem Cells in Cartilage Repair: State of the Art and Methods to monitor Cell Growth, Differentiation and Cartilage Regeneration. Curr. Med. Chem. 2010, 17, 2274–2291. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Intracellular determinants of cell aging. Mech. Ageing Dev. 1984, 28, 177–185. [Google Scholar] [CrossRef]
- Sikora, E.; Arendt, T.; Bennett, M.; Narita, M. Impact of cellular senescence signature on ageing research. Ageing Res. Rev. 2011, 10, 146–152. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Betts, D.; King, W.A.; Madan, P. Senescence or apoptosis? The choice bovine fibroblasts make in the presence of increasing concentrations of extracellular H2O2. SURG J. 2010, 3, 64–68. [Google Scholar]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T.; Petrie, J.; Kirkwood, T.B. Telomere-driven replicative senescence is a stress response. Nat. Biotechnol. 2003, 21, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gilley, D.; Herbert, B.S.; Huda, N.; Tanaka, H.; Reed, T. Factors impacting human telomere homeostasis and age-related disease. Mech. Ageing Dev. 2008, 129, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kaul, Z.; Cesare, A.J.; Huschtscha, L.I.; Neumann, A.A.; Reddel, R.R. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2011, 13, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Wnuk, M.; Przybylska, D.; Grabowska, W.; Lewinska, A.; Alster, O.; Korwek, Z.; Cmoch, A.; Myszka, A.; Pikula, S.; et al. A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta. Biogerontology 2014, 15, 47–64. [Google Scholar] [CrossRef] [PubMed]
- D’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.; D’Lima, D.D.; Hashimoto, S.; Lotz, M. Cell death in cartilage. Osteoarthr. Cartil. 2004, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vignon, E.; Arlot, M.; Patricot, L.M.; Vignon, G. The cell density of human femoral head cartilage. Clin. Orthop. Relat. Res. 1976, 121, 303–308. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Scharstuhl, A.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Reduced transforming growth factor-beta signalling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005, 7, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Hemmel, M.; Neureiter, D.; Gebhard, P.M.; Zeiler, G.; Kirchner, T.; McKenna, L. Apoptotic Cell Death Is Not a Widespread Phenomenon in Normal Aging and Osteoarthritis Human Articular Knee Cartilage: A Study of Proliferation, Programmed Cell Death (Apoptosis) and Viability of Chondrocytes in Normal and Osteoarthritic Human Knee Cartilage. Arthritis Rheum. 2001, 44, 1304–1312. [Google Scholar] [PubMed]
- Ding, C.; Cicuttini, F.; Blizzard, L.; Scott, F.; Jones, G. A longitudinal study of the effect of sex and age on rate of change in knee cartilage volume in adults. Rheumatology (Oxford) 2007, 46, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.; Davidson, C.; Morgelin, M.; Bird, J.L.E.; Bayliss, M.T.; Dudhia, J. Age-related changes in the composition, the molecular stoichiometry and the stability of proteoglycan aggregates extracted from human articular cartilage. Biochem. J. 2003, 370, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Brümmendorf, T.H.; Richter, W.; Fellenberg, J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002, 46, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Harbo, M.; Delaisse, J.M.; Kjaersgaard-Andersen, P.; Soerensen, F.B.; Koelvraa, S.; Bendix, L. The relationship between ultra-short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech. Ageing Dev. 2013, 134, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Novakofski, K.D.; Donocoff, R.S.; Liang, Y.X.; Fortier, L.A. Telomerase Activity in Articular Chondrocytes Is Lost after Puberty. Cartilage 2014, 5, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Guillot, P.V.; Gotherstrom, C.; Chan, J.; Kurata, H.; Fisk, N.M. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007, 25, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Ferrero, I.; Rustichelli, D.; Aschero, S.; Gammaitoni, L.; Aglietta, M.; Madon, E.; Fagioli, F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 2006, 97, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Fellenberg, J.; Brümmendorf, T.; Eschlbeck, A.M.; Richter, W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J. Mol. Med. 2004, 82, 49–55. [Google Scholar] [PubMed]
- Dai, S.M.; Shan, Z.Z.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K.; Yudoh, K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: Possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006, 54, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Butler, P.; Cowen, T. Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF and TGF-beta1. Connect. Tissue Res. 2007, 48, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Meyer, M.; Bechmann, V.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp. Cell Res. 2011, 317, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Harbo, M.; Bendix, L.; Bay-Jensen, A.C.; Graakjaer, J.; Soe, K.; Andersen, T.L.; Kjaersgaard-Andersen, P.; Koelvraa, S.; Delaisse, J.M. The distribution pattern of critically short telomeres in human osteoarthritic knees. Arthritis Res. Ther. 2012, 14, R12. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Söder, S.; Skhirtladze, C.; Schmitz, N.; Gebhard, P.M.; Sesselmann, S.; Aigner, T. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthr. Cartil. 2012, 20, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Hartmann, A.; Bechmann, V.; Graf, B.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011, 29, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Underwood, C.; Butler, P.E.; Cowen, T. Vulnerability to ROS-induced cell death in ageing articular cartilage: The role of antioxidant enzyme activity. Osteoarthr. Cartil. 2005, 13, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Kim, S.J. Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp. Biol. Med. 2013, 238, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; van Trieu, N.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380–R391. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.H. Regulation of senescence associated signalling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Gandhi, U.; Long, D.L.; Yin, W.; Chubinskaya, S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol. 2014, 66, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Sun, L.Y.; Pang, C.Y. Synergistic Protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M.; et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.M.; Tsai, J.L.; Lin, S.D.; Lai, C.S.; Chang, C.C. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005, 14, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Torres, Y.; Benguria, A.; Dopazo, A.; Roche, E.; Carrera-Quintanar, L.; Pérez, R.A.; Enríquez, J.A.; Torres, R.; Ramírez, J.C.; et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013, 4, e691. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Foldager, C.; Lind, M.; Zachar, V.; Søballe, K.; Koch, T. Hypoxia enhances chondrogenic differentiation of human adipose tissue-derived stromal cells in scaffold-free and scaffold systems. Cell. Tissue Res. 2014, 355, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Lomri, A. Role of reactive oxygen species and superoxide dismutase in cartilage aging and pathology. Future Reumatol. 2008, 3, 381–392. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Philipot, D.; Guerit, D.; Platano, D.; Chuchana, P.; Olivotto, E.; Espinoza, F.; Dorandeu, A.; Pers, Y.M.; Piette, J.; Borziet, R.M.; et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 2014, 16, R58. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Billinghurst, R.C.; Pidoux, I.; Antoniou, J.; Zukor, D.; Tanzer, M.; Poole, A.R. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002, 46, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.; Poole, A.R.; Reiner, A.; Mollenhauer, C.; Margulis, A.; Kuettner, K.E.; Cole, A.A. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002, 46, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Cole, A.; Murphy, G.; Bienias, J.L.; Im, H.J.; Loeser, R.F. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Blake, S.; Song, X.Y.; Lark, M.; Loeser, R.F. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res. Ther. 2008, 10, R23. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A. Effects of growth factors on articular cartilage. Ortop. Traumatol. Rehab. 2001, 3, 190–193. [Google Scholar]
- De Ceuninck, F.; Caliez, A.; Dassencourt, L.; Anract, P.; Renard, P. Pharmacological disruption of insulin-like growth factor 1 binding to IGF-binding proteins restores anabolic responses in human osteoarthritic chondrocytes. Arthritis Res. Ther. 2004, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Park, J.I.; Loeser, R.F. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 2009, 284, 31972–31981. [Google Scholar] [CrossRef] [PubMed]
- Starkman, B.G.; Cravero, J.D.; Delcarlo, M.; Loeser, R.F. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 2005, 389, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Allan, E.H.; Martin, T.J. The plasminogen activator inhibitor system in bone cell function. Clin. Orthop. Relat. Res. 1995, 313, 54–63. [Google Scholar]
- Murphy-Ullrich, J.E.; Poczatek, M. Activation of latent TGF-beta by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000, 11, 59–69. [Google Scholar] [CrossRef]
- Dangelo, M.; Sarment, D.P.; Billings, P.C.; Pacifici, M. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J. Bone Miner. Res. 2001, 16, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Albro, M.B.; Cigan, A.D.; Nims, R.J.; Yeroushalmi, K.J.; Oungoulian, S.R.; Hung, C.T.; Ateshian, G.A. Shearing of synovial fluid activates latent TGF-β. Osteoarthr. Cartil. 2012, 20, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Remst, D.F.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Parker, W.L.; ten Dijke, P.; Thorikay, M.; Philip, A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 2008, 23, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, S. Generation of novel rat and human pluripotent stem cells by reprogramming and chemical approaches. Methods Mol. Biol. 2010, 636, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, J.; Li, X.; Wang, X.; Guo, Z.; Zhang, F. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev. Biol. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Savage, R.S.; Thornalley, P.J.; Rabbani, N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 2016, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Saudek, D.M.; Kay, J. Advanced glycation endproducts and osteoarthritis. Curr. Reumatol. Rep. 2003, 5, 33–40. [Google Scholar] [CrossRef]
- Mizumura, K.; Choi, A.M.; Ryster, S.W. Emerging role of selective autophagy in human diseases. Front. Pharmacol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, V.; Bohensky, J.; Shapiro, I.M. Autophagy: A new phase in the maturation of growth plate chondrocytes is regulated by HIF, mTOR and AMP kinase. Cells Tissues Organs 2009, 189, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Layfield, R.; Lotz, M.; Settembre, C.; Whitehouse, C. Boning up on autophagy: The role of autophagy in skeletal biology. Autophagy 2014, 10, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vasheghani, F.; Li, Y.H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.P.; et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawakami, Y.; Kobayashi, M.; Greco, N.; Cummins, J.H.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. Ther. 2014, 16, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Matsushita, T.; Tabata, Y.; Saito, T.; Matsumoto, T.; Nagai, K.; Kuroda, R.; Kurosaka, M. Intra-articular administration of gelatin hydrogels incorporating rapamycin-micelles reduces the development of experimental osteoarthritis in a murine model. Biomaterials 2014, 35, 9904–9911. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, O.; Olmer, M.; Akagi, R.; Akasaki, Y.; Fisch, K.M.; Shen, T.; Su, A.I.; Lotz, M.K. Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthr. Cartil. 2016, 24, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Luo, W.; Lei, G.H. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Jt. Bone Spine 2015, 82, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, J.; Terkhorn, S.P.; Freeman, T.A.; Adams, C.S.; Garcia, J.; Shapiro, I.M.; Srinivas, V. Regulation of autophagy in humanand murine cartilage: Hypoxia-inducible factor 2 suppresses chondrocyteautophagy. Arthritis Rheum. 2009, 60, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S.; Chen, S.; Reddi, A.; Counter, C.M.; Robey, P.G.; Wang, C.Y. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat. Biotechnol. 2002, 20, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Pearce, V.P.; Sherrell, J.; Lou, Z.; Kopelovich, L.; Wright, W.E.; Shay, J.W. Immortalization of epithelial progenitor cells mediated by resveratrol. Oncogene 2008, 27, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, A.A.; Steding, C.E.; Herbert, B.S. Pharmaceutical regulation of telomerase and its clinical potential. J. Cell. Mol. Med. 2012, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-aging active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.Y.; Lam, W.S.; Ho, M.K.; Hu, Y.; Ip, F.C.; Pang, H.; Chin, A.C.; Harley, C.B.; Ip, N.Y.; Wong, Y.H. Astragaloside IV and cycloastragenol stimulate the phosphorylation of extracellular signal-regulated protein kinase in multiple cell types. Planta Med. 2012, 78, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.C.; Wang, S.; Li, Y.; Kuang, Y.Y.; Ma, C.M. Chemical constituents and pharmacologic actions of Cynomorium plants. Chin. J. Nat. Med. 2013, 11, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tichon, A.; Eitan, E.; Kurkalli, B.G.; Braiman, A.; Gazit, A.; Slavin, S.; Beith-Yannai, E.; Priel, E. Oxidative stress protection by novel telomerase activators in mesenchymal stem cells derived from healthy and diseased individuals. Curr. Mol. Med. 2013, 13, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Taka, T.; Changtam, C.; Thaichana, P.; Kaewtunjai, N.; Suksamrarn, A.; Lee, T.R.; Tuntiwechapikul, W. Curcuminoid derivatives enhance telomerase activity in an in vitro TRAP assay. Bioorg. Med. Chem. Lett. 2014, 24, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Molgora, B.; Bateman, R.; Sweeney, G.; Finger, D.; Dimler, T.; Effros, R.B.; Valenzuela, H.F. Functional assessment of pharmacological telomerase activators in human T cells. Cells 2013, 2, 57–66. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, B.B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011, 10, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective effect of resveratrol against IL-1β-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-κB signaling pathway: An “in vitro study”. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 45, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, L.; Zhang, P.; Song, J.; Liu, W. An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater. 2014, 10, 4983–4995. [Google Scholar] [CrossRef] [PubMed]
- Moussavi-Harami, F.; Duwayri, Y.; Martin, J.A.; Moussavi-Harami, F.; Buckwalter, J.A. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: Consequences for tissue engineering. Iowa Orthop. J. 2004, 24, 15–20. [Google Scholar] [PubMed]
- Martin, J.A.; Klingelhutz, A.J.; Moussavi-Harami, F.; Buckwalter, J.A. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J. Gerontol. A. Biol. Sci. Med. Sci. 2004, 59, B324–B336. [Google Scholar] [CrossRef]
- Egli, R.J.; Bastian, J.D.; Ganz, R.; Hofstetter, W.; Leunig, M. Hypoxic expansion promotes the chondrogenic potential of articular chondrocytes. J. Orthop. Res. 2008, 26, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Schrobback, K.; Klein, T.J.; Crawford, R.; Upton, Z.; Malda, J.; Leavesley, D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012, 347, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Wan Safwani, W.K. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.Z.; Choi, J.R.; Yong, K.W.; Ting, I.; Mat Adenan, N.A.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.Z.; Wong, C.W.; Yong, K.W.; Choi, J.R.; Mat Adenan, N.A.; Omar, S.Z.; Wan Abas, W.A.B.; Pingguan-Murphy, B. The effects of hypoxia and serum-free conditions on the stemness properties of human adipose-derived stem cells. Cytotechnology 2013, 68, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Hobiger, S.; Despot-Slade, E.; Pichler, M.; Zenobi-Wong, M. Hypoxia regulates RhoA and Wnt/β-catenin signaling in a context-dependent way to control re-differentiation of chondrocytes. Sci. Rep. 2017, 7, 9032. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Schätti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.; Fahy, N.; Alini, M.; Stoddart, M.J. Differences in human mesenchymal stell cell secretomes during chondrogenic induction. Eur. Cell Mater. 2016, 31, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kupcsik, L.; Yao, S.J.; Alini, M.; Stoddart, M.J. Mechanical Load Modulates Chondrogenesis of Human Mesenchymal Stem Cells through the TGFβ Pathway. J. Cell Mol. Med. 2010, 14, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yu, L.; Lim, C.G.; Goodley, A.S.; Xiao, X.; Placone, J.K.; Ferlin, K.M.; Nguyen, B.N.; Hsieh, A.H.; Fisher, J.P. Effect of Dynamic Culture and Periodic Compression on Human Mesenchymal Stem Cell Proliferation and Chondrogenesis. Ann. Biomed. Eng. 2016, 44, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Zhang, E.C.; Mauck, R.L.; Burdick, J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng. Part A 2012, 18, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.; Ge, Z.; Tan, L.P.; Hui, J.H.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Abdullah, A.A.; et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: A phase I/II study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.; Behrens, P.; Volpi, P.; de Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of Autologous Matrix Induced Chondrogenesis (AMIC) in cartilage knee surgery: Data of the AMIC Registry. Arch. Orthop. Trauma Surg. 2013, 133, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Brittberg, M.; Kiviranta, I.; Akerlund, E.L.; Lindahl, A. Autologous chon-drocyte transplantation. Biomechanics and long-term durability. Am. J. Sports Med. 2002, 30, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, X.; Zhou, J.; Wei, L. The age-related changes in cartilage and osteoarthritis. BioMed Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Grogan, S.; Schafer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. 2009, 191, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.H.; Solar-Cafaggi, S.; Quinn, T.M. Functionalization of dynamic culture surfaces with a cartilage extracellular matrix extract enhances chondrocyte phenotype against dedifferentiation. Acta Biomater. 2012, 8, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.H.; Matmati, M.; Khayat, G.; Chaudhry, S.; Hinz, B.; Quinn, T.M. Culture of primary bovine chondrocytes on a continuously expanding surface inhibits dedifferentiation. Tissue Eng. Part A 2012, 18, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Kino-Oka, M.; Yashiki, S.; Ota, Y.; Mushiaki, Y.; Sugawara, K.; Yamamoto, T.; Takezawa, T.; Taya, M. Subculture of chondrocytes on a collagen type I-coated substrate with suppressed cellular dedifferentiation. Tissue Eng. 2005, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Das, R.H.; Jahr, H.; Verhaar, J.A.; van der Linden, J.C.; van Osch, G.J.; Weinans, H. In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthr. Cartil. 2008, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Martin, I. Human articular chondrocytes culture. Methods Mol. Med. 2007, 140, 237–247. [Google Scholar] [PubMed]
- Gurusinghe, S.; Strappe, P. Gene modification of mesenchymal stem cells and articular chondrocytes to enhance chondrogenesis. BioMed Res. Int. 2014, 2014, 369528. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Girlovanu, M.; Susman, S.; Soritau, O.; Rus-Ciuca, D.; Melincovici, C.; Constantin, A.M.; Mihu, C.M. Stem cells—Biological update and cell therapy progress. Clujul. Med. 2015, 88, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 2017, 105, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Wagner, W.; Franke, W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 2008, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Emadedin, M.; Ghorbani Liastani, M.; Fazeli, R.; Mohseni, F.; Moghadasali, R.; Mardpour, S.; Hosseini, S.E.; Niknejadi, M.; Moeininia, F.; Aghahossein Fanni, A.; et al. Long-Term Follow-up of Intra-articular Injection of Autologous Mesenchymal Stem Cells in Patients with Knee, Ankle, or Hip Osteoarthritis. Arch. Iran. Med. 2015, 18, 336–344. [Google Scholar] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.C.; Lee, E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral lesions of the knee: A new one-step repair technique with bone-marrow-derived cells. J. Bone Jt. Surg. Am. 2010, 92, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Okabe, T.; Horibe, S.; Mitsuoka, T.; Saito, M.; Koyama, T.; Nawata, M.; Tensho, K.; Kato, H.; Uematsu, K.; et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J. Tissue Eng. Regen. Med. 2011, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.Y.; Anz, A.; Merican, S.; Tay, Y.G.; Ragavanaidu, K.; Jee, C.S.; McGuire, D.A. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: A report of 5 cases with histology. Arthroscopy 2011, 27, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Jo, S.B.; Kwon, O.R.; Suh, D.S.; Lee, S.W.; Park, S.H.; Choi, Y.J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.Y.; Anz, A.; Siew-Yoke Jee, C.; Merican, S.; Ching-Soong Ng, R.; Roohi, S.A.; Ragavanaidu, K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013, 29, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Fodor, P.B.; Paulseth, S.G. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet. Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-Derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Vangsness, C.T.; Farr, J.; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. J. Bone Jt. Surg. Am. 2014, 96, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martin-Ferrero, M.A.; del Canto, F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.; van den Broek, M.P.; Nizak, R.; van Rijen, M.H.; de Weger, R.A.; Dhert, W.J.; Saris, D.B. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef] [PubMed]
- De Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Nizak, R.; van Rijen, M.H.P.; Saris, D.B.F. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Rackwitz, L.; Gilbert, F.; Noth, U.; Tuan, R.S. Concise review: The clinical application of mesenchymal stem cells for musculoskeletal regeneration: Current status and perspectives. Stem Cells Transl. Med. 2012, 1, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Aristizabal, A.; Sharma, A.; Bakooshli, M.A.; Kapoor, M.; Gilbert, P.M.; Viswanathan, S.; Gandhi, R. Stage-specific differences in secretory profile of mesenchymal stromal cells (MSCs) subjected to early- vs. late-stage OA synovial fluid. Osteoarthr. Cartil. 2017, 25, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Fisher, M.B.; Huang, A.H.; Shin, J.I.; Farrell, K.M.; Mauck, R.L. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very longterm in vitro culture. J. Biomech. 2014, 47, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Perez-Silos, V.; Camacho-Morales, A.; Fuentes-Mera, L. Mesenchymal stem cells subpopulations: Application for orthopedic regenerative medicine. Stem Cells Int. 2016, 3187491. [Google Scholar] [CrossRef] [PubMed]
- Clause, K.C.; Liu, L.J.; Tobita, K. Directed stem cell differentiation: The role of physical forces. Cell Commun. Adhes. 2010, 17, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Amarpal; Sharma, G.T.; Aithal, H.P.; Kinjavdekar, P. Cartilage tissue engineering: Role of mesenchymal stem cells along with growth factors & scaffolds. Indian J. Med. Res. 2016, 144, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, T.; Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng. Part A 2012, 18, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.; Jablonski, C.L.; Leonard, C.A.; Dunn, J.F.; Raharjo, E.; Matyas, J.R.; Biernaskie, J.; Krawetz, R.J. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci. Rep. 2016, 6, 23076. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees with Cartilage Defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, G.J.; Brittberg, M.; Dennis, J.E.; Bastiaansen-Jenniskens, Y.M.; Erben, R.G.; Konttinen, Y.T.; Luyten, F.P. Cartilage repair: Past and future—Lessons for regenerative medicine. J. Cell. Mol. Med. 2009, 13, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H. Microfracture and augments. J. Knee Surg. 2012, 25, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bonzani, I.C.; George, J.H.; Stevens, M.M. Novel materials for bone and cartilage regeneration. Curr. Opin. Chem. Biol. 2006, 10, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Benthien, J.P.; Behrens, P. Autologous Matrix-Induced Chondrogenesis (AMIC): Combining Microfracturing and a Collagen I/III Matrix for Articular Cartilage Resurfacing. Cartilage 2010, 1, 65–68. [Google Scholar] [CrossRef] [PubMed]
| Author Year Study Type | Number of Patients | Stem Cell Origin | Number of Cells | Control Group | Follow-up Time | Co-Treatment | Delivery System | Assessments |
|---|---|---|---|---|---|---|---|---|
| Wakitani et al. Case and control study [142] | 12 | Autologous BM-MSCs from iliac crest | 1.3 × 107 | 12 cell free | 28–95 weeks | High tibial osteotomy; gel-cell composite; autologous periosteum | Implantation of collagen cell sheets | Arthroscopic photography; Histological samples; Clinical evaluation |
| Niejadnik et al. Cohort study [143] | 36 | Autologous BM-MSCs from iliac crest | 1.0–1.5 × 107 | 36 autologous chondrocyte implantation | 24 months | Fibrin glue | Implantation upon autologous periosteal patch | ICRS; Lysholm score; Tegner activity scale X-ray; Clinical evaluation |
| Buda et al. Case series study [144] | 20 | Autologous BM-MSCs from iliac crest | N/A (2 mL of bone marrow concentrate) | None | 24 months | Arthroscopic debridement; Hyaluronic acid membrane scaffold | Transplantation of Arthroscopic Bone-Marrow-Derived Mesenchymal Stem Cells | Clinical evaluation (IKDC, KOOS); MRI; Histochemical analysis |
| Wakitani et al. Case series [145] | 41 | Autologous BM-MSCs from iliac crest | 5.0 × 106/mL | None | 5–137 months | Gel-cell composite | Implantation cell sheets | X-ray; Clinical evaluation |
| Saw et al. Case series study [146] | 5 | Autologous peripheral blood progenitor cells | N/A (7–8 mL of peripheral blood progenitor cells | None | 10–26 months | Microfracture; Hyaluronic acid intra-articular injections | Intra-articular injection | Second-look arthroscopy; Histological samples; |
| Davatchi et al. Case series study [147] | 4 | Autologous BM-MSCs from iliac crest | 8.0–9.0 × 106 | None | 12 months | Saline with 2% human serum albumine | Intra-articular injection | X-ray; Clinical evaluation; VAS |
| Koh et al. Case and control study [148] | 25 | Autologous A-MSCs from infrapatellar fat pad | 1.2–2.3 × 106 | 25 cell free | 12–18 months | Arthroscopic debridement; Synovectomy | Intra-articular injection | Lysholm score; Tegner activity scale; VAS |
| Koh et al. Case series study [149] | 18 | Autologous A-MSCs from infrapatellar fat pad | 0.3–2.7 × 106 | None | 24–26 months | Arthroscopic debridement; Synovectomy; Platelet-rich plasma | Intra-articular injection | Lysholm score; Tegner activity scale; VAS; MRI |
| Saw et al. Randomised control trial [150] | 49 | Autologous peripheral blood progenitor cells | N/A (7–8 mL of PBPC) | 25 cell free | 24 months | Microfracture; Hyaluronic acid intra-articular injections | Intra-articular injection | IKDC; MRI; Second-look arthroscopy; Histological samples |
| Orozko et al. Case series study [151] | 12 | Autologous BM-MSCs from iliac crest | 40 × 106 | None | 12 months | Ringer lactate, human albumin, glucose | Intra-articular injection | VAS; Clinical evaluation: WOMAC; MRI |
| Wong et al. Randomized control trial [152] | 28 | Autologous BM-MSCs from iliac crest | 1.46 × 106 | 28 cell free | 24 months | Microfracture and high tibial osteotomy; Hyaluronic acid; | Intra-articular injection | IKDC; Lysholm score; Tegner activity scale; MOCART |
| Jo et al. Cohort study [153] | 18 | Autologous A-MSCs from abdominal subcutaneous fats | 1.0 × 107, 5.0 × 107, 1.0 × 108 | None | 6 months | None | Intra-articular injection | WOMAC; VAS, KSS; X-ray, MRI; Second-look arthroscopy; Histological samples |
| Akgun et al. Prospective, single-site, randomized, single-blind pilot study. [154] | 7 | Autologous synovium-derived MSCs | 4.0 × 106 | 7 matrix-induced autologous chondrocyte implantation | 24 months | Type I/III collagen membrane (2 cm × 2 cm) | Implantation of MSC preloaded collagen membrane | Clinical evaluation: VAS, KOOS; MRI |
| Davatchi et al. Case series study [138] | 4 | Autologous BM-MSCs | 8.0–9.0 × 106 | None | 60 months | Glucosamine was permitted | Intra-articular injection | X-ray; Clinical evaluation: VAS, walking time to pain |
| Fodor et al. Case series study [155] | 6 | Adipose-derived stromal vascular cells (the stromal vascular fraction of adipose tissue) | 14.0 × 106 | None | 12 months | None | Intra-articular injection | Clinical evaluation: WOMAC, VAS, ROM, TUG; MRI. |
| Koh et al. Randomized control trial [156] | 40 | Autologous A-MSCs | 5.0 × 106 | 40 Microfracture treatment | 24 months | Debridement; Microfracture, Fibrin glue | Arthroscopic implantation of MSC loaded in fibrin glue | Second-look arthroscopy; the Lysholm score, KOOS, VAS; MRI; Histological samples |
| Pers et al. Cohort study [140] | 18 | Autologous A-MSCs | 2.0 × 106, 10.0 × 106, 50.0 × 106 | None | 6 months | None | Intra-articular injection | WOMAC, VAS, PGA, SAS, KOOS; Histological samples |
| Author Year Study Type | Number of Patients | Stem Cell Origin | Number of Cells | Control Group | Follow-up Time | Co-Treatment | Delivery System | Assessments |
|---|---|---|---|---|---|---|---|---|
| Vangsness et al. Randomized, double-blind, controlled study [157] | 36 | Allogenic BM-MSCs from 18–30-year old donors | 5.0 × 107, 1.5 × 108 | 20 cell free vehicle control | 24 months | Partial medial meniscectomy | Intra-articular injection | Measurement of immune cell markers; MRI; VAS; Lysholm score |
| Vega et al. Randomized control trial [158] | 15 | Allogenic BM-MSCs | 40 × 106 | 15 Hyaluronic acid (60 mg, single dose) | 12 months | None | Intra-articular injection | VAS, WOMAC, SF-12; MRI |
| De Windt et al. Case series study [159] | 10 | Allogenic BM-MSCs from the iliac crest of 2 healthy donors (age 2 and 5) | N/A. Mixed cells in the fibrinogen component of fibrin glue at 1.5–2 × 106 cells/mL | 10% or 20% autologous chondrons (a standard or a high yield mixture) | 12 months | None | Defect site-specific implantation | KOOS, VAS; Second-look arthroscopy; Histological analysis; |
| De Windt et al. Case series study [160] | 35 | Allogenic cryopreserved BM-MSCs | N/A. Mixed cells in the fibrinogen component of fibrin glue at 1.5–2 × 106 cells/mL. Approximately 0.9 mL cell product/cm2 defect | 10% or 20% autologous chondrons (a standard or a high yield mixture) | 18 months | None | Defect site-specific implantation | KOOS, VAS; Second-look arthroscopy; Histological analysis; |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Osowski, A.; Wojtkiewicz, J. Articular Cartilage Aging-Potential Regenerative Capacities of Cell Manipulation and Stem Cell Therapy. Int. J. Mol. Sci. 2018, 19, 623. https://doi.org/10.3390/ijms19020623
Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W, Osowski A, Wojtkiewicz J. Articular Cartilage Aging-Potential Regenerative Capacities of Cell Manipulation and Stem Cell Therapy. International Journal of Molecular Sciences. 2018; 19(2):623. https://doi.org/10.3390/ijms19020623
Chicago/Turabian StyleKrajewska-Włodarczyk, Magdalena, Agnieszka Owczarczyk-Saczonek, Waldemar Placek, Adam Osowski, and Joanna Wojtkiewicz. 2018. "Articular Cartilage Aging-Potential Regenerative Capacities of Cell Manipulation and Stem Cell Therapy" International Journal of Molecular Sciences 19, no. 2: 623. https://doi.org/10.3390/ijms19020623
APA StyleKrajewska-Włodarczyk, M., Owczarczyk-Saczonek, A., Placek, W., Osowski, A., & Wojtkiewicz, J. (2018). Articular Cartilage Aging-Potential Regenerative Capacities of Cell Manipulation and Stem Cell Therapy. International Journal of Molecular Sciences, 19(2), 623. https://doi.org/10.3390/ijms19020623







