Size-Dependent Affinity of Glycine and Its Short Oligomers to Pyrite Surface: A Model for Prebiotic Accumulation of Amino Acid Oligomers on a Mineral Surface
Abstract
:1. Introduction
2. Results
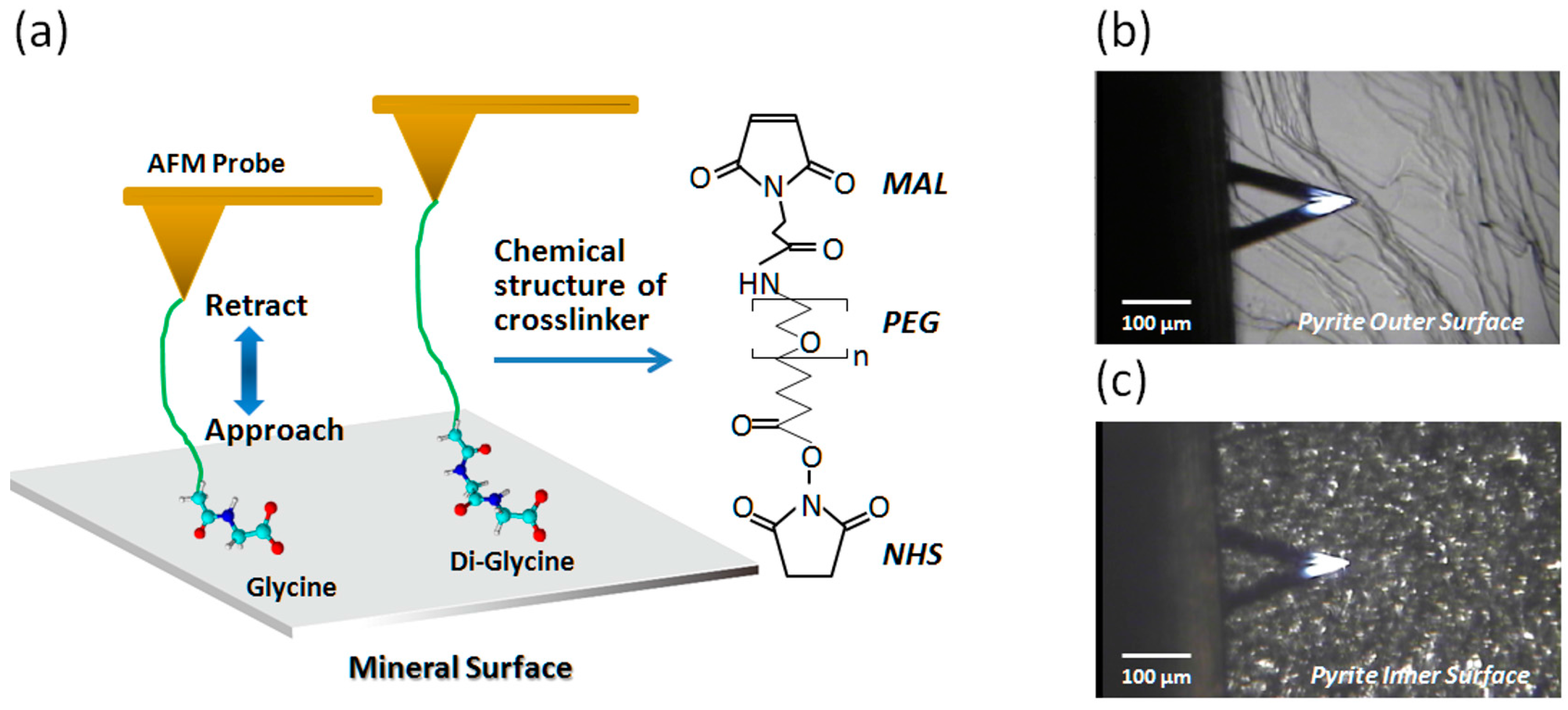
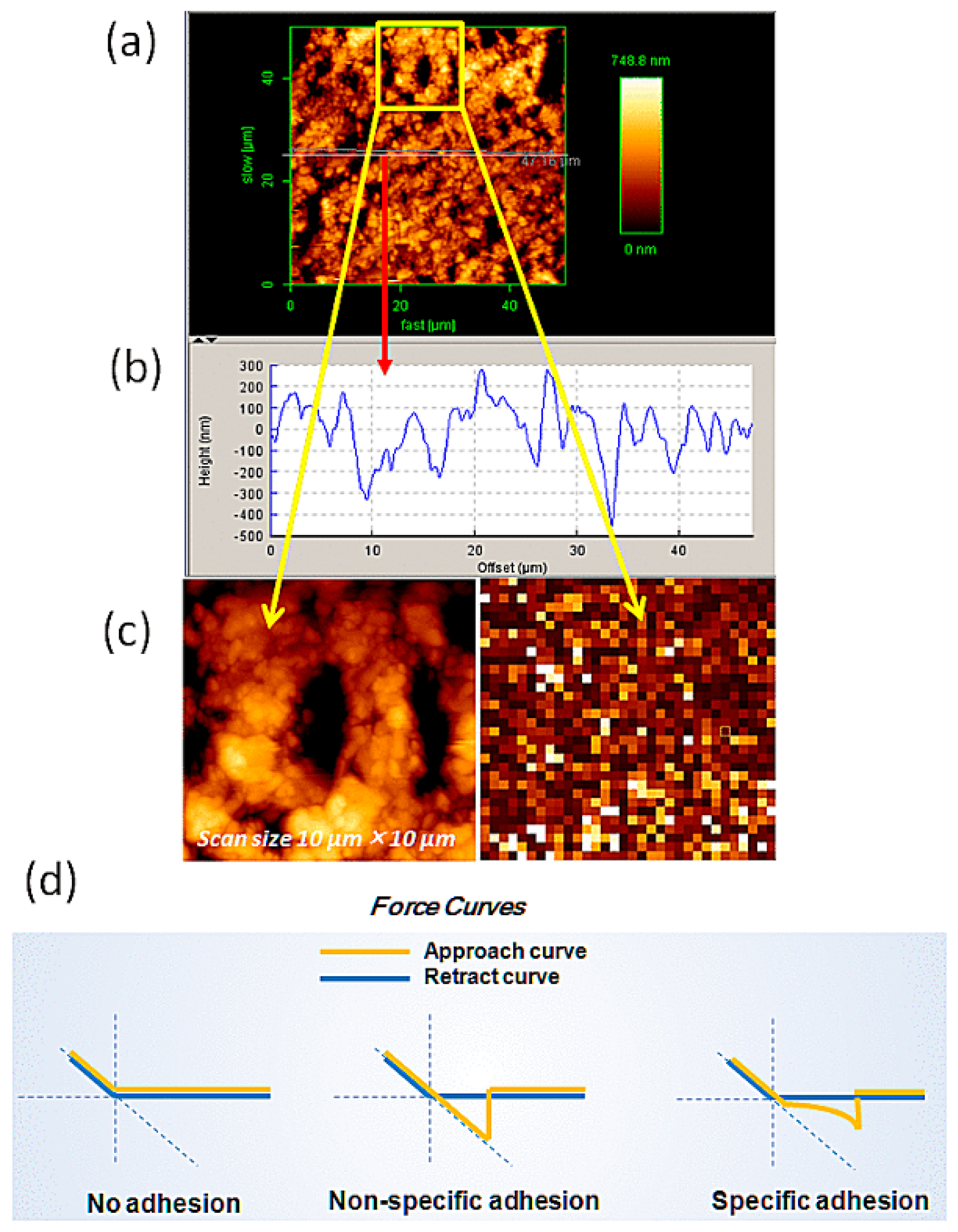
2.1. Imaging of Pyrite
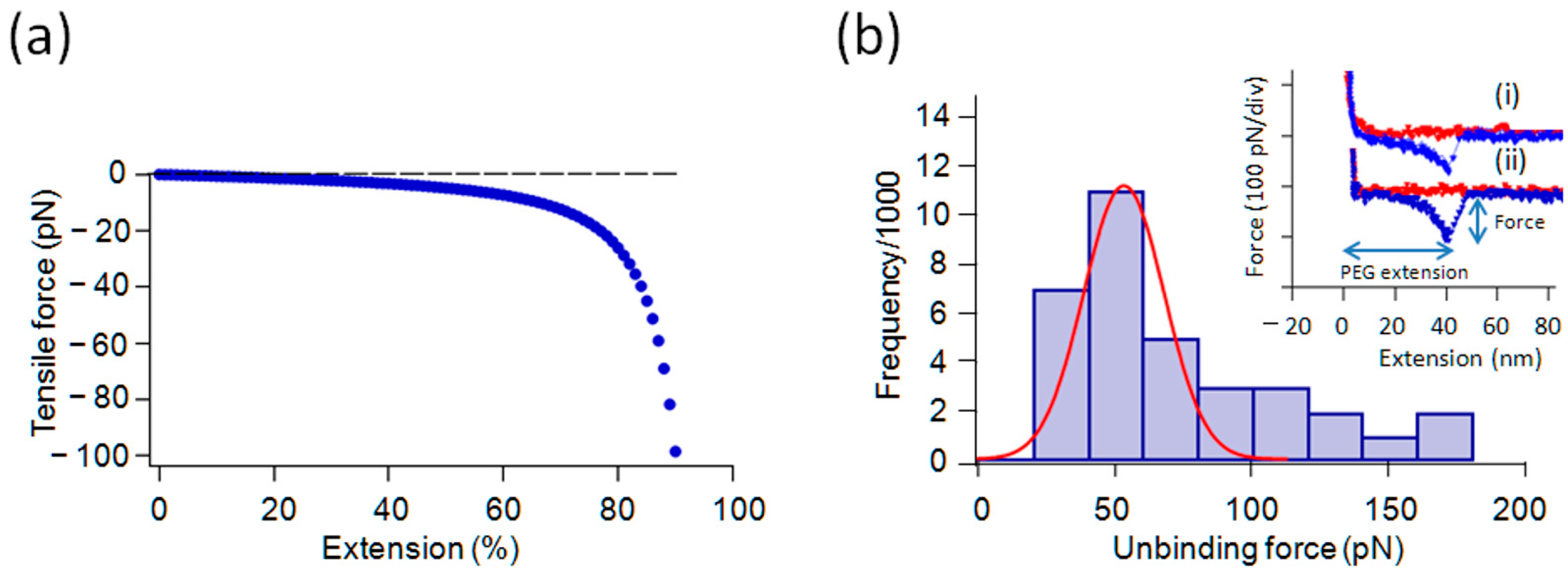
2.2. Force Measurements with Gly and Its Oligomers in 1/10× PBS
2.3. Effect of Salt Concentrations on the Interaction
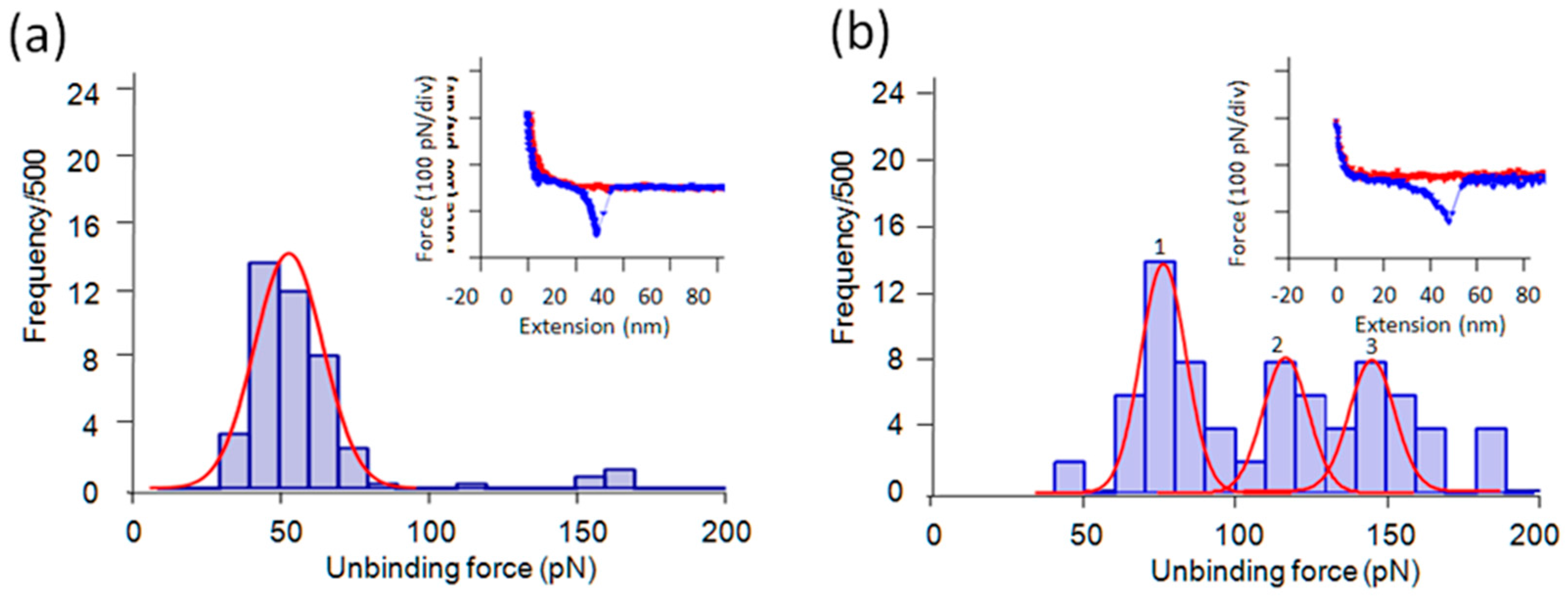
2.4. Tri-Gly and Penta-Gly
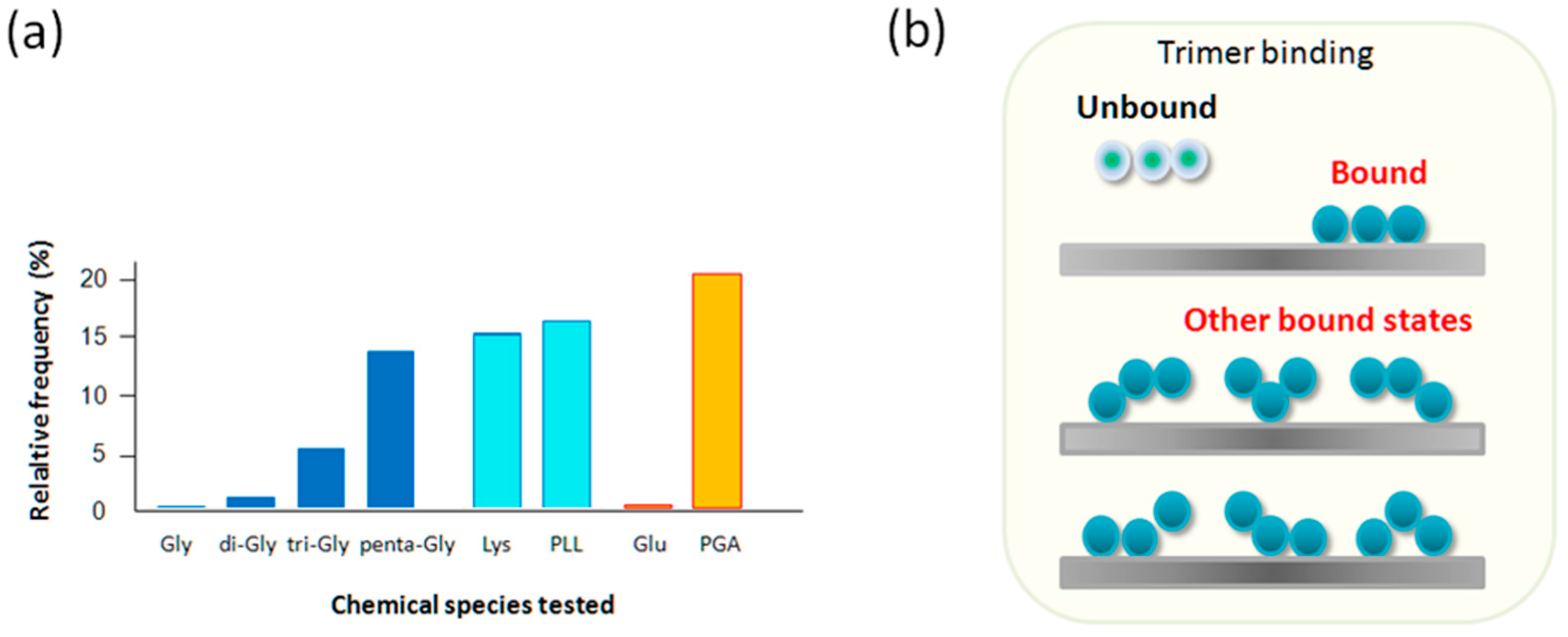
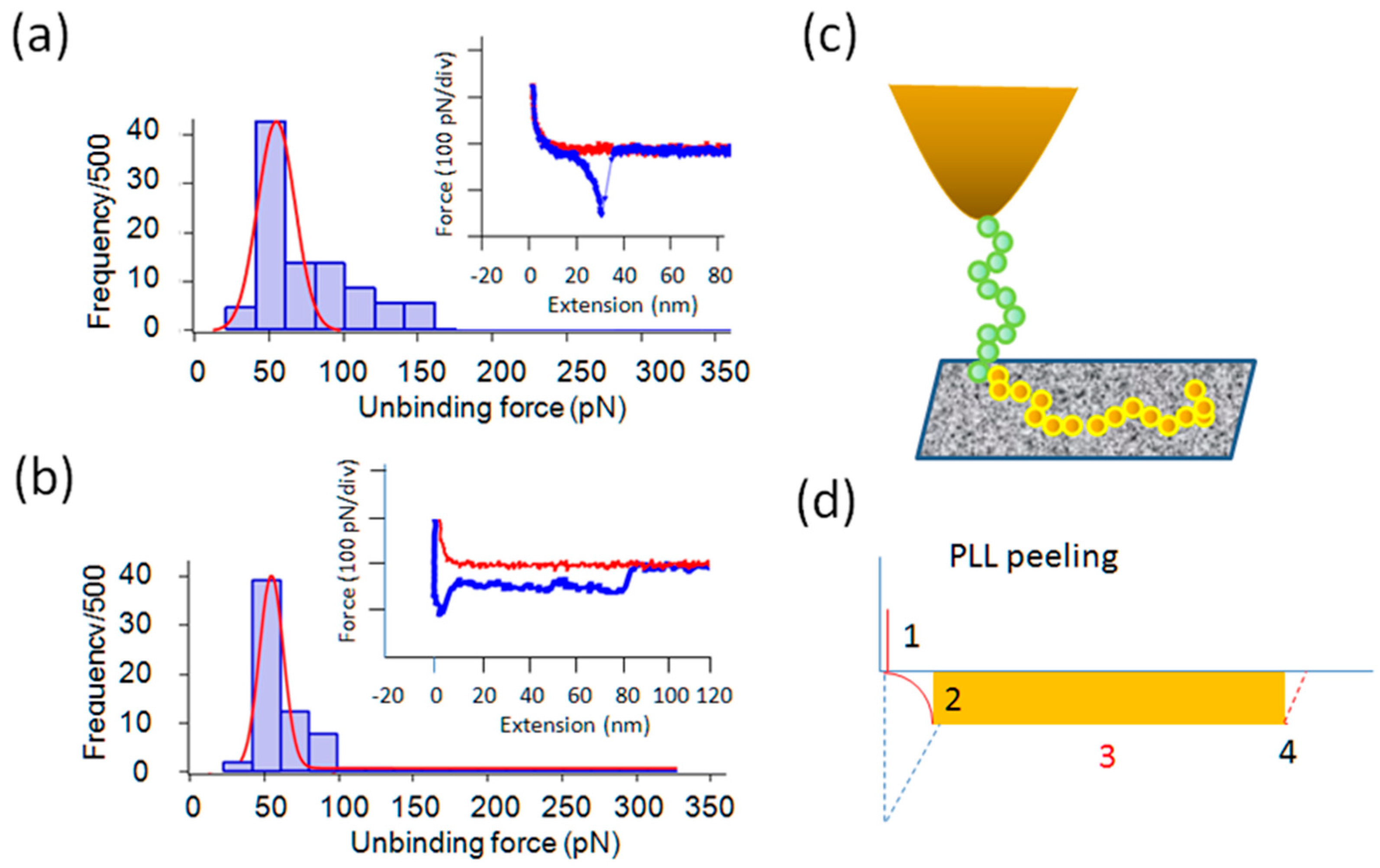
2.5. Lys and PLL, Glu vs. PGA
3. Discussion
4. Materials and Methods
4.1. Natural Pyrite and Amino Acids for AFM Operations
4.2. Modification of AFM Probes with Gly and Its Oligomers
4.3. AFM Imaging & Force-Curve Measurements
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Gly | glycine |
| di-Gly | diglycine |
| tri-Gly | triglycine |
| penta-Gly | pentaglycine |
| Lys | lysine |
| pLL | poly- l-lysine |
| Glu | glutamic acid |
| PGA | polyglutamic acid |
| PBS | phosphate buffered saline |
| MPF | most probable force |
| MAL-PEG-NHS | Poly(ethylene glycol) ( N-hydroxysuccinimide 5-pentanoate) ether N′-(3-maleimidopropionyl)aminoethane |
References
- Hazen, R.M.; Filley, T.R.; Goodfriend, G.A. Selective adsorption of l- and d-amino acids on calcite: Implications for biochemical homochirality. Proc. Natl. Acad. Sci. USA 2001, 98, 5487–5490. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H. Chapter 10: Role of clay minerals in chemical evolution and the origins of life. In Clay Minerals in Nature—Their Characterization, Modification and Application; Valaškova, M., Martynkova, G.S., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Lane, N. The Vital Question: Why is Life the Way It Is? Profile Books Ltd.: London, UK, 2016. [Google Scholar]
- Oparin, A.I. Origin of Life; Macmillan: New York, NY, USA, 1938. [Google Scholar]
- Haldane, J.B.S. What is Life? Boni and Caer: New York, NY, USA, 1947. [Google Scholar]
- Bracher, P.J. Origin of life: Primordial soup that cooks itself. Nat. Chem. 2015, 7, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. The origin of life. Ration. Annu. 1929, 148, 3–10. [Google Scholar]
- Flegmann, A.W.; Scholefield, D. Thermodynamics of peptide bond formation at clay mineral surfaces. J. Mol. Evol. 1978, 12, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.D. Significance of carbonaceous meteorites in theories on the origin of life. Nature 1961, 190, 129–131. [Google Scholar] [CrossRef]
- Lahav, N.; Chang, S. The possible role of solid surface area in condensation reactions during chemical evolution: Reevaluation. J. Mol. Evol. 1976, 8, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Basiuk, V.A.; Gromovoy, T.; Khil’chevskaya, E.G. Adsorption of small biological molecules on silica from diluted aqueous solutions: Quantitative characterization and implications to the bernal’s hypothesis. Orig. Life Evol. Biosph. 1995, 25, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.F. Adsorption and polymerization of amino acids on mineral surfaces: A review. Orig. Life Evol. Biosph. 2008, 38, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Smith, A.G. Genetic Takeover; Cambridge University Press: Cambridge, UK, 1982; p. 133. [Google Scholar]
- Wolthers, M.; Di Tommaso, D.; Dub, Z.; de Leeuwb, N.H. Calcite surface structure and reactivity: Molecular dynamics simulations and macroscopic surface modelling of the calcite—Water interface. Phys. Chem. Chem. Phys. 2012, 14, 15145–15157. [Google Scholar] [CrossRef] [PubMed]
- Bebie, J.; Schoonen, M.A.A. Pyrite surface interaction with selected organic aqueous species under anoxic conditions. Geochem. Trans. 2000, 1, 47–53. [Google Scholar] [CrossRef]
- Lowe, M.; Yadav, T.P.; Fournee, V.; Ledieu, J.; McGrath, R.; Sharma, H.R. Influence of leaching on surface composition, microstructure, and valence band of single grain icosahedral Al-Cu-Fe quasicrystal. J. Chem. Phys. 2015, 142, 094703. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Bowman, K.; Ohara, S.; Sverjensky, D.A.; Hazen, R.M.; Cleaves, H.J. Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochim. Cosmochim. Acta 2010, 74, 5852–5861. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar]
- Ikai, A. The World of Nano-Biomechanics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Wächtershäuse, G. Origin of life. Life as we don’t know it. Science 2000, 289, 1307–1308. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Discussing the origin of life. Science 2002, 298, 747–749. [Google Scholar] [PubMed]
- Beinert, H.; Holm, R.H.; Münck, E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.N.; Schreiner, E.; Marx, D. Glycine at the pyrite-water interface: The role of surface defects. J. Am. Chem. Soc. 2006, 128, 13815–13826. [Google Scholar] [CrossRef] [PubMed]
- Noren, K.; Loring, J.S.; Persson, P. Adsorption of alpha amino acids at the water/goethite interface. J. Colloid Interface Sci. 2008, 319, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Tao, J.; Xu, X.; Tang, R. Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level. Langmuir 2007, 23, 8972–8981. [Google Scholar] [CrossRef] [PubMed]
- Shanker, U.; Bhushan, B.; Bhattacharjee, G.; Kamaluddin. Oligomerization of glycine and alanine catalyzed by iron oxides: Implications for prebiotic chemistry. Orig. Life Evol. Biosph. 2012, 42, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.F.; Jaber, M.; Georgelin, T.; Stievano, L. A comparative study of the catalysis of peptide bond formation by oxide surfaces. Phys. Chem. Chem. Phys. 2013, 15, 13371–13380. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Corno, M.; Garza, J.; Ugliengo, P. Ab initio modelling of protein-biomaterial interactions: Influence of amino acid polar side chains on adsorption at hydroxyapatite surfaces. Philos. Trans. A Math. Phys. Eng. Sci. 2012, 370, 1478–1498. [Google Scholar] [CrossRef] [PubMed]
- Boehme, C.; Dominik, M. Glycine on a wet pyrite surface at extreme conditions. J. Am. Chem. Soc. 2003, 125, 13362–13363. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arenillasa, M.; Mateo-Marti, E. Pyrite surface environment drives molecular adsorption: Cystine on pyrite (100) investigated by X-ray photoemission spectroscopy and low energy electron diffraction. Phys. Chem. Chem. Phys. 2016, 18, 27219–27225. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, N.; Matsuzaki, N.; Nakazawa, Y.; Afrin, R.; Aono, M.; Yano, T.; Hayashi, T.; Hara, M. Surface force analysis of pyrite (FeS2): Its reactivity to amino acid adsorption. Adv. Mater. Phys. Chem. 2016, 6, 167–176. [Google Scholar] [CrossRef]
- Razvag, Y.; Gutkin, V.; Reches, M. Probing the interaction of individual amino acids with inorganic surfaces using atomic force spectroscopy. Langmuir 2013, 29, 10102–10109. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Stievano, L.; Lambert, J.F. Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 2004, 20, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J.; Michalkova, S.A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.M.; Jonsson, C.L.; Sverjensky, D.A.; Cleaves, H.J.; Hazen, R.M. Attachment of l-glutamate to rutile (α-TiO2): A potentiometric, adsorption, and surface complexation study. Langmuir 2009, 25, 12127–12135. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-López, A.; del Ángel-Meraz, E.; Colín-García, M.; Ramos-Bernal, S.; Negrón-Mendoza, A.; Heredia, A. Ultraviolet irradiation of glycine in presence of pyrite as a model of chemical evolution: An experimental and molecular modelling approach. Int. J. Astrobiol. 2017, 16, 237–243. [Google Scholar] [CrossRef]
- Rubinson, K.A.; Krueger, S. Poly(ethylene glycol)s 2000–8000 in water may be planar: A small-angle neutron scattering (SANS) structure study. Polymer 2009, 50, 4852–4858. [Google Scholar] [CrossRef]
- Bustamante, C.; Marko, J.F.; Siggia, E.D.; Smith, S. Entropic elasticity of lambda-phage DNA. Science 1994, 265, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Raible, M.; Evstigneev, M.; Bartels, F.W.; Eckel, R.; Nguyen-Duong, M.; Merkel, R.; Ros, R.; Anselmetti, D.; Reimann, P. Theoretical analysis of single-molecule force spectroscopy experiments: Heterogeneity of chemical bonds. Biophys. J. 2006, 90, 3851–3864. [Google Scholar] [CrossRef] [PubMed]
- Wonnacott, T.H.; Wonnacott, R.J. Introductory Statistics; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Baumann, C.G.; Bloomfield, V.A.; Smith, S.B.; Bustamante, C.; Wang, M.D.; Block, S.M. Stretching of single collapsed DNA molecules. Biophys. J. 2000, 78, 1965–1978. [Google Scholar] [CrossRef]
- Manohar, S.; Mantz, A.R.; Bancroft, K.E.; Hui, C.Y.; Jagota, A.; Vezenov, D.V. Peeling single-stranded DNA from graphite surface to determine oligonucleotide binding energy by force spectroscopy. Nano Lett. 2008, 8, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Grandbois, M.; Beyer, M.; Rief, M.; Clausen-Schaumann, H.; Gaub, H.E. How strong is a covalent bond? Science 1999, 283, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Yakubovicha, A.V.; Solov’yov, I.A.; Solov’yov, A.V.; Greiner, W. Conformational changes in glycine tri- and hexapeptide. Eur. Phys. J. D 2006, 39, 23–34. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; de Mendonça Silva, J.C.; Anderson Duarte, H.L.A. Pyrite oxidation mechanism by oxygen in aqueous medium. J. Phys. Chem. C 2016, 120, 2760–2768. [Google Scholar] [CrossRef]
- Hukkanen, E.J.; Wieland, J.A.; Gewirth, A.; Leckband, D.E.; Braatz, R.D. Multiple-bond kinetics from single-molecule pulling experiments: Evidence for multiple NCAM bonds. Biophys. J. 2005, 89, 3434–3445. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Golenhofen, N.; Grundhofer, N.; Wiegand, J.; Drenckhahn, D. Ca2+ dependency of N-cadherin function probed by laser tweezer and atomic force microscopy. J. Neurosci. 2003, 23, 11008–11014. [Google Scholar] [PubMed]
- Kienberger, F.; Kada, G.; Mueller, H.; Hinterdorfer, P. Single molecule studies of antibody-antigen interaction strength versus intra-molecular antigen stability. J. Mol. Biol. 2005, 347, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Hinterdorfer, P.; Baumgartner, W.; Gruber, H.J.; Schilcher, K.; Schindler, H. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 3477–3481. [Google Scholar] [CrossRef] [PubMed]
- Folkers, J.P.; Laibinis, P.E.; Whitesiedes, G.M. Self-assembled monolayers of alkanethiols on gold: The adsorption and wetting properties of monolayers derived from two components with alkane chains of different lengths. J. Adhes. Sci. Technol. 1992, 6, 1397–1410. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic force microscope tips. Rev. Sci. Instrum. 1993, 7, 1868–1873. [Google Scholar] [CrossRef]
- Butt, H.J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Puech, P.H.; Poole, K.; Knebel, D.; Muller, D.J. A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy 2006, 106, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Xu, S.; Oliveira, C.L.P.; Pedersen, J.S.; Thiel, S.; Besenbacher, F.; Vorup-Jensen, T. Conformational Changes in Mannan-Binding Lectin Bound to Ligand Surfaces. J. Immunol. 2007, 178, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Lazar, P.; Zhang, S.; Safářová, K.; Li, Q.; Froning, J.P.; Granatier, J.; Hobza, P.; Zbořil, R.; Besenbacher, F.; Dong, M.; et al. Quantification of the Interaction Forces between Metals and Graphene by Quantum Chemical Calculations and Dynamic Force Measurements under Ambient Conditions. ACS Nano 2013, 7, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Aslan, H.; Besenbacher, F.; Dong, M. Quantitative biomolecular imaging by dynamic nanomechanical mapping. Chem. Soc. Rev. 2014, 43, 7412–7429. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Sahin, O. A nanomechanical interface to rapid single-molecule interactions. Nat. Commun. 2011, 2, 247. [Google Scholar] [CrossRef] [PubMed]

| Molecular Species | MPF * Obtained by AFM (pN) | Relative Frequency of Interaction (%) |
|---|---|---|
| Gly | − ** | <1 |
| Di-Gly | 50 | 3‒5 |
| Tri-Gly | 55 | 7‒10 |
| Penta-Gly | 76, 110, 140 | 10‒15 |
| Lys | 57 | 10‒15 |
| PLL | 55 | 15‒20 |
| Glu | − ** | <1 |
| PGA | 65 | 20‒25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, R.; Ganbaatar, N.; Aono, M.; Cleaves II, H.J.; Yano, T.-a.; Hara, M. Size-Dependent Affinity of Glycine and Its Short Oligomers to Pyrite Surface: A Model for Prebiotic Accumulation of Amino Acid Oligomers on a Mineral Surface. Int. J. Mol. Sci. 2018, 19, 365. https://doi.org/10.3390/ijms19020365
Afrin R, Ganbaatar N, Aono M, Cleaves II HJ, Yano T-a, Hara M. Size-Dependent Affinity of Glycine and Its Short Oligomers to Pyrite Surface: A Model for Prebiotic Accumulation of Amino Acid Oligomers on a Mineral Surface. International Journal of Molecular Sciences. 2018; 19(2):365. https://doi.org/10.3390/ijms19020365
Chicago/Turabian StyleAfrin, Rehana, Narangerel Ganbaatar, Masashi Aono, H. James Cleaves II, Taka-aki Yano, and Masahiko Hara. 2018. "Size-Dependent Affinity of Glycine and Its Short Oligomers to Pyrite Surface: A Model for Prebiotic Accumulation of Amino Acid Oligomers on a Mineral Surface" International Journal of Molecular Sciences 19, no. 2: 365. https://doi.org/10.3390/ijms19020365
APA StyleAfrin, R., Ganbaatar, N., Aono, M., Cleaves II, H. J., Yano, T.-a., & Hara, M. (2018). Size-Dependent Affinity of Glycine and Its Short Oligomers to Pyrite Surface: A Model for Prebiotic Accumulation of Amino Acid Oligomers on a Mineral Surface. International Journal of Molecular Sciences, 19(2), 365. https://doi.org/10.3390/ijms19020365