Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation
Abstract
1. Introduction
1.1. Leptin Plays a Canonical Role on Appetite Control
1.2. Leptin’s Non-Appetite Controlling Characteristic
1.3. Fish Is a New Low Vertebrate Model for Leptin-Associated Study
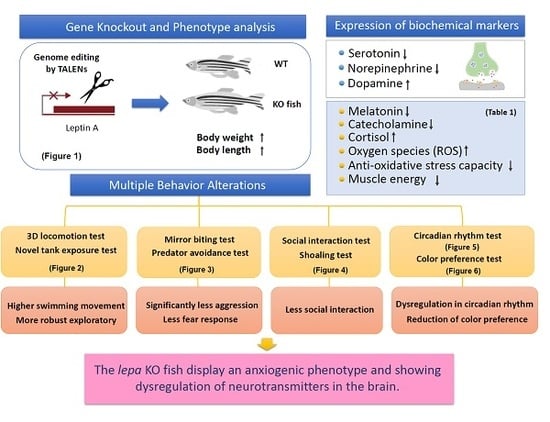
2. Results
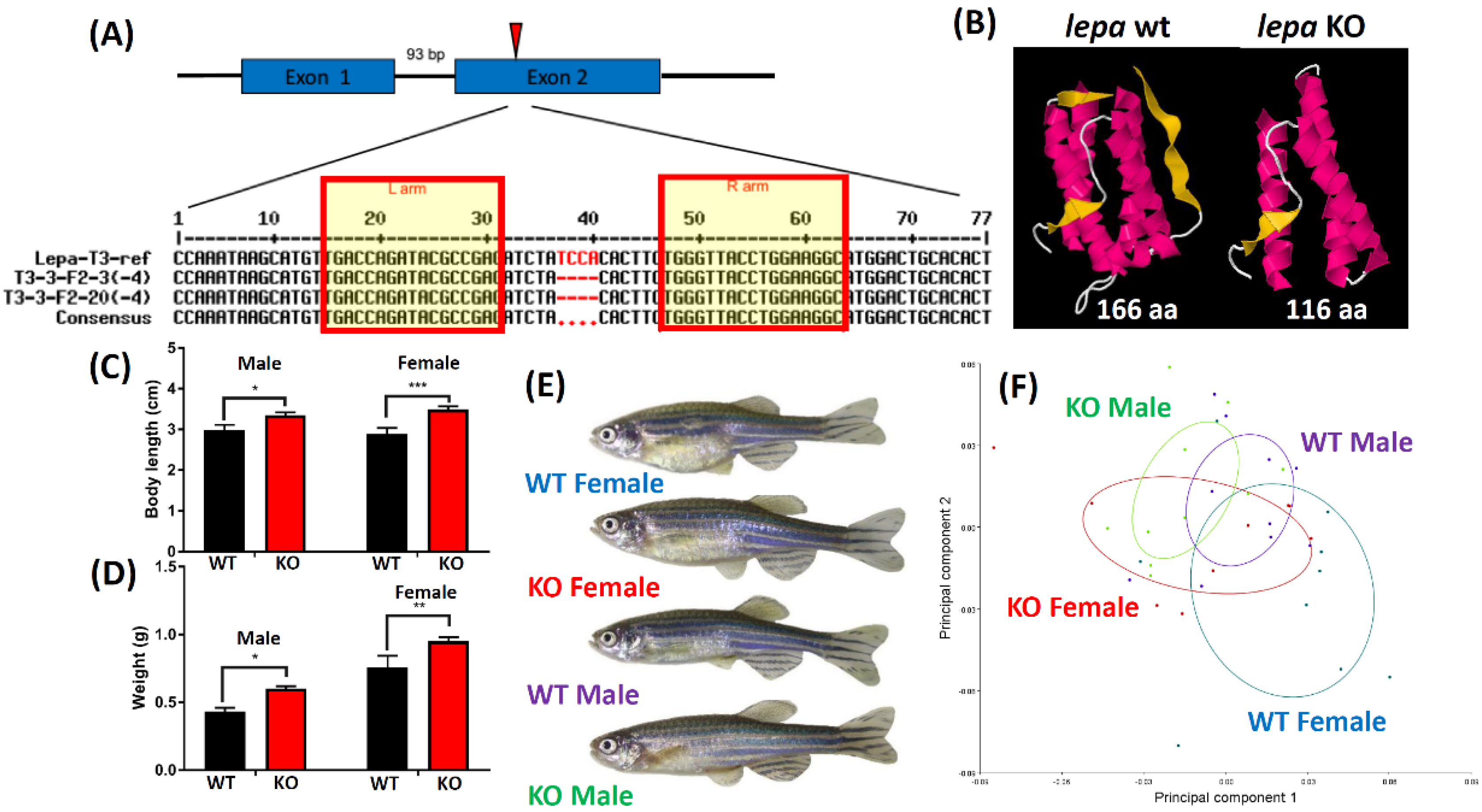
2.1. Lepa KO Zebrafish Displayed Typical Obesity Phenotype
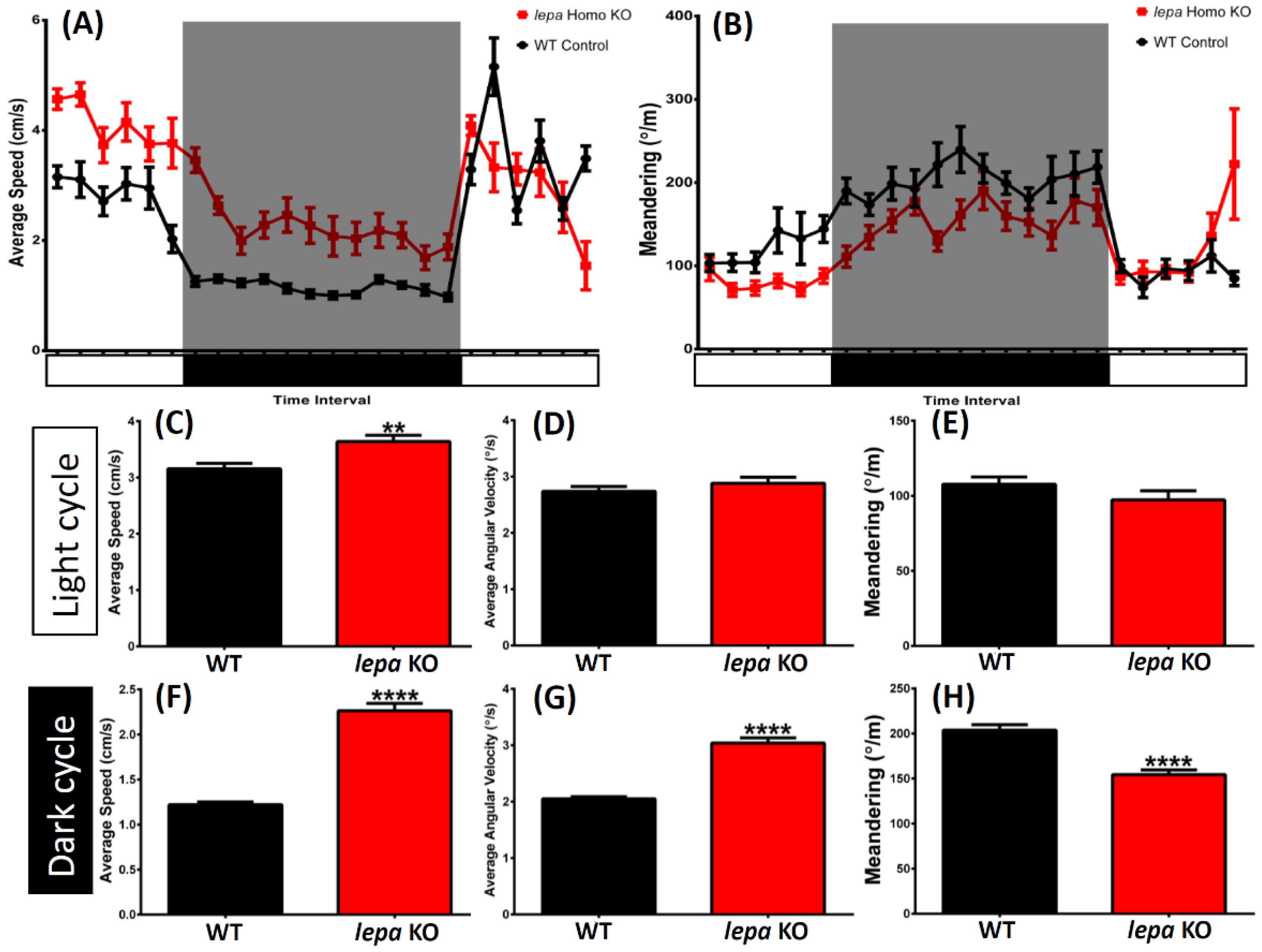
2.2. Lepa KO Zebrafish Display Hyperactivity with Faster Locomotion and Robust Exploratory Behaviors
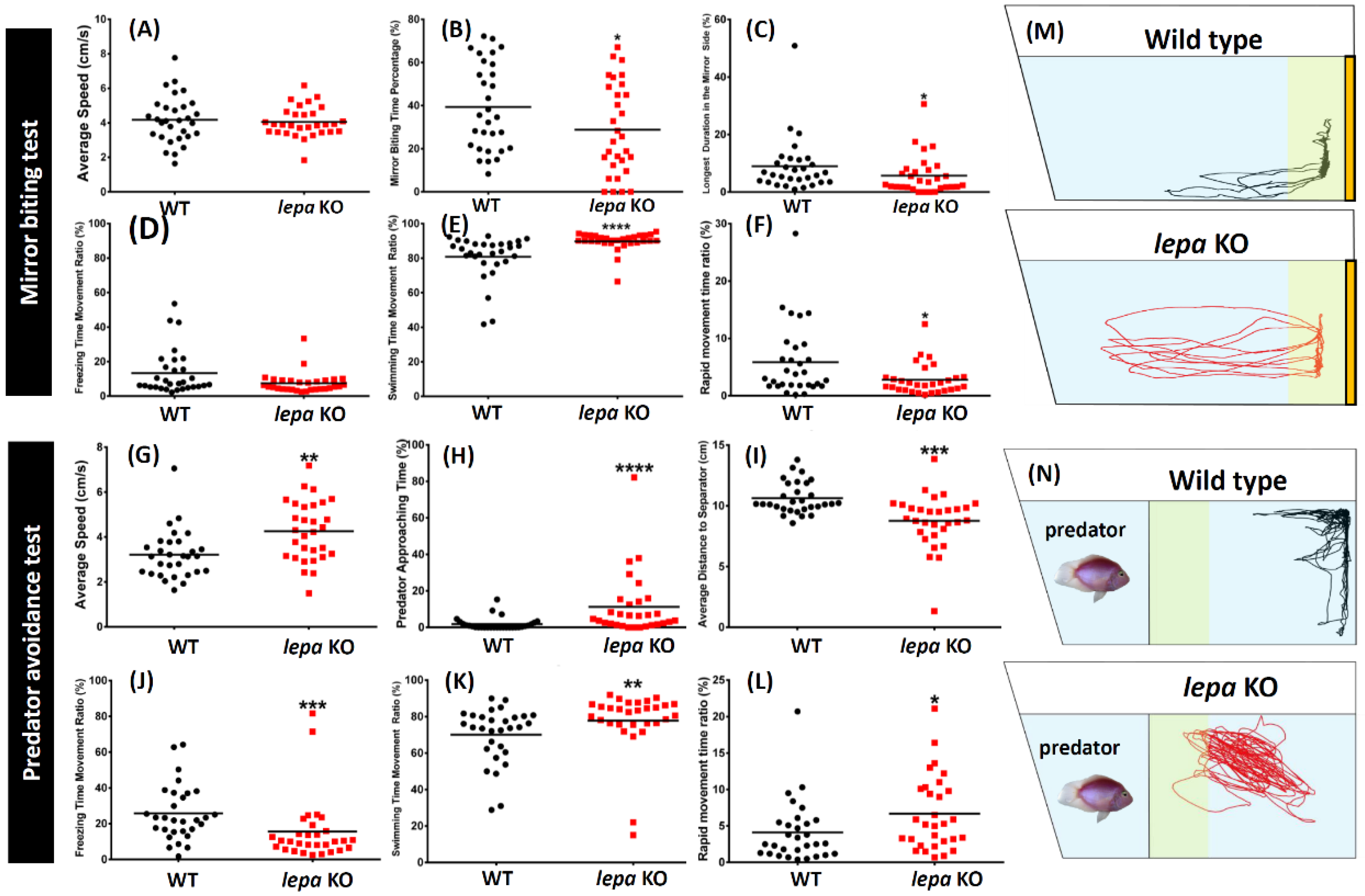
2.3. Lepa KO Zebrafish Displayed Less Aggression and Less Fear to Predator
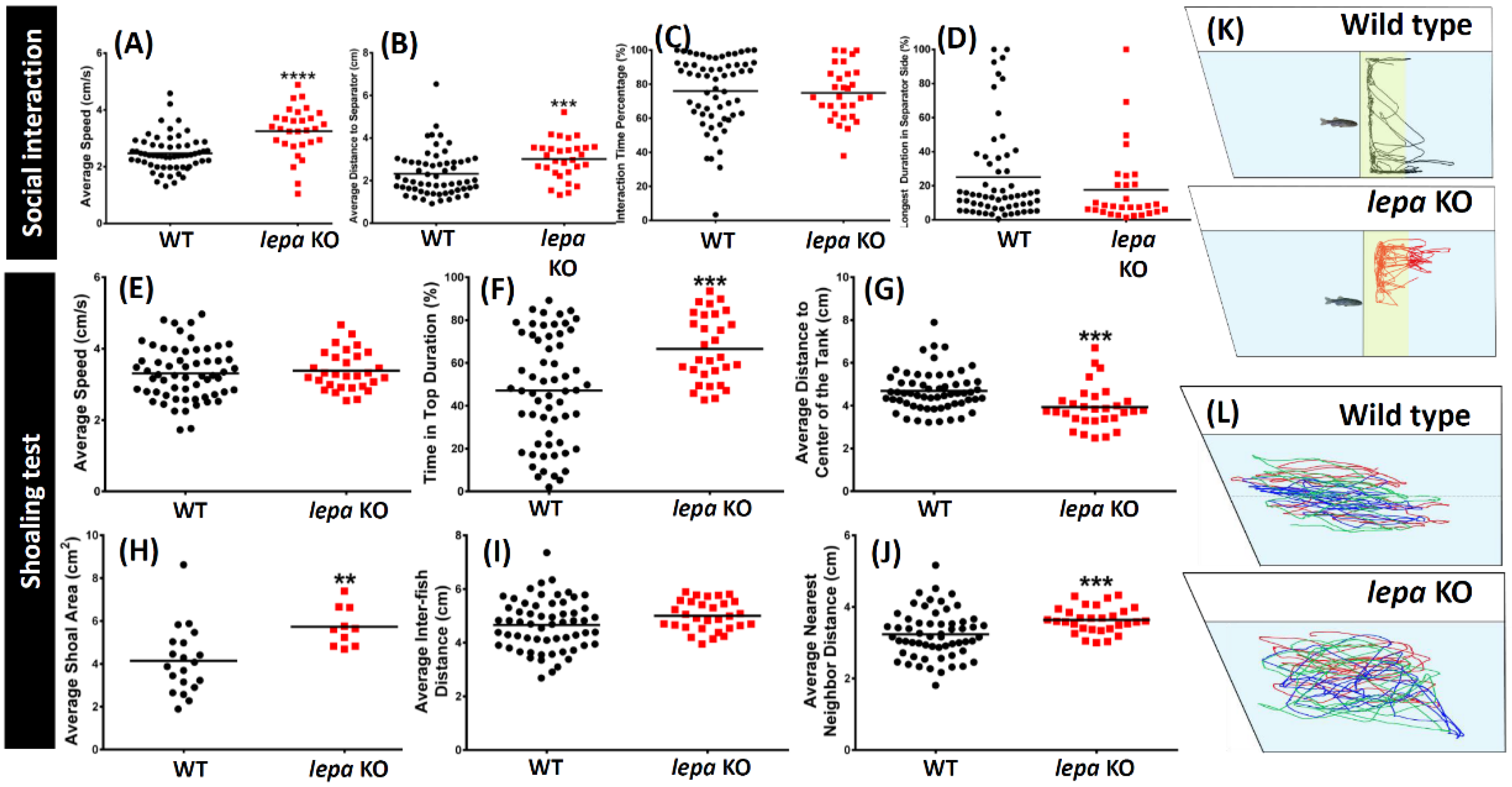
2.4. Lepa KO Zebrafish Displayed Less Social Interaction and Loose Shoaling
2.5. Lepa KO Zebrafish Display Dysregulation in Circadian Rhythm
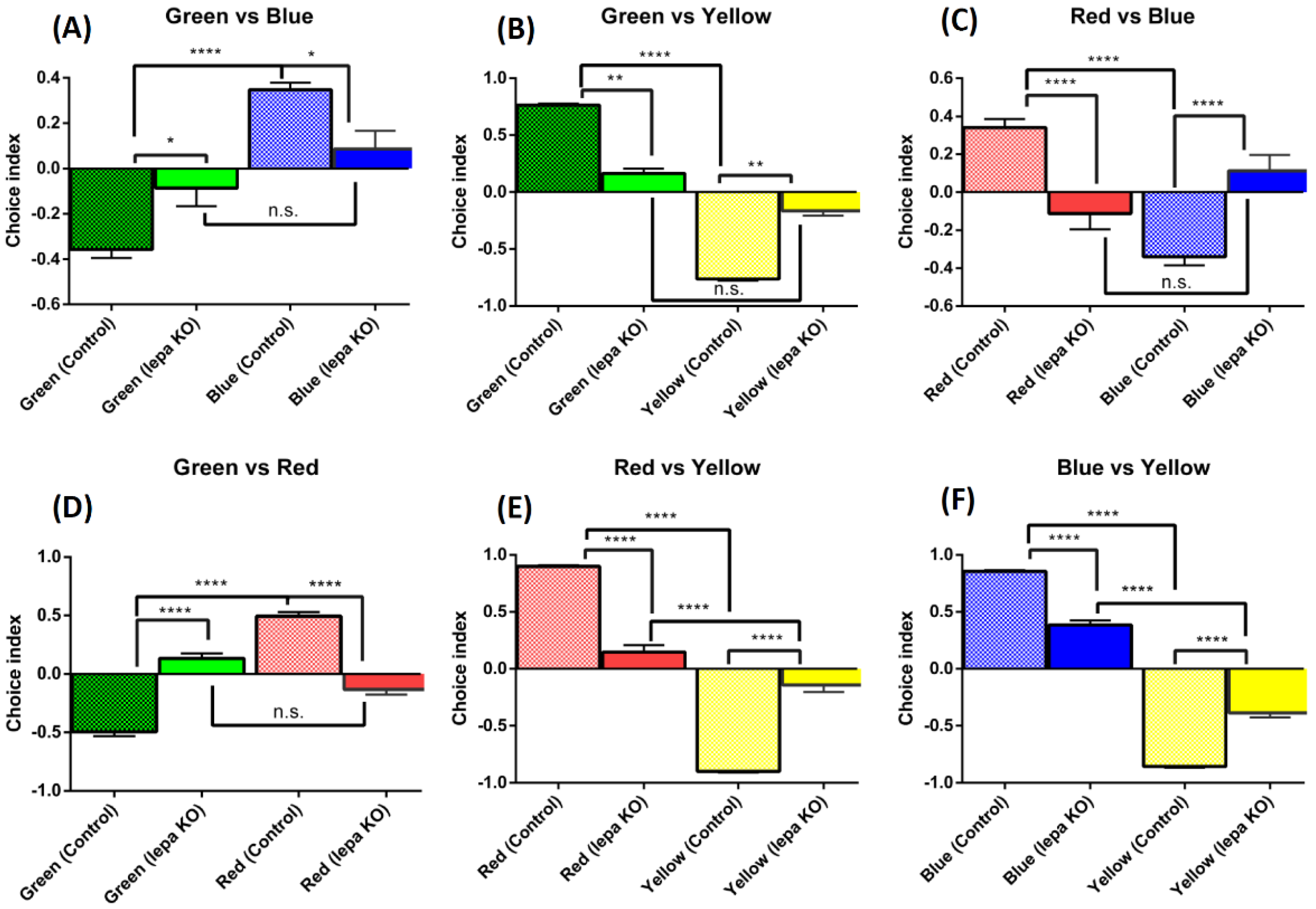
2.6. Lepa KO Zebrafish Displayed Reduction of Color Preference
2.7. Lepa KO Zebrafish Display Normal Short-Term Memory
2.8. Dysregulation of Appetite-Controlling Hormones in the Lepa KO Fish
2.9. Dysregulation of Neurotransmitter in the Lepa KO Fish Brain
2.10. Leptin Deficient Zebrafish Exhibited Greater Oxidative Damage in the Brain
3. Discussion
3.1. Leptin and Leptin Receptor Mutant Fish
3.2. Behavior Alterations in Lepa KO Fish
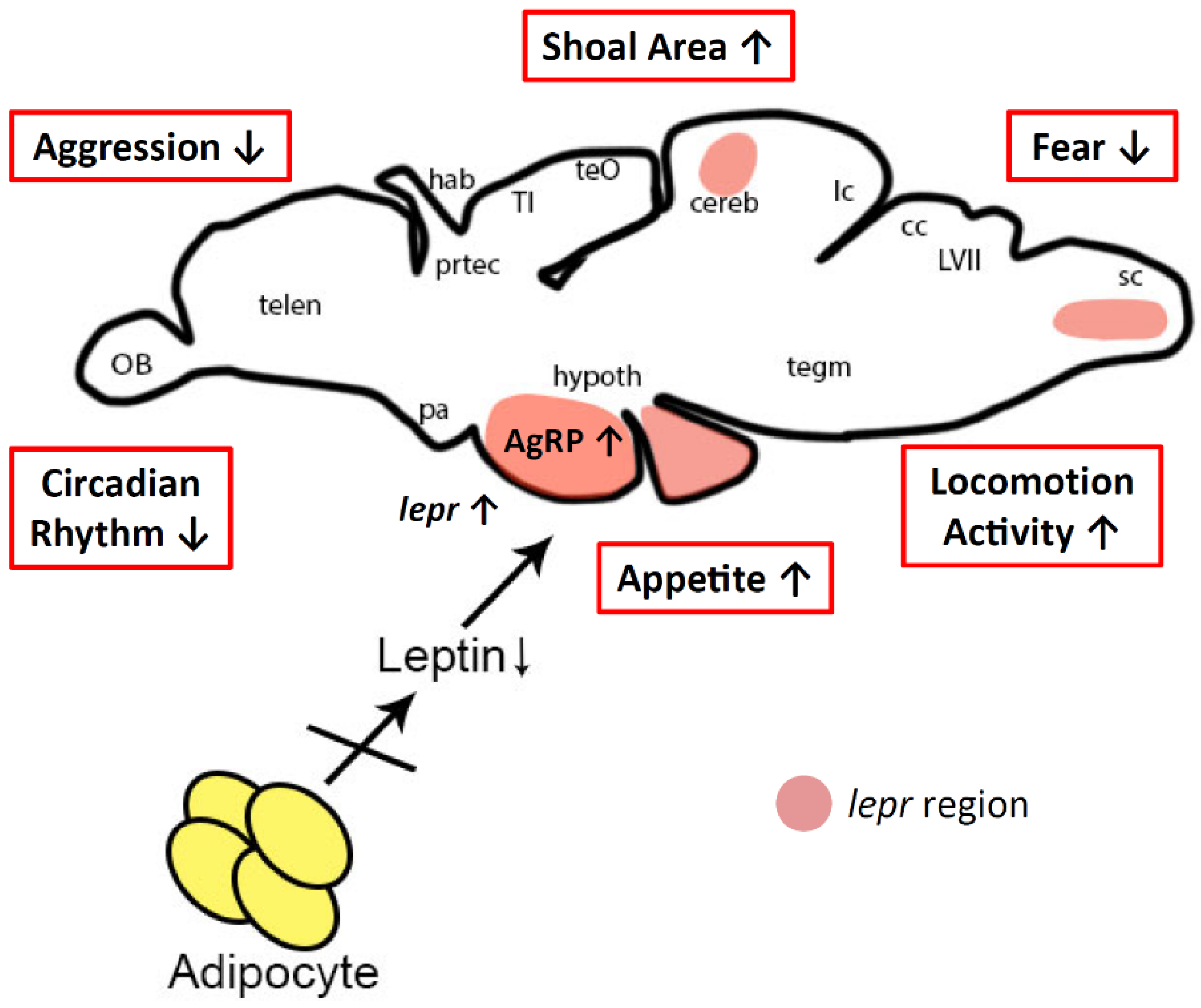
3.3. Possible Mechanism for Behavior Alterations in Lepa KO Fish
4. Material and Methods
4.1. Animal Ethics and Rearing
4.2. Production of TALEN mRNA
4.3. Microinjection of Zebrafish Embryos
4.4. Identification of Indel and Targeted Mutations by HRM Assay
4.5. Genotyping of Lepa KO Fish
4.6. Body Length and Body Weight Measurement
4.7. Zebrafish 3D Locomotor Activity
4.8. Passive Avoidance Test (Short-Term Memory Test)
4.9. Aggressiveness Test
4.10. Circadian Rhythm Test
4.11. Predator Avoidance Test
4.12. Color Preferences Assay
4.13. Determination of Appetite-Controlling Hormone, Neurotransmitter, Oxidative, Anti-Oxidative Capacity, Lipid Peroxidation, DNA Damage, Stress Hormone and Inflammation Markers
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Appendix A

References
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Prokop, J.W.; Duff, R.J.; Ball, H.C.; Copeland, D.L.; Londraville, R.L. Leptin and leptin receptor: Analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides 2012, 38, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob rna in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Lollmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Cone, R.D. Knockout models resulting in the development of obesity. Trends Genet. 2001, 17, S50–S54. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.; Delibasi, T.; Wong, M.L.; Licinio, J. Congenital leptin deficiency: Diagnosis and effects of leptin replacement therapy. Arq. Bras. Endocrinol. Metabol. 2010, 54, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Bluher, S.; Shah, S.; Mantzoros, C.S. Leptin deficiency: Clinical implications and opportunities for therapeutic interventions. J. Investig. Med. 2009, 57, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the t-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Murakami, M.; Ogawa, Y.; Masuzaki, H.; Tanaka, M.; Ozaki, S.; Nakao, K.; Mimori, T. Leptin inhibits stress-induced apoptosis of t lymphocytes. Clin. Exp. Immunol. 2002, 128, 21–26. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.; Franco, C.B.; Wang, K.B.; Wong, M.L.; Licinio, J. Leptin: Molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq. Bras. Endocrinol. Metabol. 2012, 56, 597–607. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernandez, C.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Barb, C.R.; Lents, C.A. Leptin and reproductive function. Biochimie 2012, 94, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Maymo, J.; Duenas, J.L.; Varone, C.; Sanchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Maeda, T.; Kawane, T.; Matsunuma, A.; Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin d3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 2010, 25, 1711–1723. [Google Scholar] [CrossRef]
- Ferron, M.; Lacombe, J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch. Biochem. Biophys 2014, 561, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J. Leptin: A diverse regulator of neuronal function. J. Neurochem. 2007, 100, 307–313. [Google Scholar] [CrossRef]
- Guimond, D.; Diabira, D.; Porcher, C.; Bader, F.; Ferrand, N.; Zhu, M.; Appleyard, S.M.; Wayman, G.A.; Gaiarsa, J.L. Leptin potentiates gabaergic synaptic transmission in the developing rodent hippocampus. Front. Cell. Neurosci. 2014, 8, 235. [Google Scholar] [CrossRef]
- Irving, A.J.; Harvey, J. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130155. [Google Scholar] [CrossRef]
- Ates, M.; Dayi, A.; Kiray, M.; Sisman, A.R.; Agilkaya, S.; Aksu, I.; Baykara, B.; Buyuk, E.; Cetinkaya, C.; Cingoz, S.; et al. Anxiety- and depression-like behavior are correlated with leptin and leptin receptor expression in prefrontal cortex of streptozotocin-induced diabetic rats. Biotech. Histochem. 2014, 89, 161–171. [Google Scholar] [CrossRef]
- Rebolledo-Solleiro, D.; Roldan-Roldan, G.; Diaz, D.; Velasco, M.; Larque, C.; Rico-Rosillo, G.; Vega-Robledo, G.B.; Zambrano, E.; Hiriart, M.; Perez de la Mora, M. Increased anxiety-like behavior is associated with the metabolic syndrome in non-stressed rats. PLoS ONE 2017, 12, e0176554. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Boozer, C.; Blair-West, J.; Fidahusein, N.; Denton, D.A.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8878–8883. [Google Scholar] [CrossRef]
- Liu, J.; Garza, J.C.; Bronner, J.; Kim, C.S.; Zhang, W.; Lu, X.Y. Acute administration of leptin produces anxiolytic-like effects: A comparison with fluoxetine. Psychopharmacology 2010, 207, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, S.L.; Li, K.; Chen, Y.; Jiang, B.; Li, Y.K.; Xiao, J.L.; Yang, S.; Chen, T.; Chen, J.G.; et al. Leptin: A potential anxiolytic by facilitation of fear extinction. CNS Neurosci. Ther. 2015, 21, 425–434. [Google Scholar] [CrossRef]
- Licinio, J.; Negrao, A.B.; Wong, M.L. Plasma leptin concentrations are highly correlated to emotional states throughout the day. Transl. Psychiatry 2014, 4, e475. [Google Scholar] [CrossRef] [PubMed]
- Veniant, M.M.; LeBel, C.P. Leptin: From animals to humans. Curr. Pharm. Des. 2003, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Barb, C.R.; Kraeling, R.R.; Rampacek, G.B.; Hausman, G.J. The role of neuropeptide y and interaction with leptin in regulating feed intake and luteinizing hormone and growth hormone secretion in the pig. Reproduction 2006, 131, 1127–1135. [Google Scholar] [CrossRef]
- Londraville, R.L.; Prokop, J.W.; Duff, R.J.; Liu, Q.; Tuttle, M. On the molecular evolution of leptin, leptin receptor, and endospanin. Front. Endocrinol. 2017, 8, 58. [Google Scholar] [CrossRef]
- Bates, S.H.; Myers, M.G. The role of leptin-->stat3 signaling in neuroendocrine function: An integrative perspective. J. Mol. Med. 2004, 82, 12–20. [Google Scholar] [CrossRef]
- Niswender, K.D.; Morton, G.J.; Stearns, W.H.; Rhodes, C.J.; Myers, M.G., Jr.; Schwartz, M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001, 413, 794–795. [Google Scholar] [CrossRef]
- Su, H.; Jiang, L.; Carter-Su, C.; Rui, L. Glucose enhances leptin signaling through modulation of ampk activity. PLoS ONE 2012, 7, e31636. [Google Scholar] [CrossRef] [PubMed]
- Mania, M.; Maruccio, L.; Russo, F.; Abbate, F.; Castaldo, L.; D’Angelo, L.; de Girolamo, P.; Guerrera, M.C.; Lucini, C.; Madrigrano, M.; et al. Expression and distribution of leptin and its receptors in the digestive tract of dio (diet-induced obese) zebrafish. Ann. Anat. 2017, 212, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dalman, M.R.; Liu, Q.; King, M.D.; Bagatto, B.; Londraville, R.L. Leptin expression affects metabolic rate in zebrafish embryos (d. Rerio). Front. Physiol. 2013, 4, 160. [Google Scholar] [CrossRef] [PubMed]
- Imrie, D.; Sadler, K.C. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev. Dyn. 2010, 239, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a crispr nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Liu, J.; Yu, Z.; Zhang, B.; Gao, G.; Jiao, R. TALEN or cas9–rapid, efficient and specific choices for genome modifications. J. Genet. Genom. 2013, 40, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Wang, Y.; Campbell, J.M.; Poshusta, T.L.; Starker, C.G.; Krug, R.G., II; Tan, W.; Penheiter, S.G.; Ma, A.C.; Leung, A.Y.J.N. In vivo genome editing using a high-efficiency talen system. Nature 2012, 491, 114. [Google Scholar] [CrossRef]
- Kawakami, K.; Shima, A.; Kawakami, N. Identification of a functional transposase of the tol2 element, an ac-like element from the japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 11403–11408. [Google Scholar] [CrossRef]
- Kawakami, K.J. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007, 8, S7. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Gorissen, M.; Bernier, N.J.; Nabuurs, S.B.; Flik, G.; Huising, M.O. Two divergent leptin paralogues in zebrafish (danio rerio) that originate early in teleostean evolution. J. Endocrinol. 2009, 201, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Takács, P.; Vitál, Z.; Ferincz, Á.; Staszny, Á. Repeatability, reproducibility, separative power and subjectivity of different fish morphometric analysis methods. PLoS ONE 2016, 11, e0157890. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3d locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Fole, A.; Martín, M.; Morales, L.; Cano, M.V.; Ruiz-Gayo, M.; Del Olmo, N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol. Learn. Mem. 2013, 106, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Greco, S.J.; Bryan, K.J.; Sarkar, S.; Zhu, X.; Smith, M.A.; Ashford, J.W.; Johnston, J.M.; Tezapsidis, N.; Casadesus, G. Leptin reduces pathology and improves memory in a transgenic mouse model of alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J. Serotonin and appetite. Neuropharmacology 1984, 23, 1537–1551. [Google Scholar] [CrossRef]
- Halford, J.C.; Blundell, J.E. Separate systems for serotonin and leptin in appetite control. Ann. Med. 2000, 32, 222–232. [Google Scholar] [CrossRef]
- Leibowitz, S.F.; Alexander, J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry 1998, 44, 851–864. [Google Scholar] [CrossRef]
- Yadav, V.K.; Oury, F.; Suda, N.; Liu, Z.-W.; Gao, X.-B.; Confavreux, C.; Klemenhagen, K.C.; Tanaka, K.F.; Gingrich, J.A.; Guo, X.E. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 2009, 138, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Halford, J.C.; Harrold, J.A.; Lawton, C.L.; Blundell, J.E. Serotonin (5-ht) drugs: Effects on appetite expression and use for the treatment of obesity. Curr. Drug Targets 2005, 6, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Halford, J.C. Serotonin and appetite regulation. CNS Drugs 1998, 9, 473–495. [Google Scholar] [CrossRef]
- Weiss, J.M.; Goodman, P.A.; Losito, B.G.; Corrigan, S.; Charry, J.M.; Bailey, W.H. Behavioral depression produced by an uncontrollable stressor: Relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res. Rev. 1981, 3, 167–205. [Google Scholar] [CrossRef]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.; Arizzi, M.; Sandoval, M.; Cervone, K.; Aberman, J. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: Contrast between the effects of skf 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology 2002, 160, 371–380. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between er stress, oxidative stress, and inflammation in health and disease. In Stress Responses; Springer: Berlin, Germany, 2015; pp. 205–214. [Google Scholar]
- Fulton, S.; Woodside, B.; Shizgal, P. Modulation of brain reward circuitry by leptin. Science 2000, 287, 125–128. [Google Scholar] [CrossRef]
- Carr, K.D. Augmentation of drug reward by chronic food restriction: Behavioral evidence and underlying mechanisms. Physiol. Behav. 2002, 76, 353–364. [Google Scholar] [CrossRef]
- Shalev, U.; Yap, J.; Shaham, Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J. Neurosci. 2001, 21, RC129. [Google Scholar] [CrossRef] [PubMed]
- Figlewicz, D.P.; Woods, S.C. Adiposity signals and brain reward mechanisms. Trends Pharmacol. Sci. 2000, 21, 235–236. [Google Scholar] [CrossRef]
- Tecott, L.H. Serotonin and the orchestration of energy balance. Cell MeTable 2007, 6, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Martin-Gronert, M.S.; Stocker, C.J.; Wargent, E.T.; Cripps, R.L.; Garfield, A.S.; Jovanovic, Z.; D’Agostino, G.; Yeo, G.S.; Cawthorne, M.A.; Arch, J.R. 5-HT2A and 5-HT2C receptors as hypothalamic targets of developmental programming in male rats. Dis. Models Mech. 2016. [Google Scholar] [CrossRef]
- Romanova, I.V.; Derkach, K.V.; Mikhrina, A.L.; Sukhov, I.B.; Mikhailova, E.V.; Shpakov, A.O. The leptin, dopamine and serotonin receptors in hypothalamic pomc-neurons of normal and obese rodents. Neurochem. Res. 2018, 43, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Page-McCaw, P.S.; Chen, W.; Cone, R.D. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, 3084–3089. [Google Scholar] [CrossRef]
- Chisada, S.-I.; Kurokawa, T.; Murashita, K.; Rønnestad, I.; Taniguchi, Y.; Toyoda, A.; Sakaki, Y.; Takeda, S.; Yoshiura, Y. Leptin receptor-deficient (knockout) medaka, oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen. Comp. Endocrinol. 2014, 195, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; von Schnurbein, J.; Moepps, B.; Lahr, G.; Strauss, G.; Barth, T.F.; Kassubek, J.; Muhleder, H.; Möller, P.; Debatin, K.-M. A new missense mutation in the leptin gene causes mild obesity and hypogonadism without affecting t cell responsiveness. J. Clin. Endocrinol. MeTable 2010, 95, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.-Y. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of gsk-3β/β-catenin signaling. Mol. Psychiatry 2012, 17, 790. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.J.; Trainor, B.C. Neural mechanisms of aggression. Nat. Rev. Neurosci. 2007, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J. Encyclopedia of Gender and Society; SAGE Publications: Newcastle upon Tyne, UK, 2008. [Google Scholar]
- Haagensen, A.M.J.; Sørensen, D.B.; Sandøe, P.; Matthews, L.R.; Birck, M.M.; Fels, J.J.; Astrup, A. High fat, low carbohydrate diet limit fear and aggression in göttingen minipigs. PLoS ONE 2014, 9, e93821. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin, Germany, 2012; pp. 231–246. [Google Scholar]
- Piato, Â.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (danio rerio): Behavioral and physiological responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Greene, K.; Dydinski, A.; Gerlai, R. Effects of nicotine and alcohol on zebrafish (danio rerio) shoaling. Behav. Brain Res. 2013, 240, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol. Biochem. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef]
- Prentice, A.M.; Moore, S.E.; Collinson, A.C.; O’Connell, M.A. Leptin and undernutrition. Nutr. Rev. 2002, 60, S56–S67. [Google Scholar] [CrossRef]
- Bass, S.L.; Gerlai, R. Zebrafish (danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008, 186, 107–117. [Google Scholar] [CrossRef]
- Arble, D.M.; Vitaterna, M.H.; Turek, F.W. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS ONE 2011, 6, e25079. [Google Scholar] [CrossRef]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian dysfunction induces leptin resistance in mice. Cell MeTable 2015, 22, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Murakami, D.; Horwitz, B.A.; Fuller, C.A. Circadian rhythms of temperature and activity in obese and lean zucker rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 269, R1038–R1043. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell MeTable 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Sun, M.; Zhang, Q.; Li, T.; Li, X.; Xu, J.; Zhao, X.; Chen, D.; Feng, X. Reversal of reserpine-induced depression and cognitive disorder in zebrafish by sertraline and traditional chinese medicine (TCM). Behav. Brain Funct. BBF 2018, 14. [Google Scholar] [CrossRef]
- Korkmaz, S.; Özer, Ö.; Kaya, Ş.; Kazgan, A.; Atmaca, M. The correlation between color choices and impulsivity, anxiety and depression. Eur. J. Gen. Med. 2016, 13, 47–50. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Holmes, A.; Murphy, D.L.; Crawley, J.N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol. Psychiatry 2003, 54, 953–959. [Google Scholar] [CrossRef]
- Lira, A.; Zhou, M.; Castanon, N.; Ansorge, M.S.; Gordon, J.A.; Francis, J.H.; Bradley-Moore, M.; Lira, J.; Underwood, M.D.; Arango, V. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry 2003, 54, 960–971. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W.J.H. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Bouret, S.G. Neurodevelopmental actions of leptin. Brain Res. 2010, 1350, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Copeland, D.; Ball, H.; Duff, R.J.; Rockich, B.; Londraville, R.L. Expression of leptin receptor gene in developing and adult zebrafish. Gen. Comp. Endocrinol. 2010, 166, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tong, Q.J. Expanding neurotransmitters in the hypothalamic neurocircuitry for energy balance regulation. Protein Cell 2011, 2, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Roubos, E.W.; Dahmen, M.; Kozicz, T.; Xu, L. Leptin and the hypothalamo-pituitary–adrenal stress axis. Gen. Comp. Endocrinol. 2012, 177, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pitchot, W.; Herrera, C.; Ansseau, M. Hpa axis dysfunction in major depression: Relationship to 5-ht(1a) receptor activity. Neuropsychobiology 2001, 44, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Ida, I.; Yuuki, N.; Oshima, A.; Kumano, H.; Takahashi, K.; Fukuda, M.; Oriuchi, N.; Endo, K.; Matsuda, H.; et al. Hpa axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. Neuroimaging 2007, 155, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Haesemeyer, M.; Schier, A.F. The study of psychiatric disease genes and drugs in zebrafish. Curr. Opin. Neurobiol. 2015, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Hewagalamulage, S.; Lee, T.; Clarke, I.; Henry, B.A. Stress, cortisol, and obesity: A role for cortisol responsiveness in identifying individuals prone to obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Moller-Leimkuhler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, A.; Riley, M.; Koyama, M.; Monen, J.; Satou, C.; Kimura, Y.; Higashijima, S.-I.; Fetcho, J. A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proc. Natl. Acad. Sci. USA 2011, 108, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Kao, Y.-H.; Fetcho, J. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Proc. Natl. Acad. Sci. USA 1996, 17, 1145–1155. [Google Scholar] [CrossRef]
- Asakawa, K.; Suster, M.L.; Mizusawa, K.; Nagayoshi, S.; Kotani, T.; Urasaki, A.; Kishimoto, Y.; Hibi, M.; Kawakami, K. Genetic dissection of neural circuits by tol2 transposon-mediated gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 2008, 105, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.K.; Mason, L.; Arrenberg, A.B.; Ziv, L.; Gosse, N.J.; Xiao, T.; Chi, N.C.; Asakawa, K.; Kawakami, K.; Baier, H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 2007, 4, 323. [Google Scholar] [CrossRef] [PubMed]
- Baier, H.; Scott, E.K. Genetic and optical targeting of neural circuits and behavior—Zebrafish in the spotlight. Curr. Opin. Neurobiol. 2009, 19, 553–560. [Google Scholar] [CrossRef]
- Blank, M.; Guerim, L.D.; Cordeiro, R.F.; Vianna, M.R. A one-trial inhibitory avoidance task to zebrafish: Rapid acquisition of an nmda-dependent long-term memory. Neurobiol. Learn. Mem. 2009, 92, 529–534. [Google Scholar] [CrossRef]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. The effects of early and adult social environment on zebrafish (danio rerio) behavior. Environ. Biol. Fishes 2007, 80, 91–101. [Google Scholar] [CrossRef]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. Idtracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | WT | lepa KO | Unit | Significance | p Value |
|---|---|---|---|---|---|
| Brain | |||||
| leptin | 0.020 ± 0.002 | 0.010 ± 0.002 | ng/μg total protein | YES | p = 0.0024 |
| Leptin receptor | 0.094 ± 0.012 | 0.213 ± 0.039 | ng/μg total protein | YES | p = 0.0421 |
| AgRP | 0.145 ± 0.009 | 0.286 ± 0.021 | ng/μg total protein | YES | p = 0.0036 |
| Gehirn | 0.118 ± 0.014 | 0.453 ± 0.058 | ng/μg total protein | YES | p = 0.0051 |
| Glucose | 0.172 ± 0.020 | 0.358 ± 0.018 | mmol/μg total protein | YES | p = 0.0024 |
| Insulin | 0.857±0.108 | 0.428 ± 0.098 | U/μg total protein | YES | p = 0.0423 |
| Serotonin (5-HT) | 0.187 ± 0.034 | 0.0153 ± 0.003 | ng/μg total protein | YES | p = 0.0076 |
| Norepinephrine | 0.731 ± 0.094 | 0.063 ± 0.010 | ng/μg total protein | YES | p = 0.0021 |
| Dopamine | 5.530 ± 0.308 | 10.130 ± 1.138 | pg/μg total protein | YES | p = 0.0175 |
| GABA | 0.020 ± 0.004 | 0.021 ± 0.005 | ug/μg total protein | No | p = 0.8789 |
| Glutamate | 0.141 ± 0.007 | 0.053 ± 0.013 | ug/μg total protein | YES | p = 0.0038 |
| Glycine | 0.239 ± 0.010 | 0.999 ± 0.170 | ug/μg total protein | YES | p = 0.0110 |
| ACh | 6.150 ± 0.746 | 0.979 ± 0.118 | U/μg total protein | YES | p = 0.0024 |
| AChE | 1.289 ± 0.159 | 0.541 ± 0.061 | U/μg total protein | YES | p = 0.0117 |
| Catecholamine | 1.633 ± 0.086 | 0.589 ± 0.142 | ng/μg total protein | YES | p = 0.0016 |
| Cortisol | 3.500 ± 0.325 | 5.155 ± 0.398 | pg/μg total protein | YES | p = 0.0092 |
| Histamine | 0.061 ± 0.006 | 0.109 ± 0.026 | ng/μg total protein | No | p = 0.1456 |
| TNF-α | 1.310 ± 0.337 | 0.412 ± 0.046 | pg/μg total protein | No | p = 0.0575 |
| IL1β | 0.054 ± 0.021 | 0.011 ± 0.004 | ng/μg total protein | No | p = 0.1151 |
| Catalase | 1.135 ± 0.150 | 0.052 ± 0.007 | U/μg total protein | YES | p = 0.0019 |
| ROS | 1.632 ± 0.153 | 3.720 ± 0.475 | U/μg total protein | YES | p = 0.0138 |
| Melatonin | 0.970 ± 0.144 | 0.081 ± 0.007 | pg/μg total protein | YES | p = 0.0035 |
| Amyloid beta | 1.839 ± 0.209 | 1.334 ± 0.174 | ug/μg total protein | No | p = 0.1366 |
| p-Tau | 1.723 ± 0.181 | 1.383 ± 0.130 | pg/μg total protein | No | p = 0.2014 |
| Hif-1α | 2.672 ± 0.216 | 3.225 ± 0.241 | pg/μg total protein | No | p = 0.1619 |
| creatinine | 0.850 ± 0.023 | 0.774 ± 0.019 | ug/μg total protein | No | p = 0.0620 |
| Muscle | |||||
| leptin | 0.096 ± 0.006 | 0.023 ± 0.001 | ng/μg total protein | YES | p = 0.0003 |
| Creatine kinase | 4.120 ± 0.368 | 1.100 ± 0.132 | ng/μg total protein | YES | p = 0.0015 |
| ATP | 143.300 ± 11.380 | 48.960 ± 5.780 | ng/μg total protein | YES | p = 0.0018 |
| ROS | 3.917 ± 0.741 | 7.427 ± 0.769 | U/μg total protein | YES | p = 0.0303 |
| Hif-1α | 5.708 ± 0.245 | 8.714 ± 0.522 | pg/μg total protein | YES | p = 0.0065 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Sarasamma, S.; Chen, J.-R.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Hsieh, M.-C.; Hsiao, C.-D. Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation. Int. J. Mol. Sci. 2018, 19, 4038. https://doi.org/10.3390/ijms19124038
Audira G, Sarasamma S, Chen J-R, Juniardi S, Sampurna BP, Liang S-T, Lai Y-H, Lin G-M, Hsieh M-C, Hsiao C-D. Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation. International Journal of Molecular Sciences. 2018; 19(12):4038. https://doi.org/10.3390/ijms19124038
Chicago/Turabian StyleAudira, Gilbert, Sreeja Sarasamma, Jung-Ren Chen, Stevhen Juniardi, Bonifasius Putera Sampurna, Sung-Tzu Liang, Yu-Heng Lai, Geng-Ming Lin, Ming-Chia Hsieh, and Chung-Der Hsiao. 2018. "Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation" International Journal of Molecular Sciences 19, no. 12: 4038. https://doi.org/10.3390/ijms19124038
APA StyleAudira, G., Sarasamma, S., Chen, J.-R., Juniardi, S., Sampurna, B. P., Liang, S.-T., Lai, Y.-H., Lin, G.-M., Hsieh, M.-C., & Hsiao, C.-D. (2018). Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation. International Journal of Molecular Sciences, 19(12), 4038. https://doi.org/10.3390/ijms19124038