Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content
Abstract
1. Introduction
2. Results
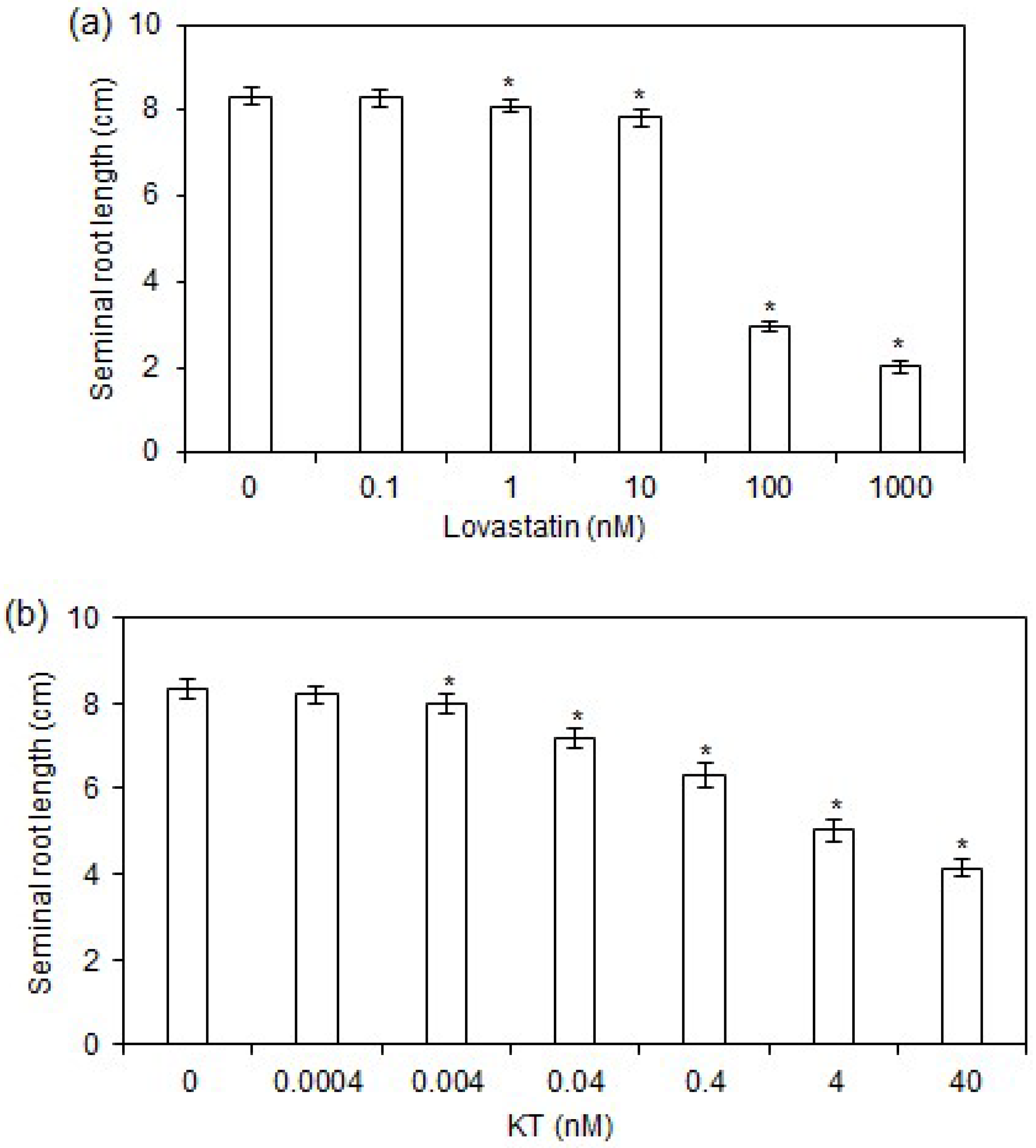
2.1. Effects of Lovastatin and KT Treatments on Rice Seminal Root Growth
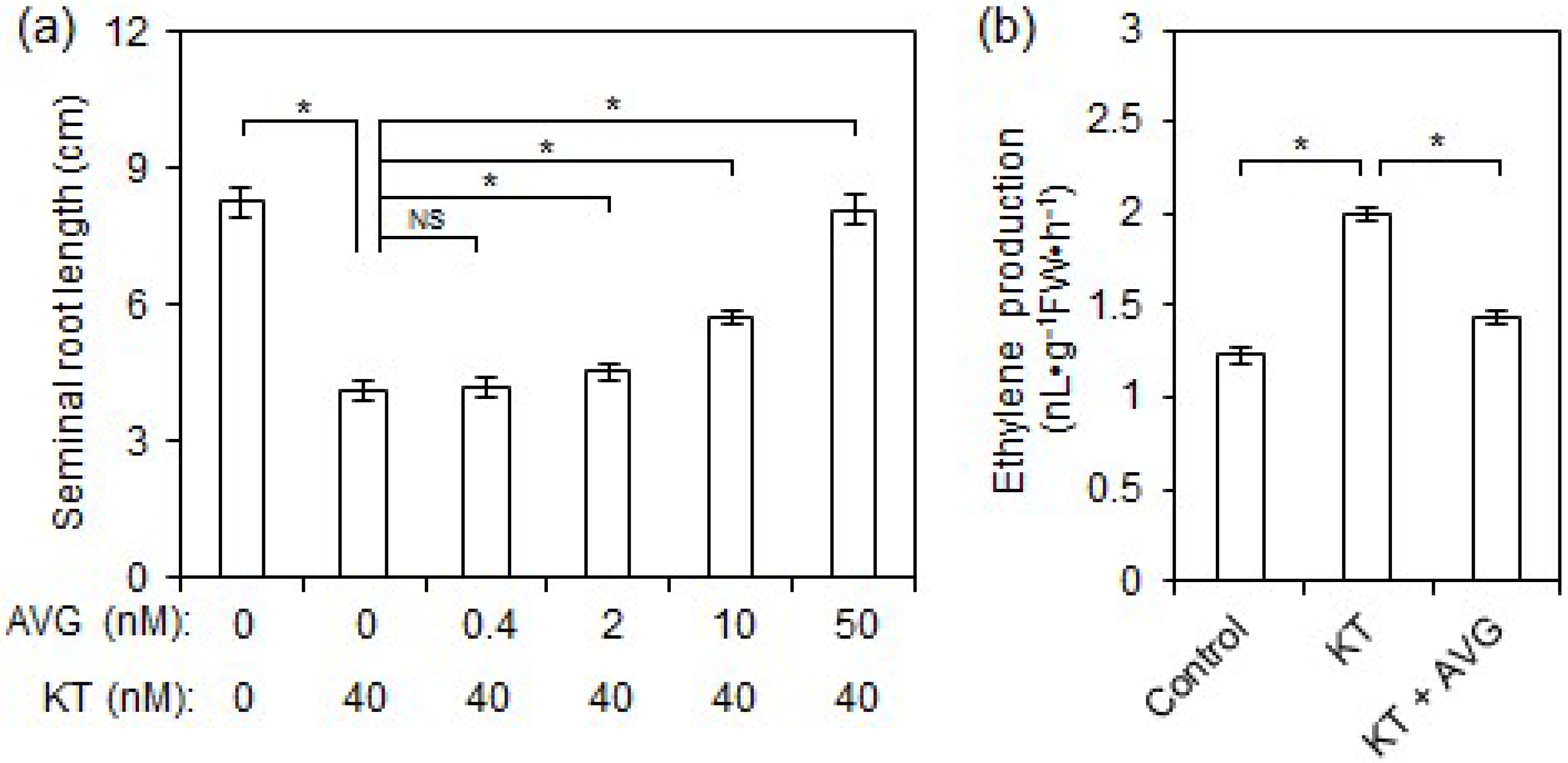
2.2. Exogenous CK Inhibited Rice Seminal Root Growth by Promoting Ethylene Production
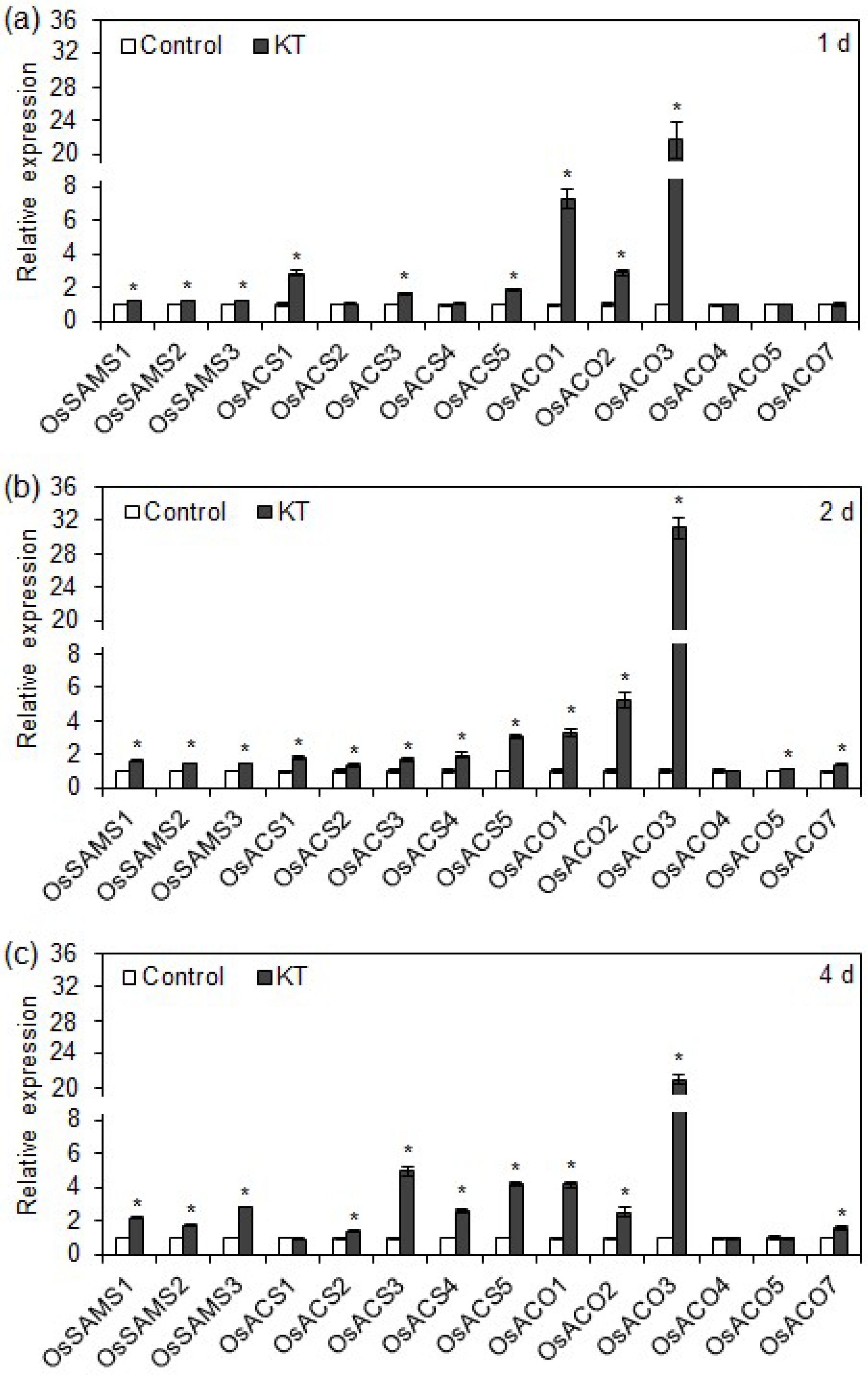
2.3. Exogenous CK Upregulated Transcription of Ethylene Biosynthesis Genes in Rice Seminal Roots
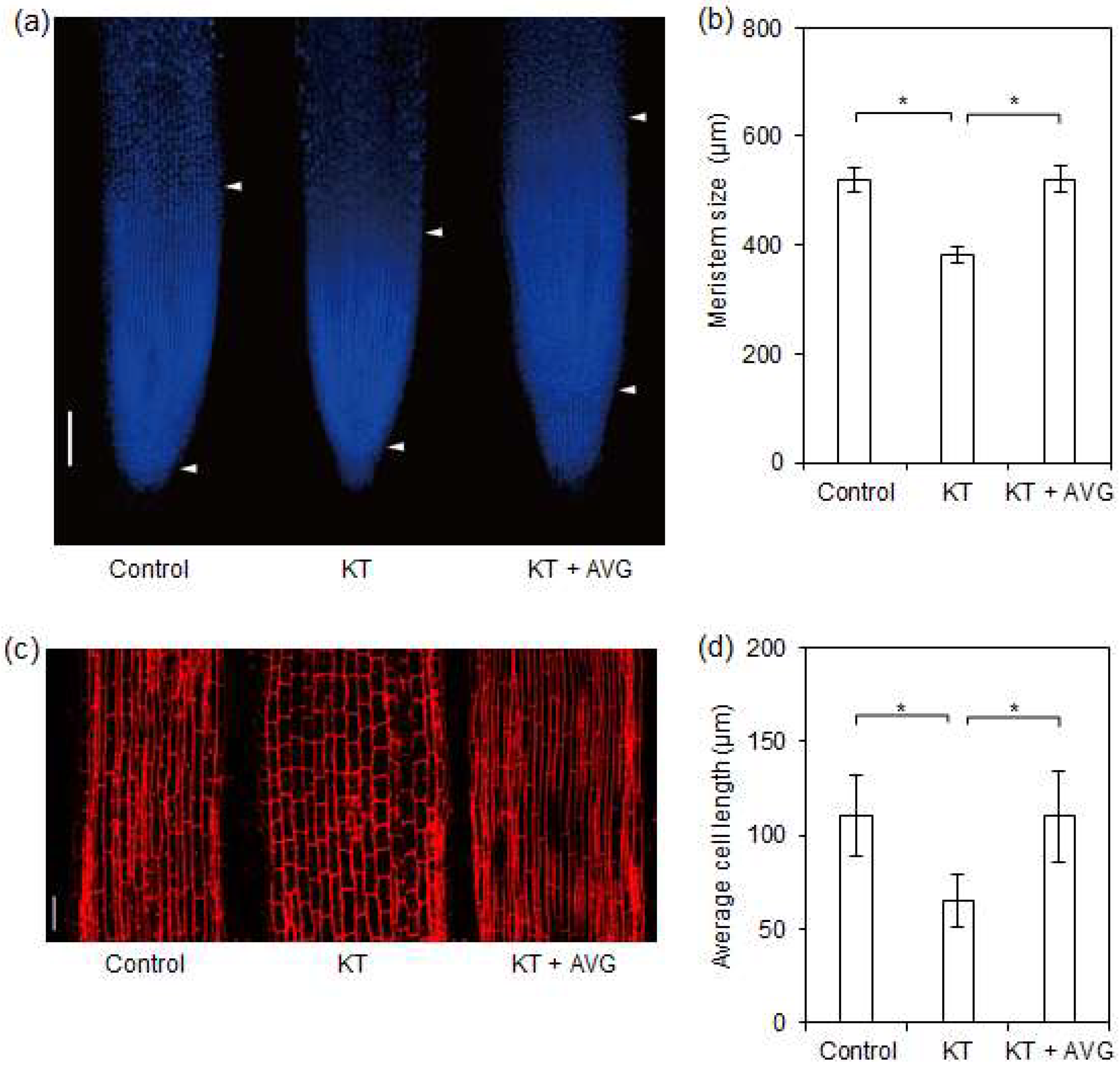
2.4. CK-Induced Ethylene Reduced Meristem Size and Cell Length of Rice Seminal Roots
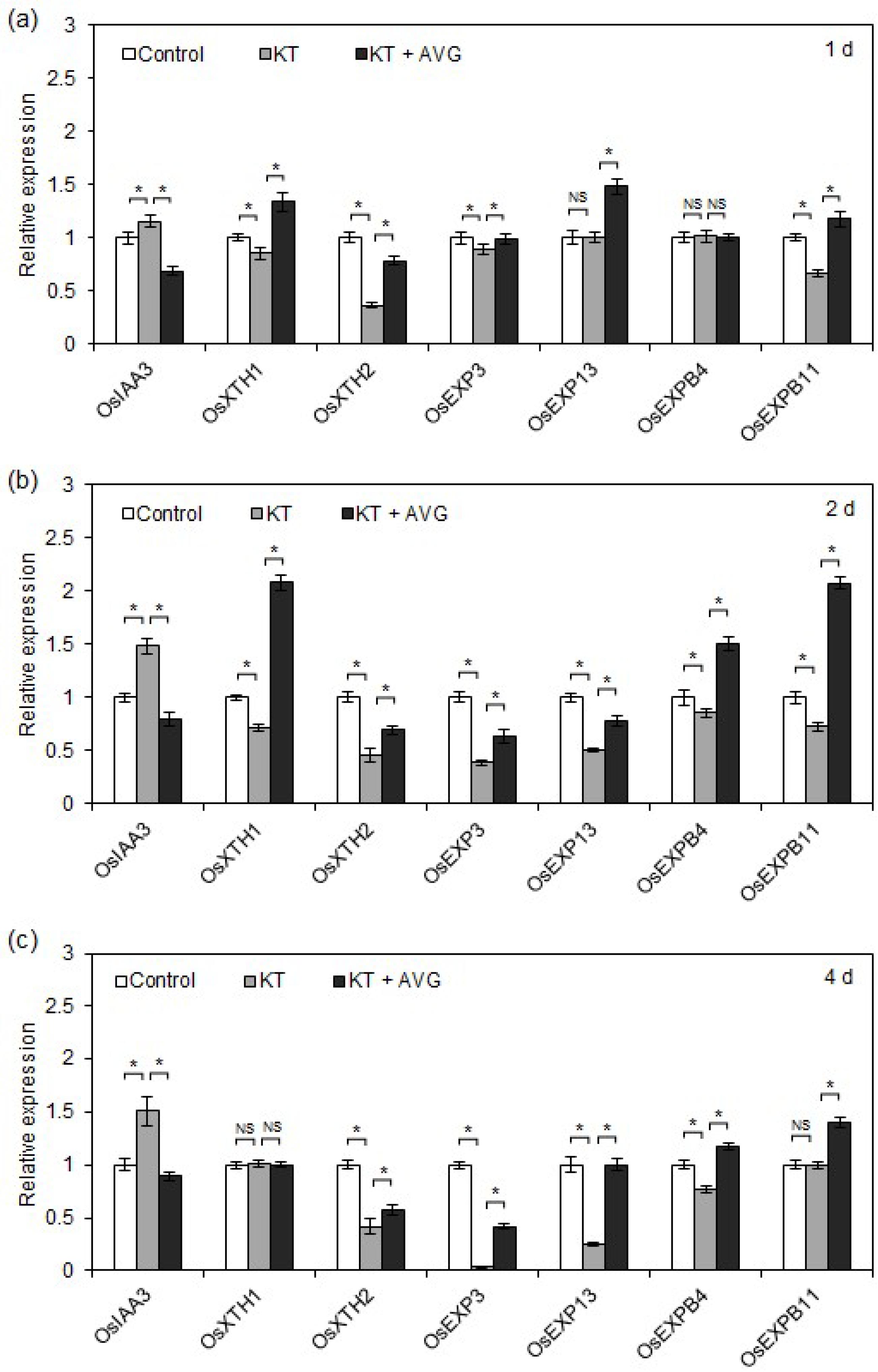
2.5. CK-Induced Ethylene Promoted OsIAA3 Transcription and Inhibited Transcription of Cell Elongation-Related Genes in Rice Seminal Roots
3. Discussion
3.1. Threshold Content of CK Is Required for Rapid Growth of Rice Seminal Roots
3.2. Supraoptimal CK Content Inhibits Rice Seminal Root Growth
3.3. Supraoptimal CK Content Inhibits Rice Seminal Root Growth by Promoting Ethylene Biosynthesis
3.4. CK-Induced Ethylene Inhibits Rice Seminal Root Growth by Reducing Meristem Size and Cell Length
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Chemicals and Treatments
4.3. Ethylene Production Measurements
4.4. Extraction, Purification and Quantification of CKs
4.5. RNA Isolation and qRT-PCR Analysis
4.6. Examination of Root Meristem Size and Cell length
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | Cytokinin |
| KT | Kinetin |
| AVG | Aminoethoxyvinylglycine |
| XTH | Xyloglucan endotransglucosylase/hydrolase |
| SAMS | S-adenosyl-L-methionine synthetase |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ACS | 1-aminocyclopropane-1-carboxylic acid synthase |
| ACO | 1-aminocyclopropane-1-carboxylic acid oxidase |
| Z | zeatin |
| DZ | dihydrozeatin |
| SE | Standard error |
| NS | No significance |
| iP | isopentenyladenine |
| ND | below detection limits |
| CKX | Cytokinin oxidase dehydrogenase |
| IPT | Isopentenyltransferase |
| DMSO | Dimethyl sulfoxide |
| FW | Fresh weight |
| qRT-PCR | Quantitative real-time PCR |
References
- Hopping, M.E. Effect of exogenous auxins, gibberellins, and cytokinins on fruit development in Chinese gooseberry (Actinidia chinensis Planch.). N. Z. J. Bot. 1976, 14, 69–75. [Google Scholar] [CrossRef]
- Miyoshi, K.; Sato, T. The effects of kinetin and gibberellin on the germination of dehusked seeds of indica and japonica rice (Oryza sativa L.) under anaerobic and aerobic conditions. Ann. Bot. 1997, 80, 479–483. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Zeng, H.; Cai, Q.; Zhou, X.; Yin, C. Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J. Plant Growth Regul. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Chaudhury, A.M.; Letham, S.; Craig, S.; Dennis, E.S. amp1—A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993, 4, 907–916. [Google Scholar] [CrossRef]
- Cary, A.J.; Liu, W.; Howell, S.H. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995, 107, 1075–1082. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Onckelen, H.V.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmülling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef]
- Ioio, R.D.; Galinha, C.; Fletcher, A.G.; Grigg, S.P.; Molnar, A.; Willemsen, V.; Scheres, B.; Sabatini, S.; Baulcombe, D.; Maini, P.K. A PHABULOSA/cytokinin feedback loop controls root growth in Arabidopsis. Curr. Biol. 2012, 22, 1699–1704. [Google Scholar] [CrossRef]
- López-García, C.M.; Raya-González, J.; López-Bucio, J.S.; Guevara-García, Á.A.; López-Bucio, J. ALTERED MERISTEM PROGRAM 1 plays a role in seed coat development, root growth, and post-embryonic epidermal cell elongation in Arabidopsis. J. Plant Growth Regul. 2016, 35, 1–18. [Google Scholar] [CrossRef]
- Köllmer, I.; Novák, O.; Strnad, M.; Schmülling, T.; Werner, T. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 2014, 78, 359–371. [Google Scholar] [CrossRef]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wu, Q.; Zeng, H.; Xia, K.; Xu, J.; Li, R. Endogenous auxin is required but supraoptimal for rapid growth of rice (Oryza sativa L.) seminal roots, and auxin inhibition of rice seminal root growth is not caused by ethylene. J. Plant Growth Regul. 2011, 30, 20–29. [Google Scholar] [CrossRef]
- Růzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Ioio, R.D.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Dello, I.R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Nakamura, A.; Umemura, I.; Gomi, K.; Hasegawa, Y.; Kitano, H.; Sazuka, T.; Matsuoka, M. Production and characterization of auxin-insensitive rice by overexpression of a mutagenized rice IAA protein. Plant J. 2006, 46, 297–306. [Google Scholar] [CrossRef]
- Beemster, G.T.; Baskin, T.I. Stunted plant 1 mediates effects of cytokinin, but not of auxin, on cell division and expansion in the root of Arabidopsis. Plant Physiol. 2000, 124, 1718–1727. [Google Scholar] [CrossRef]
- Yokoyama, R. A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiol. 2004, 134, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Kende, H. Expression of α-expansin and expansin-like genes in deepwater rice. Plant Physiol. 2002, 130, 1396–1405. [Google Scholar] [CrossRef]
- Bertell, G.; Eliasson, L. Cytokinin effects on root growth and possible interactions with ethylene and indolecetic acid. Physiol. Plantarum 1992, 84, 255–261. [Google Scholar] [CrossRef]
- Kende, H. Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 344, 283–307. [Google Scholar] [CrossRef]
- Wang, K.L.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef]
- Rzewuski, G.; Sauter, M. Ethylene biosynthesis and signaling in rice. Plant Sci. 2008, 175, 32–42. [Google Scholar] [CrossRef]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef]
- Iwai, T.; Miyasaka, A.; Seo, S.; Ohashi, Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol. 2006, 142, 1202–1215. [Google Scholar] [CrossRef]
- Lee, J.H.; Chae, H.S.; Lee, J.H.; Hwang, B.; Hahn, K.W.; Kang, B.G.; Kim, W.T. Structure and expression of two cDNAs encoding S-adenosyl-L-methionine synthetase of rice (Oryza sativa L.). BBA-Gene Struct. Expr. 1997, 1354, 13–18. [Google Scholar] [CrossRef]
- Li, W.; Han, Y.; Tao, F.; Chong, K. Knockdown of SAMS genes encoding S-adenosyl-l-methionine synthetases causes methylation alterations of DNAs and histones and leads to late flowering in rice. J. Plant Physiol. 2011, 168, 1837–1843. [Google Scholar] [CrossRef]
- Chae, H.S.; Cho, Y.G.; Park, M.Y.; Lee, M.C.; Eun, M.Y.; Kang, B.G.; Kim, W.T. Hormonal cross-talk between auxin and ethylene differentially regulates the expression of two members of the 1-aminocyclopropane-1-carboxylate oxidase gene family in rice (Oryza sativa L.). Plant Cell Physiol. 2000, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Luo, W.; Li, W.; Chen, N.; Zhang, D.; Chong, K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence, in rice. Plant Physiol. 2013, 163, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Shiono, K.; Nagano, M.; Fukazawa, A.; Ando, M.; Takamure, I.; Mori, H.; Nishizawa, N.K.; Kawai-Yamada, M.; Tsutsumi, N.; et al. Ethylene biosynthesis is promoted by very-long-chain fatty acids during lysigenous aerenchyma formation in rice roots. Plant Physiol. 2015, 169, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Pischke, M.S.; Mähönen, A.P.; Miyawaki, K.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Shinozaki, K.; Kato, T.; Tabata, S.; et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 2004, 101, 8821–8826. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Makita, N.; Kojima, M.; Kamada-Nobusada, T.; Sakakibara, H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007, 48, 523–539. [Google Scholar] [CrossRef]
- Mason, M.G.; Mathews, D.E.; Argyros, D.A.; Maxwell, B.B.; Kieber, J.J.; Alonso, J.M.; Ecker, J.R.; Schaller, G.E. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 2005, 17, 3007–3718. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, K.; Tarkowski, P.; Matsumotokitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Sato, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Howell, S.H. The effects of cytokinin and light on hypocotyl elongation in Arabidopsis seedlings are independent and additive. Plant Physiol. 1995, 108, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Woeste, K.E.; Theologis, A.; Kieber, J.J. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 1998, 95, 4766–4771. [Google Scholar] [CrossRef]
- Massot, N.; Nicander, B.; Barceló, J.; Poschenrieder, Ch.; Tillberg, E. A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+-induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.). Plant Growth Regul. 2002, 37, 105–112. [Google Scholar] [CrossRef]
- Chae, H.S.; Faure, F.; Kieber, J.J. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 2003, 15, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Street, I.H.; Aman, S.; Zubo, Y.; Ramzan, A.; Wang, X.; Shakeel, S.N.; Kieber, J.J.; Schaller, G.E. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol. 2015, 169, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zou, X.; Zhao, S.; Xia, C.; Qian, K.; Wang, P.; Yin, C. Fluridone induces leaf bleaching by inhibiting pigment biosynthesis via downregulated transcription levels of pigment biosynthetic genes in rice (Oryza sativa, L.). J. Plant Growth Regul. 2018, 37, 1385–1395. [Google Scholar] [CrossRef]
- Crowell, D.N.; Salaz, M.S. Inhibition of growth of cultured tobacco cells at low concentrations of lovastatin is reversed by cytokinin. Plant Physiol. 1992, 100, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Kato, H.; Muranaka, T.; Yoshida, S. Amyloplast formation in cultured tobacco BY-2 cells requires a high cytokinin content. Plant Cell Physiol. 2002, 43, 1534–1541. [Google Scholar] [CrossRef]
- Hartig, K.; Beck, E. Assessment of lovastatin application as tool in probing cytokinin-mediated cell cycle regulation. Physiol. Plantarum 2005, 125, 260–267. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Chi, G.; Pua, E.; Gph, C. Role of Ethylene on de Novo shoot regeneration from cotyledonary explants of Brassica campestris ssp. pekinensis (Lour) olsson in Vitro. Plant Physiol. 1991, 96, 178–183. [Google Scholar] [CrossRef]
- Lim, T.; Chitra, T.R.; Han, P.; Pua, E.C.; Yu, H. Cloning and characterization of Arabidopsis and Brassica juncea flavin-containing amine oxidases. J. Exp. Bot. 2006, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Xiao, J.; Wang, S. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 2012, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Thomann, A.; Lechner, E.; Hansen, M.; Dumbliauskas, E.; Parmentier, Y.; Kieber, J.; Scheres, B.; Genschik, P. Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanism. PLoS Genet. 2009, 5, e1000328. [Google Scholar] [CrossRef] [PubMed]
| Treatment | KT (ng·g−1 FW) | Z (ng·g−1 FW) | DZ (ng·g−1 FW) | iP (ng·g−1 FW) | Total (ng·g−1 FW) |
|---|---|---|---|---|---|
| Control | ND | 89.88 ± 2.02 | 2.11 ± 0.03 | ND | 91.99 |
| 0.1 nM LOV | ND | 88.97 ± 0.74 | 2.07 ± 0.06 | ND | 91.04 |
| 1 nM LOV | ND | 84.86 ± 1.05 | 1.87 ± 0.06 | ND | 86.73 |
| 10 nM LOV | ND | 61.40 ± 1.80 | 1.23 ± 0.04 | ND | 62.63 |
| 100 nM LOV | ND | 43.30 ± 1.19 | 0.92 ± 0.03 | ND | 44.22 |
| 1000 nM LOV | ND | 29.60 ± 1.64 | 0.65 ± 0.03 | ND | 30.25 |
| 0.0004 nM KT | ND | 89.12 ± 1.70 | 2.11 ± 0.08 | ND | 91.23 |
| 0.004 nM KT | 4.39 ± 0.14 | 89.24 ± 0.74 | 2.06 ± 0.08 | ND | 95.69 |
| 0.04 nM KT | 5.95 ± 0.12 | 89.70 ± 1.40 | 2.06 ± 0.10 | ND | 97.71 |
| 0.4 nM KT | 6.57 ± 0.50 | 89.95 ± 1.30 | 2.08 ± 0.06 | ND | 98.6 |
| 4 nM KT | 9.82 ± 0.26 | 89.33 ± 0.16 | 2.03 ± 0.09 | ND | 101.18 |
| 40 nM KT | 18.42 ± 0.40 | 89.69 ± 0.77 | 2.06 ± 0.07 | ND | 110.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Shao, J.; Wang, Q.; Chen, P.; Zhu, Y.; Yin, C. Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content. Int. J. Mol. Sci. 2018, 19, 4051. https://doi.org/10.3390/ijms19124051
Zou X, Shao J, Wang Q, Chen P, Zhu Y, Yin C. Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content. International Journal of Molecular Sciences. 2018; 19(12):4051. https://doi.org/10.3390/ijms19124051
Chicago/Turabian StyleZou, Xiao, Junwei Shao, Qi Wang, Peisai Chen, Yanchun Zhu, and Changxi Yin. 2018. "Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content" International Journal of Molecular Sciences 19, no. 12: 4051. https://doi.org/10.3390/ijms19124051
APA StyleZou, X., Shao, J., Wang, Q., Chen, P., Zhu, Y., & Yin, C. (2018). Supraoptimal Cytokinin Content Inhibits Rice Seminal Root Growth by Reducing Root Meristem Size and Cell Length via Increased Ethylene Content. International Journal of Molecular Sciences, 19(12), 4051. https://doi.org/10.3390/ijms19124051





