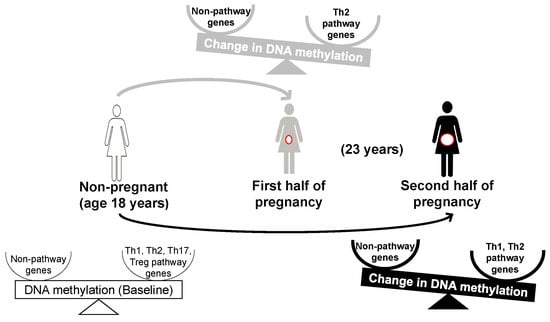
Changes in DNA Methylation from Age 18 to Pregnancy in Type 1, 2, and 17 T Helper and Regulatory T-Cells Pathway Genes
Abstract
1. Introduction
2. Results
3. Discussion
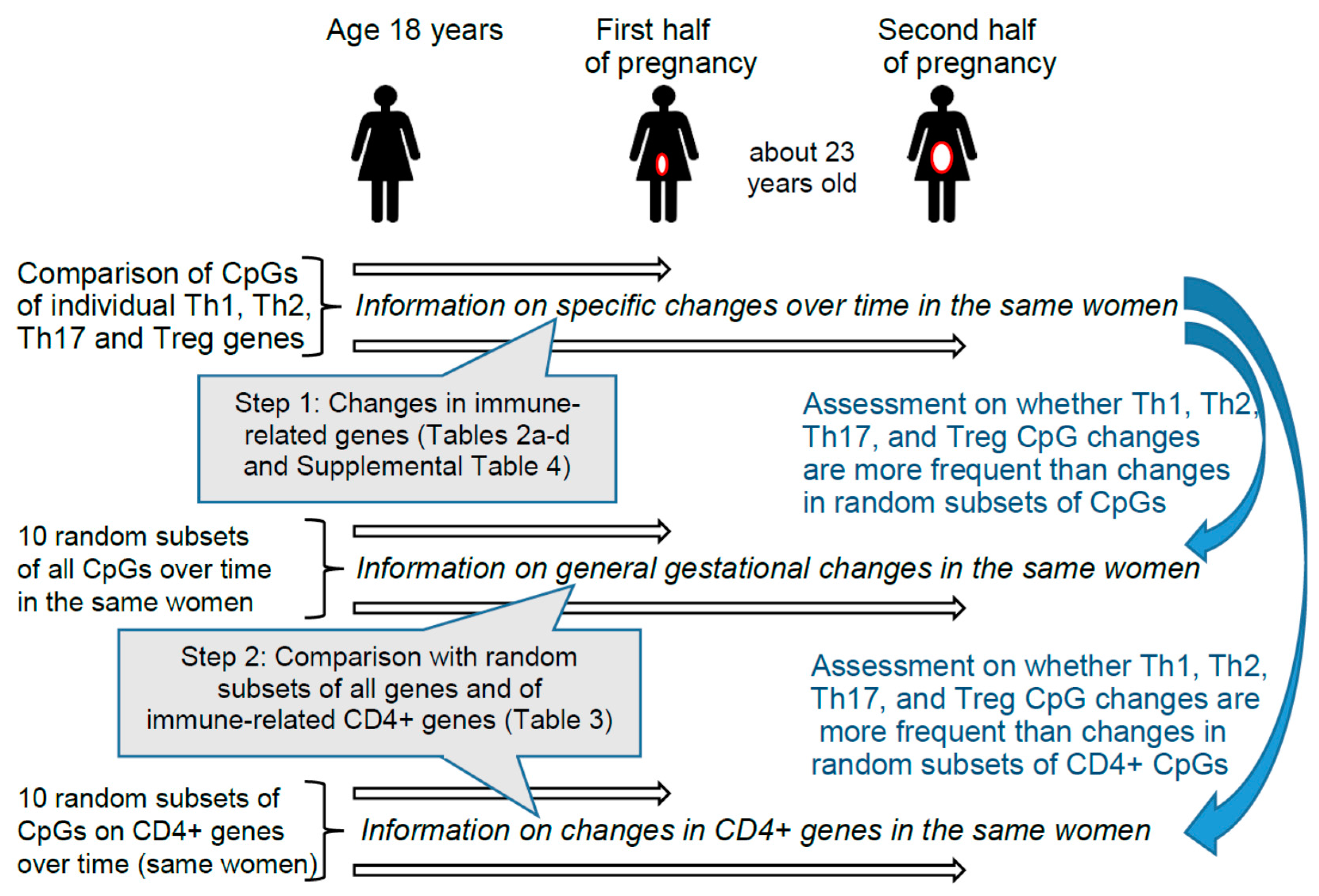
4. Participants and Methods
4.1. The Isle of Wight Birth Cohort
4.2. Ethics
4.3. Study Design
4.4. DNA Methylation
4.5. Variables Used for the Description of the Population Samples
4.6. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Vince, G.S.; Johnson, P.M. Is there a Th2 bias in human pregnancy? J. Reprod. Immunol. 1996, 32, 101–104. [Google Scholar] [CrossRef]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Romero, R.; Aldo, P.B.; Abrahams, V.M. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit. Rev. Immunol. 2005, 25, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.T.; Miller, E.M. The roles of the immune system in women’s reproduction: Evolutionary constraints and life history trade-offs. Am. J. Phys. Anthropol. 2011, 146, 134–154. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P. How immune mechanisms are affected by pregnancy. Vaccine 2003, 21, 3352–3357. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Silver, R.; Dalton, J. Immunology of normal pregnancy. Semin. Fetal Neonatal Med. 2006, 11, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.; Mellins, E.D. Plasticity of T-cell phenotype and function: The T helper type 17 example. Immunology 2010, 129, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Crome, S.Q.; Wang, A.Y.; Levings, M.K. Translational mini-review series on Th17 cells: Function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010, 159, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L. Estrogen, a double-edged sword: Modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug Targets Inflamm. Allergy 2004, 3, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Halonen, M.; Lohman, I.C.; Stern, D.A.; Spangenberg, A.; Anderson, D.; Mobley, S.; Ciano, K.; Peck, M.; Wright, A.L. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J. Immunol. 2009, 182, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.M. Epigenetic regulation of Th1 and Th2 cell development. Brain Behav. Immun. 2006, 20, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.L.; Huang, J.L.; Yeh, K.W.; Li, P.S.; Hsieh, K.H. Evaluation of Th1/Th2 ratio and cytokine production profile during acute exacerbation and convalescence in asthmatic children. Ann. Allergy Asthma Immunol. 2001, 86, 272–276. [Google Scholar] [CrossRef]
- Barnes, P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001, 2, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Khaled, W.T.; Read, E.K.; Nicholson, S.E.; Baxter, F.O.; Brennan, A.J.; Came, P.J.; Sprigg, N.; McKenzie, A.N.; Watson, C.J. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development 2007, 134, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, L.H.; Murphy, K.M. Lineage commitment in the immune system: The T helper lymphocyte grows up. Genes Dev. 2000, 14, 1693–1711. [Google Scholar] [PubMed]
- Fujio, K.; Okamura, T.; Sumitomo, S.; Yamamoto, K. The functions of CD4+CD25-LAG3+ regulatory T cells and Egr2 in the regulation of autoimmunity. Jpn. J. Clin. Immunol. 2014, 37, 69–73. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, H.; Tanaka, T.; Suzuki, Y.; Iwamura, C.; Ohkubo, S.; Endoh, K.; Kato, M.; Endo, Y.; Onodera, A.; Tumes, D.J.; et al. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proc. Natl. Acad. Sci. USA 2013, 110, 4691–4696. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Ma, X.; Tsuneyama, K.; Huang, S.; Takahashi, T.; Chalasani, N.P.; Bowlus, C.L.; Yang, G.X.; Leung, P.S.; Ansari, A.A.; et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: Implications for therapy. Hepatology 2014, 59, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hawkins, G.A.; Ampleford, E.J.; Moore, W.C.; Li, H.; Hastie, A.T.; Howard, T.D.; Boushey, H.A.; Busse, W.W.; Calhoun, W.J.; et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J. Allergy Clin. Immunol. 2013, 132, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Saglani, S. T cells in asthma: Influences of genetics, environment, and T-cell plasticity. J. Allergy Clin. Immunol. 2013, 131, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, X.; Holloway, J.W.; Rezwan, F.I.; Lockett, G.A.; Patil, V.; Ray, M.; Everson, T.M.; Soto-Ramirez, N.; Arshad, S.H.; et al. The interplay of DNA methylation over time with Th2 pathway genetic variants on asthma risk and temporal asthma transition. Clin. Epigenetics 2014, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Sengupta, T.K.; Ruiz, D.C.; Yang, E.; Ivashkiv, L.B. IL-4 selectively inhibits IL-2-triggered Stat5 activation, but not proliferation, in human T cells. J. Immunol. 1999, 162, 1261–1269. [Google Scholar] [PubMed]
- Kim, E.G.; Shin, H.J.; Lee, C.G.; Park, H.Y.; Kim, Y.K.; Park, H.W.; Cho, S.H.; Min, K.U.; Cho, M.L.; Park, S.H.; et al. DNA methylation and not allelic variation regulates STAT6 expression in human T cells. Clin. Exp. Med. 2010, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Yamashita, M.; Abe, R.; Tada, T.; Okumura, K.; Ransom, J.T.; Nakayama, T. CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4-mediated Th2 differentiation. J. Immunol. 1999, 163, 2432–2442. [Google Scholar] [PubMed]
- Yamashita, M.; Katsumata, M.; Iwashima, M.; Kimura, M.; Shimizu, C.; Kamata, T.; Shin, T.; Seki, N.; Suzuki, S.; Taniguchi, M.; et al. T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signaling complex. J. Exp. Med. 2000, 191, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Ziyab, A.H.; Davies, G.A.; Ewart, S.; Hopkin, J.M.; Schauberger, E.M.; Wills-Karp, M.; Holloway, J.W.; Arshad, S.H.; Zhang, H.; Karmaus, W. Interactive effect of STAT6 and IL13 gene polymorphisms on eczema status: Results from a longitudinal and a cross-sectional study. BMC Med. Genet. 2013, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Ashino, S.; Takeda, K.; Li, H.; Taylor, V.; Joetham, A.; Pine, P.R.; Gelfand, E.W. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J. Allergy Clin. Immunol. 2014, 133, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jager, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Jiang, Q.; Zhang, L.; Su, L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol. Res. 2008, 41, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, M.; Jutel, M. Human T regulatory cells: On the way to cognition. Arch. Immunol. Ther. Exp. 2013, 61, 229–236. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Le Gros, G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol. Cell Biol. 1999, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chaouat, G.; Dubanchet, S.; Ledee, N. Cytokines: Important for implantation? J. Assist. Reprod. Genet. 2007, 24, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Aldo, P.B.; Mor, G. Toll-like receptors and pregnancy: Trophoblast as modulators of the immune response. J. Obstet. Gynaecol. Res. 2009, 35, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Ito, M.; Yoneda, S.; Shiozaki, A.; Hidaka, T.; Saito, S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am. J. Reprod. Immunol. 2010, 63, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; Fazekas de St Groth, B.; Nanan, R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef] [PubMed]
- Druckmann, R.; Druckmann, M.A. Progesterone and the immunology of pregnancy. J. Steroid Biochem. Mol. Biol. 2005, 97, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993, 151, 4562–4573. [Google Scholar] [PubMed]
- Saito, S.; Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 2003, 59, 161–173. [Google Scholar] [CrossRef]
- Chaouat, G.; Ledee-Bataille, N.; Zourbas, S.; Ostojic, S.; Dubanchet, S.; Martal, J.; Frydman, R. Cytokines, implantation and early abortion: Re-examining the Th1/Th2 paradigm leads to question the single pathway, single therapy concept. Am. J. Reprod. Immunol. 2003, 50, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.D.; Quenby, S.; Takakuwa, K.; Johnson, P.M.; Vince, G.S. Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? Hum. Reprod. 2002, 17, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Janson, P.C.; Winerdal, M.E.; Winqvist, O. At the crossroads of T helper lineage commitment-Epigenetics points the way. Biochim. Biophys. Acta 2009, 1790, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Kim, S.T.; Spilianakis, C.G.; Fields, P.E.; Flavell, R.A. T helper cell differentiation: Regulation by cis elements and epigenetics. Immunity 2006, 24, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Clifton, V. Asthma and pregnancy: Emerging evidence of epigenetic interactions in utero. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Ho, S.M. Environmental epigenetics and asthma: Current concepts and call for studies. Am. J. Respir. Crit. Care Med. 2008, 177, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Hide, D.W. Effect of environmental factors on the development of allergic disorders in infancy. J. Allergy Clin. Immunol. 1992, 90, 235–241. [Google Scholar] [CrossRef]
- Zhang, H.; Nestor, C.E.; Zhao, S.; Lentini, A.; Bohle, B.; Benson, M.; Wang, H. Profiling of human CD4+ T-cell subsets identifies the TH2-specific noncoding RNA GATA3-AS1. J. Allergy Clin. Immunol. 2013, 132, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Storey, J.D. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 2007, 3, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerder, S.; Defrance, M.; Calonne, E.; Denis, H.; Sotiriou, C.; Fuks, F. Evaluation of the Infinium Methylation 450K technology. Epigenomics 2011, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sebra, R.; Pullman, B.S.; Qiao, W.; Peter, I.; Desnick, R.J.; Geyer, C.R.; DeCoteau, J.F.; Scott, S.A. Quantitative and multiplexed DNA methylation analysis using long-read single-molecule real-time bisulfite sequencing (SMRT-BS). BMC Genom. 2015, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Roessler, J.; Ammerpohl, O.; Gutwein, J.; Hasemeier, B.; Anwar, S.L.; Kreipe, H.; Lehmann, U. Quantitative cross-validation and content analysis of the 450 k DNA methylation array from Illumina, Inc. BMC Res. Notes 2012, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Ronn, T.; Volkov, P.; Davegardh, C.; Dayeh, T.; Hall, E.; Olsson, A.H.; Nilsson, E.; Tornberg, A.; Dekker Nitert, M.; Eriksson, K.F.; et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013, 9, e1003572. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Volkov, P.; Dayeh, T.; Esguerra, J.L.; Salo, S.; Eliasson, L.; Ronn, T.; Bacos, K.; Ling, C. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol 2014, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Glossop, J.R.; Nixon, N.B.; Emes, R.D.; Haworth, K.E.; Packham, J.C.; Dawes, P.T.; Fryer, A.A.; Mattey, D.L.; Farrell, W.E. Epigenome-wide profiling identifies significant differences in DNA methylation between matched-pairs of T- and B-lymphocytes from healthy individuals. Epigenetics 2013, 8, 1188–1197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabriel, A.S.; Lafta, F.M.; Schwalbe, E.C.; Nakjang, S.; Cockell, S.J.; Iliasova, A.; Enshaei, A.; Schwab, C.; Rand, V.; Clifford, S.C.; et al. Epigenetic landscape correlates with genetic subtype but does not predict outcome in childhood acute lymphoblastic leukemia. Epigenetics 2015, 10, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Reinius, L.E.; Greco, D.; Gref, A.; Orsmark-Pietras, C.; Persson, H.; Pershagen, G.; Hedlin, G.; Melen, E.; Scheynius, A.; et al. Risk of childhood asthma is associated with CpG-site polymorphisms, regional DNA methylation and mRNA levels at the GSDMB/ORMDL3 locus. Hum. Mol. Genet. 2015, 24, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ramirez, N.; Ziyab, A.H.; Karmaus, W.; Zhang, H.; Kurukulaaratchy, R.J.; Ewart, S.; Arshad, S.H. Epidemiologic methods of assessing asthma and wheezing episodes in longitudinal studies: Measures of change and stability. J. Epidemiol. Jpn. Epidemiol. Assoc. 2013, 23, 399–410. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yan, L.; Hu, Q.; Sucheston, L.E.; Higgins, M.J.; Ambrosone, C.B.; Johnson, C.S.; Smiraglia, D.J.; Liu, S. IMA: An R package for high-throughput analysis of Illumina’s 450 K Infinium methylation data. Bioinformatics 2012, 28, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Kuan, P.F.; Wang, S.; Zhou, X.; Chu, H. A statistical framework for Illumina DNA methylation arrays. Bioinformatics 2010, 26, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhang, X.; Huang, C.C.; Jafari, N.; Kibbe, W.A.; Hou, L.; Lin, S.M. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform. 2010, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Karmaus, W.; Kurukulaaratchy, R.; Sadeghnejad, A.; Huebner, M.; Ewart, S. Polymorphisms in the interleukin 13 and GATA binding protein 3 genes and the development of eczema during childhood. Br. J. Dermatol. 2008, 158, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Touleimat, N.; Tost, J. Complete pipeline for Infinium((R)) Human Methylation 450 K BeadChip data processing using subset quantile normalization for accurate DNA methylation estimation. Epigenomics 2012, 4, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar]
| Pathway | Number of Genes | Genes Coding Cytokines, Proteins, or Receptors | Number of CpG Sites |
|---|---|---|---|
| Th1 | 19 | IFN-γ family [12,13,14,15,16,17,18,19,20,21,22,23] TNF-α family [12,13,14,15,16,17,18,19,20,21,22,23] IL-2 [12,13,14,17,20,22,23] IL-12 family [13,18,24] | 155 |
| Th2 | 12 | IL-4 [12,13,15,16,17,18,19,20,22,26,27,28,29,30,31,32] IL4R [29,32,33] IL-5 5 [12,13,16,17,18,19,20,22,23,26,27] IL-9 [17,20,22] IL-13 family [12,13,16,17,18,19,20,22,23,25,26,27,29,31,34] GATA3 [23,29] STAT6 [18,23,29,31,32,34] JAK1 [35] JAK3 [32,33,35] IL1RL1 [12,19,22] | 77 |
| Th17 | 15 | IL17 family [12,19,22] IL21 family [22,36] IL22 family [19,22] | 106 |
| Treg | 2 | FOXP3 [37,38] CTLA4 [39] | 10 |
| Th1 Gene Names | Total Number of CpGs | Significant CpGs in First Half of Pregnancy b | Significant CpGs in Second Half of Pregnancy c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Significant CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | No. of Significant CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | ||
| IFNG | 5 | 1 | cg01940810 | 0.15 | 0.034 | 0.145 | 1 | ||||
| cg26227465 | 0.13 | 0.014 | 0.056 | ||||||||
| IFNGR1 | 3 | 1 | cg26668632 | −0.14 | 0.045 | 0.163 | 1 | ||||
| cg07401792 | −0.25 | 0.011 | 0.044 | ||||||||
| IFNGR2 | 10 | 3 | cg08173915 | −0.49 | 1.00 × 10−32 | <1.0 × 10−6 | 4 | cg08173915 | −0.44 | <1 × 10−16 | <1.0 × 10−6 |
| cg17356733 | −0.5 | 4.44 × 10−16 | <1.0 × 10−6 | cg17356733 | −0.43 | 3.48 × 10−11 | <1.0 × 10−6 | ||||
| cg22669060 | −0.49 | 1.01 × 10−12 | <1.0 × 10−6 | cg22669060 | −0.47 | 1.05 × 10−10 | <1.0 × 10−6 | ||||
| cg27469991 | −0.15 | 0.018 | 0.066 | ||||||||
| IL12A | 7 | 3 | cg09362366 | −0.56 | 6.60 × 10−4 | 0.004 | 2 | ||||
| cg20515136 | −0.31 | 4.34 × 10−6 | 1.1 × 10−5 | cg20515136 | −0.5 | 1.16 × 10−7 | <1.0 × 10−6 | ||||
| cg25829945 | −0.24 | 0.041 | 0.150 | cg25829945 | −0.18 | 0.039 | 0.113 | ||||
| IL12B | 9 | 1 | cg06111286 | −0.39 | 2.31 × 10−5 | 2.4 × 10−5 | 1 | cg06111286 | −0.45 | 3.16 × 10−9 | <1.0 × 10−6 |
| IL12RB1 | 6 | 1 | cg12123019 | −0.5 | 1.35 × 10−4 | 0.001 | 2 | cg12123019 | −0.33 | 6.963 × 10−4 | 0.005 |
| cg18307303 | −0.14 | 0.016 | 0.059 | ||||||||
| IL12RB2 | 10 | 7 | cg02566391 | 0.46 | 1.48 × 10−12 | <1.0 × 10−6 | 8 | cg02566391 | 0.49 | <1 × 10−16 | <1.0 × 10−6 |
| cg06952660 | −0.29 | 0.030 | 0.140 | cg06952660 | −0.32 | 3.54 × 10−4 | 0.003 | ||||
| cg09018107 | 0.48 | 7.67 × 10−10 | <1.0 × 10−6 | cg09018107 | 0.37 | 2.44 × 10−6 | 2.0 × 10−6 | ||||
| cg12633410 | 0.21 | 0.031 | 0.140 | cg12633410 | 0.16 | 0.025 | 0.081 | ||||
| cg14849855 | 0.32 | 1.53 × 10−7 | <1.0 × 10−6 | cg14849855 | 0.19 | 0.004 | 0.021 | ||||
| cg19745415 | −0.49 | 4.40 × 10−4 | 0.003 | cg19745415 | −0.41 | 0.003 | 0.016 | ||||
| cg20253742 | −0.53 | 7.27 × 10−5 | 0.001 | cg20253742 | −0.25 | 0.0023 | 0.012 | ||||
| cg11132246 | −0.27 | 0.021 | 0.073 | ||||||||
| IL2 | 1 | 0 | 0 | ||||||||
| IL2RA | 2 | 0 | 0 | ||||||||
| IL2RB | 4 | 1 | cg21307484 | 0.27 | 6.63 × 10−5 | 0.001 | 3 | cg21307484 | 0.18 | 0.002 | 0.011 |
| cg24509815 | 0.18 | 2.13 × 10−9 | <1.0 × 10−6 | ||||||||
| cg26757673 | 0.23 | 4.09 × 10−7 | <1.0 × 10−6 | ||||||||
| TNF | 11 | 4 | cg01360627 | 0.16 | 0.006 | 0.031 | 6 | cg01360627 | 0.3 | 2.42 × 10−11 | <1.0 × 10−6 |
| cg04425624 | −0.27 | 0.002 | 0.011 | ||||||||
| cg10650821 | −0.33 | 1.43 × 10−4 | 0.001 | cg10650821 | −0.3 | 7.45 × 10−4 | 0.005 | ||||
| cg10717214 | −0.25 | 0.002 | 0.011 | ||||||||
| cg17755321 | 0.15 | 0.027 | 0.088 | ||||||||
| cg15989608 | 0.21 | 0.043 | 0.122 | ||||||||
| cg23384708 | 0.22 | 6.21 × 10−5 | 0.001 | ||||||||
| cg26736341 | 0.14 | 0.032 | 0.099 | ||||||||
| TNFAIP1 | 5 | 1 | cg22640868 | −0.5 | 2.29 × 10−12 | <1.0 × 10−6 | 4 | cg22640868 | −0.46 | 1.27 × 10−13 | <1.0 × 10−6 |
| cg26663469 | 0.16 | 0.033 | 0.101 | ||||||||
| cg11814826 | −0.24 | 0.010 | 0.031 | ||||||||
| cg13290523 | −0.18 | 0.031 | 0.097 | ||||||||
| TNFAIP2 | 8 | 2 | cg03021690 | 0.12 | 0.036 | 0.145 | 4 | cg03021690 | 0.14 | 0.002 | 0.015 |
| cg13144594 | 0.23 | 0.012 | 0.058 | cg13144594 | 0.26 | 0.016 | 0.059 | ||||
| cg03572388 | −0.12 | 0.049 | 0.135 | ||||||||
| cg04264002 | 0.14 | 0.007 | 0.033 | ||||||||
| TNFAIP3 | 13 | 4 | cg06779945 | 0.17 | 0.039 | 0.049 | 2 | ||||
| cg08919597 | −0.84 | 0.0000 | <1.0 × 10−6 | cg08919597 | −0.43 | 0.003 | 0.018 | ||||
| cg12200164 | −0.19 | 0.035 | 0.145 | ||||||||
| cg18264753 | −0.13 | 0.018 | 0.085 | ||||||||
| cg12214665 | −0.14 | 0.011 | 0.044 | ||||||||
| TNFAIP6 | 4 | 1 | cg03406844 | −0.49 | 1.79 × 10−12 | <1.0 × 10−6 | 1 | cg03406844 | −0.51 | 4.44 × 10−16 | <1.0 × 10−6 |
| TNFAIP8 | 22 | 9 | cg00524900 | −0.71 | 1.03 × 10−9 | <1.0 × 10−6 | 7 | cg00524900 | −0.88 | <1 × 10−16 | <1.0 × 10−6 |
| cg01057573 | −0.73 | <1 × 10−16 | <1.0 × 10−6 | cg01057573 | −0.72 | <1 × 10−16 | <1.0 × 10−6 | ||||
| cg03665078 | −0.21 | 0.001 | 0.007 | cg03665078 | −0.17 | 0.009 | 0.040 | ||||
| cg07398791 | −0.31 | 4.40 × 10−6 | 1.1 × 10−5 | cg07398791 | −0.35 | 1.83 × 10−7 | <1.0 × 10−6 | ||||
| cg11846226 | 0.22 | 1.34 × 10−4 | 0.001 | ||||||||
| cg12148675 | −0.18 | 0.037 | 0.145 | ||||||||
| cg15408889 | −0.2 | 0.010 | 0.053 | cg15408889 | −0.2 | 0.010 | 0.042 | ||||
| cg21239001 | −0.45 | 6.10 × 10−12 | <1.0 × 10−6 | cg21239001 | −0.49 | 8.88 × 10−16 | <1.0 × 10−6 | ||||
| cg07086380 | 0.22 | 2.67 × 10−4 | 0.002 | cg07086380 | 0.27 | 6.82 × 10−5 | 0.001 | ||||
| TNFAIP8L | 30 | 4 | cg21544402 | −0.33 | 4.61 × 10−4 | 0.003 | 10 | ||||
| cg05503460 | −0.26 | 2.16 × 10−4 | 0.002 | cg05503460 | −0.3 | 3.251 × 10−5 | 3.1 × 10−5 | ||||
| cg12122631 | −0.15 | 0.039 | 0.149 | ||||||||
| cg23343680 | −0.15 | 0.034 | 0.145 | cg23343680 | −0.15 | 0.006 | 0.001 | ||||
| cg02436098 | −0.2 | 0.007 | 0.032 | ||||||||
| cg11708963 | −0.17 | 0.0237 | 0.079 | ||||||||
| cg22754389 | −0.15 | 0.036 | 0.108 | ||||||||
| cg23612220 | −0.44 | 0.005 | 0.026 | ||||||||
| cg02233197 | 0.18 | 0.008 | 0.038 | ||||||||
| cg02346713 | −0.25 | 1.37 × 10−5 | 1.2 × 10−5 | ||||||||
| cg03454639 | 0.22 | 0.024 | 0.081 | ||||||||
| cg22038124 | 0.1 | 0.039 | 0.113 | ||||||||
| Total | 155 | 43 | 56 | ||||||||
| Th2 Genes | Total Number of CpGs | Significant CpGs in First Half of Pregnancy b | Significant CpGs in Second Half of Pregnancy c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | ||
| GATA3 | 17 | 6 | cg00463367 | 0.18 | 0.025 | 0.095 | 4 | cg00463367 | 0.29 | 2.94 × 10−6 | 2.0 × 10−6 |
| cg01255894 | −0.27 | 0.014 | 0.055 | ||||||||
| cg03669298 | −0.32 | 1 × 10−4 | 0.001 | cg03669298 | −0.28 | 2 × 10−4 | 0.001 | ||||
| cg10008757 | −0.15 | 0.007 | 0.034 | cg10008757 | −0.2 | 0.006 | 0.027 | ||||
| cg11430077 | 0.38 | 0.001 | 0.005 | ||||||||
| cg22770911 | 0.24 | 2 × 10−4 | 0.002 | cg22770911 | 0.12 | 0.021 | 0.074 | ||||
| IL13 | 4 | 1 | cg15329179 | 0.19 | 0.005 | 0.024 | 1 | ||||
| cg13566430 | −0.13 | 0.042 | 0.130 | ||||||||
| IL13RA1 | 6 | 5 | cg01080862 | 1.8 | 1.27 × 10−9 | <1.0 × 10−6 | 5 | cg01080862 | 1.77 | 1.41 × 10−12 | <1.0 × 10−6 |
| cg22817042 | 1.19 | 6.81 × 10−10 | <1.0 × 10−6 | cg22817042 | 0.94 | 7.48 × 10−10 | <1.0 × 10−6 | ||||
| cg23508470 | −0.13 | 0.039 | 0.126 | cg23508470 | −0.16 | 0.012 | 0.047 | ||||
| cg25968748 | 1.06 | 9.49 × 10−13 | <1.0 × 10−6 | cg25968748 | 0.76 | 3.99 × 10−8 | <1.0 × 10−6 | ||||
| cg27501007 | 1.92 | <1 × 10−16 | <1.0 × 10−6 | cg27501007 | 1.76 | <1 × 10−16 | <1.0 × 10−6 | ||||
| IL13RA2 | 2 | 1 | cg03244736 | −0.93 | 0.001 | 0.005 | 1 | cg03244736 | −0.53 | 0.013 | 0.049 |
| IL1RL1 | 4 | 2 | cg11916609 | −0.35 | 0.002 | 0.014 | 1 | cg11916609 | −0.36 | 6.14 × 10−7 | <1.0 × 10−6 |
| cg17738684 | 0.15 | 0.048 | 0.138 | ||||||||
| IL4 | 1 | 0 | 0 | ||||||||
| IL4R | 8 | 4 | cg01165142 | −0.34 | 1.63 × 10−6 | 2.0 × 10−6 | 4 | cg01165142 | −0.39 | 9.78 × 10−8 | <1.0 × 10−6 |
| cg05903710 | −0.14 | 0.0427 | 0.126 | ||||||||
| cg16649560 | −0.54 | 5.50 × 10−13 | <1.0 × 10−6 | cg16649560 | −0.53 | <1 × 10−16 | <1.0 × 10−6 | ||||
| cg26937798 | −0.32 | 0.042 | 0.126 | cg26937798 | −0.3 | 0.033 | 0.112 | ||||
| cg05729093 | 0.21 | 0.008 | 0.035 | ||||||||
| IL5 | 2 | 0 | 1 | cg16184131 | −0.39 | 4.52 × 10−8 | <1.0 × 10−6 | ||||
| IL5RA | 7 | 3 | cg01310029 | 0.19 | 0.034 | 0.120 | 3 | ||||
| cg23032421 | 0.56 | 5.21 × 10−4 | 0.004 | cg23032421 | 0.58 | 7.72 × 10−12 | <1.0 × 10−6 | ||||
| cg23828301 | −0.68 | 2.64 × 10−13 | <1.0 × 10−6 | cg23828301 | −0.67 | <1 × 10−16 | <1.0 × 10−6 | ||||
| cg08404225 | 0.25 | 0.001 | 0.005 | ||||||||
| IL9 | 3 | 0 | 0 | ||||||||
| JAK1 | 11 | 3 | cg00153395 | −0.22 | 5.77 × 10−6 | 1.0 × 10−5 | 3 | cg00153395 | −0.38 | 6.29 × 10−10 | <1.0 × 10−6 |
| cg12444684 | −0.16 | 0.029 | 0.107 | ||||||||
| cg26315985 | −0.27 | 0.013 | 0.055 | ||||||||
| cg07798602 | 0.14 | 0.014 | 0.052 | ||||||||
| cg25020373 | −0.28 | 0.001 | 0.003 | ||||||||
| JAK3 | 7 | 1 | cg06655414 | 0.34 | 0.039 | 0.126 | 2 | ||||
| cg02285920 | −0.27 | 0.049 | 0.145 | ||||||||
| cg25623545 | −0.25 | 0.008 | 0.035 | ||||||||
| STAT6 | 5 | 1 | cg12693595 | 0.16 | 0.012 | 0.053 | 1 | ||||
| cg25157914 | −0.15 | 0.036 | 0.114 | ||||||||
| Total | 77 | 27 | 26 | ||||||||
| Th17 Gene Names | Total Number of CpGs | Significant CpGs in First Half of Pregnancy b | Significant CpGs in Second Half of Pregnancy c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | ||
| IL17A | 2 | 1 | cg05884768 | −0.21 | 0.046 | 0.155 | 0 | ||||
| IL17B | 7 | 1 | cg01579636 | 0.21 | 0.002 | 0.014 | 2 | cg01579636 | 0.13 | 0.011 | 0.047 |
| cg05860978 | −0.12 | 0.014 | 0.059 | ||||||||
| IL17C | 5 | 3 | cg08155347 | −0.21 | 0.012 | 0.058 | 2 | ||||
| cg26686608 | 0.14 | 3.27 × 10−4 | 0.003 | cg26686608 | 0.14 | 0.009 | 0.042 | ||||
| cg27132152 | 0.18 | 0.009 | 0.050 | cg27132152 | 0.23 | 0.004 | 0.020 | ||||
| IL17D | 13 | 1 | cg09985351 | 0.2 | 0.047 | 0.155 | 2 | ||||
| cg12475590 | −0.27 | 0.002 | 0.017 | ||||||||
| cg02792322 | −0.17 | 0.006 | 0.031 | ||||||||
| IL17F | 5 | 0 | 0 | ||||||||
| IL17RA | 8 | 5 | cg01085328 | 0.17 | 0.011 | 0.055 | 4 | ||||
| cg01760983 | −0.65 | 1.23 × 10−4 | 0.001 | cg01760983 | −0.24 | 0.039 | 0.130 | ||||
| cg02866761 | −0.16 | 0.001 | 0.009 | ||||||||
| cg16389078 | −0.52 | 2.72 × 10−8 | <1.0 × 10−6 | cg16389078 | −0.36 | 0.001 | 0.005 | ||||
| cg19901866 | 0.24 | 0.032 | 0.118 | ||||||||
| cg13595439 | 0.15 | 0.015 | 0.061 | ||||||||
| cg15502903 | −0.19 | 1.76 × 10−4 | 0.002 | ||||||||
| IL17RC | 2 | 0 | 0 | ||||||||
| IL17RD | 15 | 4 | cg00770158 | −0.09 | 0.029 | 0.110 | 5 | ||||
| cg01797381 | −0.29 | 0.005 | 0.027 | cg01797381 | −0.32 | 4.18 × 10−4 | 0.004 | ||||
| cg03435901 | −0.48 | 5.82 × 10−5 | 0.001 | cg03435901 | −0.4 | 0.003 | 0.020 | ||||
| cg10882522 | −0.28 | 0.001 | 0.009 | cg10882522 | −0.18 | 0.019 | 0.073 | ||||
| cg09429700 | 0.11 | 0.004 | 0.020 | ||||||||
| cg00743540 | 0.19 | 0.002 | 0.014 | ||||||||
| IL17RE | 8 | 4 | cg02968508 | −0.11 | 0.025 | 0.101 | 2 | ||||
| cg05253480 | 0.2 | 0.017 | 0.070 | ||||||||
| cg06619959 | −0.16 | 0.003 | 0.016 | cg06619959 | −0.1 | 0.003 | 0.011 | ||||
| cg15095327 | 0.13 | 0.043 | 0.151 | cg15095327 | 0.19 | 0.031 | 0.116 | ||||
| IL17REL | 12 | 5 | cg00692279 | 0.12 | 4.28 × 10−4 | 0.004 | 4 | ||||
| cg04485799 | 0.14 | 0.026 | 0.101 | cg04485799 | 0.13 | 0.039 | 0.130 | ||||
| cg12009803 | 0.11 | 0.013 | 0.061 | ||||||||
| cg26206185 | 0.55 | 2.3 × 10−5 | 3.5 × 10−5 | ||||||||
| cg27068297 | −0.14 | 0.003 | 0.016 | ||||||||
| cg00090674 | 0.23 | 2.16 × 10−4 | 0.002 | ||||||||
| cg13563334 | 0.15 | 1.96 × 10−5 | 3.5 × 10−5 | ||||||||
| cg00692279 | 0.13 | 0.007 | 0.032 | ||||||||
| IL21 | 3 | 1 | cg00136405 | −0.16 | 0.014 | 0.062 | 1 | cg00136405 | −0.16 | 0.004 | 0.020 |
| IL21R | 12 | 7 | cg02656594 | −0.53 | 2.86 × 10−14 | <1.0 × 10−6 | 7 | cg02656594 | −0.52 | 2.89 × 10−15 | <1.0 × 10−6 |
| cg00050618 | 0.26 | 0.001 | 0.006 | cg00050618 | 0.22 | 3.05 × 10−6 | 6.0 × 10−6 | ||||
| cg05814654 | −0.86 | 5.46 × 10−6 | 1.2 × 10−5 | cg05814654 | −0.82 | 1.01 × 10−4 | 0.002 | ||||
| cg02983090 | −0.72 | 9.87 × 10−6 | 1.7 × 10−5 | cg02983090 | −0.73 | 2.14 × 10−7 | 1.0 × 10−6 | ||||
| cg08282819 | −1.07 | 5.33 × 10−9 | <1.0 × 10−6 | cg08282819 | −0.96 | 4.29 × 10−14 | <1.0 × 10−6 | ||||
| cg19423311 | −0.48 | 0.048 | 0.15494 | cg19423311 | −0.41 | 0.035 | 0.125 | ||||
| cg27027151 | −0.47 | 4.7 × 10−11 | <1.0 × 10−6 | ||||||||
| IL22 | 8 | 0 | 1 | cg13851647 | −0.22 | 0.046 | 0.149 | ||||
| IL22RA1 | 4 | 1 | cg21293216 | 0.28 | 0.009 | 0.050 | 3 | cg21293216 | 0.4 | 1.55 × 10−4 | 0.002 |
| cg09152089 | 0.18 | 0.032 | 0.116 | ||||||||
| cg11651446 | 0.17 | 0.002 | 0.014 | ||||||||
| IL22RA2 | 2 | 1 | cg00415333 | 0.14 | 0.050 | 0.155 | 0 | ||||
| Total number | 106 | 34 | 33 | ||||||||
| Treg Gene Names | Total Number of CpGs | Significant CpGs in First Half of Pregnancy b | Significant CpGs in Second Half of Pregnancy c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | No. of Sign. CpGs | CpG Sites | Parameter Estimates | p-Value | FDR-Adjusted p-Value | ||
| FOXP3 | 7 | 3 | cg01905377 | −1.01 | 2.19 × 10−8 | <1.0 × 10−6 | 4 | cg01905377 | −1.28 | 7.46 × 10−11 | <1.0 × 10−6 |
| cg06767008 | 0.42 | 7.46 × 10−12 | <1.0 × 10−6 | cg06767008 | 0.37 | 2.22 × 10−6 | 0.10 | ||||
| cg15614573 | −0.41 | 0.033 | 0.11 | cg15614573 | −0.81 | 0.001 | 1.0 × 10−5 | ||||
| cg04920616 | 0.21 | 0.040 | 0.004 | ||||||||
| CTLA4 | 3 | 0 | 0 | ||||||||
| Total | 10 | 3 | 4 | ||||||||
| Time | First Half of Pregnancy | Second Half of Pregnancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Association Based on Original p-Value | Association Based on FDR-Tested p-Value | Association Based on Original p-Value | Association Based on FDR-Tested p-Value | |||||||||
| Pathway (Total Number of CpGs) | Proportion of Significant CpGs | Risk Ratio | 95% Confidence Interval | Proportion of Significant CpGs | Risk Ratio | 95% Confidence Interval | Proportion of Significant CpGs | Risk Ratio | 95% Confidence Interval | Proportion of Significant CpGs | Risk Ratio | 95% Confidence Interval |
| Compared to 10 random subsets based on the whole genome | ||||||||||||
| Th1 (155) | 27.7 | 1.15 | 0.84–1.57 | 18.7 | 1.45 | 0.95–2.22 | 36.1 | 1.43 | 1.08–1.88 | 25.2 | 1.68 | 1.16–2.44 |
| Th2 (77) | 35.1 | 1.45 | 1.02–2.07 | 20.9 | 1.61 | 0.96–2.69 | 33.8 | 1.33 | 0.93–1.91 | 26.0 | 1.74 | 1.11–2.73 |
| Th17 (106) | 32.1 | 1.33 | 0.95–1.85 | 18.9 | 1.46 | 0.90–2.36 | 31.1 | 1.23 | 0.88–1.72 | 22.6 | 1.52 | 0.98–2.33 |
| Treg (10) | 30.0 | 1.24 | 0.47–3.26 | 20.0 | 1.55 | 0.44–5.51 | 40.0 | 1.58 | 0.72–3.44 | 30.0 | 2.01 | 0.75–5.35 |
| Random (Median proportion) | 24.1 | 12.9 | 24.6 | 14.9 | ||||||||
| Compared to 10 random subsets based on the genes expressed in CD4+ cells | ||||||||||||
| Th1 (155) | 27.7 | 1.16 | 0.84–1.59 | 18.7 | 1.30 | 0.86–1.97 | 36.1 | 1.35 | 1.03–1.78 | 25.2 | 1.72 | 1.18–2.49 |
| Th2 (77) | 35.1 | 1.46 | 1.02–2.09 | 20.9 | 1.45 | 0.87–2.40 | 33.8 | 1.26 | 0.88–1.81 | 26.0 | 1.77 | 1.13–2.79 |
| Th17 (106) | 32.1 | 1.34 | 0.96–1.87 | 18.9 | 1.31 | 0.82–2.10 | 31.1 | 1.17 | 0.84–1.62 | 22.6 | 1.55 | 1.00–2.38 |
| Treg (10) | 30.0 | 1.25 | 0.48–3.28 | 20.0 | 1.39 | 0.39–4.94 | 40.0 | 1.50 | 0.69–3.26 | 30.0 | 2.05 | 0.77–5.46 |
| Random (Median proportion) | 24.0 | 10.4 | 26.7 | 9.80 | ||||||||
| Position of CpGs on Gene a | Th1, Th2, Th17 and Treg Gene CpGs (Total 348) n (%) | Random CpGs from Whole Genome (Total 3480) n (%) | Random CpGs from Genes Expressed in CD4+ Cells (Total 3480) n (%) | Random CpGs from Whole Genome Excluding CpGs Situated in Intergenic Region (Total 348) n (%) |
|---|---|---|---|---|
| Body, 3′UTR, and Promoter | 0 (0) | 1 (0.03) | 0 (0) | 1 (0.03) |
| Body, 5′UTR, and Promoter | 6 (1.7) | 33 (1.0) | 74 (2.1) | 37 (1.1) |
| Body and 3′UTR | 3 (0.9) | 23 (0.7) | 26 (0.8) | 34 (1.0) |
| Body and 5′UTR | 21 (6.0) | 148 (4.3) | 197 (5.7) | 180 (5.2) |
| Body and Promoter | 4 (1.2) | 134 (3.9) | 293 (8.4) | 161 (4.6) |
| Body | 129 (37.1) | 1140 (32.8) | 1375 (39.5) | 1489 (42.8) |
| 3′UTR and Promoter | 1 (0.3) | 5 (0.1) | 15 (0.4) | 9 (0.1) |
| 5′UTR and Promoter | 8 (2.3) | 55 (1.6) | 116 (3.3) | 65 (1.9) |
| Promoter | 117 (33.6) | 854 (24.5) | 1005 (28.9) | 1114 (32.0) |
| 3′UTR and 5′UTR | 0 (0) | 0 (0) | 1 (0.03) | 0 (0) |
| 3′UTR | 26 (7.5) | 124 (3.6) | 149 (4.1) | 153 (4.4) |
| 5′UTR | 33 (9.5) | 200 (5.7) | 229 (6.6) | 237 (6.8) |
| Intergenic | 0 (0) | 763 (21.9) | 0 (0) | 0 (0) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.; Lockett, G.A.; Holloway, J.W.; Arshad, S.H.; Zhang, H.; Kaushal, A.; Tetali, S.R.; Mukherjee, N.; Karmaus, W.J.J. Changes in DNA Methylation from Age 18 to Pregnancy in Type 1, 2, and 17 T Helper and Regulatory T-Cells Pathway Genes. Int. J. Mol. Sci. 2018, 19, 477. https://doi.org/10.3390/ijms19020477
Iqbal S, Lockett GA, Holloway JW, Arshad SH, Zhang H, Kaushal A, Tetali SR, Mukherjee N, Karmaus WJJ. Changes in DNA Methylation from Age 18 to Pregnancy in Type 1, 2, and 17 T Helper and Regulatory T-Cells Pathway Genes. International Journal of Molecular Sciences. 2018; 19(2):477. https://doi.org/10.3390/ijms19020477
Chicago/Turabian StyleIqbal, Sabrina, Gabrielle A. Lockett, John W. Holloway, S. Hasan Arshad, Hongmei Zhang, Akhilesh Kaushal, Sabarinath R. Tetali, Nandini Mukherjee, and Wilfried J. J. Karmaus. 2018. "Changes in DNA Methylation from Age 18 to Pregnancy in Type 1, 2, and 17 T Helper and Regulatory T-Cells Pathway Genes" International Journal of Molecular Sciences 19, no. 2: 477. https://doi.org/10.3390/ijms19020477
APA StyleIqbal, S., Lockett, G. A., Holloway, J. W., Arshad, S. H., Zhang, H., Kaushal, A., Tetali, S. R., Mukherjee, N., & Karmaus, W. J. J. (2018). Changes in DNA Methylation from Age 18 to Pregnancy in Type 1, 2, and 17 T Helper and Regulatory T-Cells Pathway Genes. International Journal of Molecular Sciences, 19(2), 477. https://doi.org/10.3390/ijms19020477