Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives
Abstract
1. Introduction
2. Results
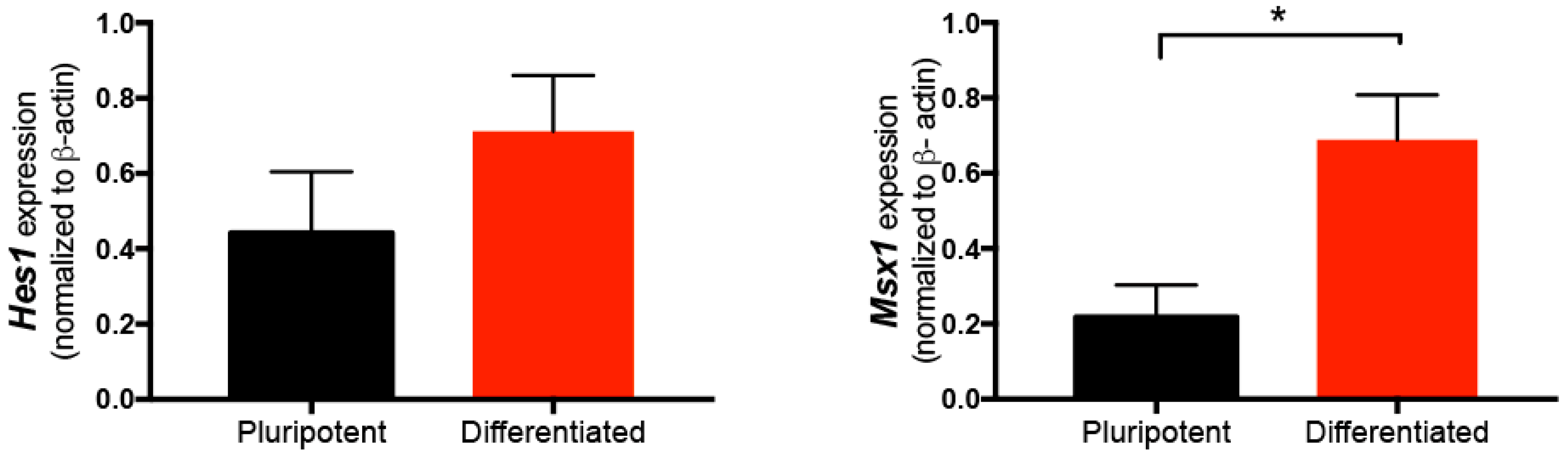
2.1. Expression of Hes1 and Msx1 in Wild-type ES Cells in Pluripotent Conditions and after NC Differentiation
2.2. HES-1-GR and MSX-1-GR Translocate to the Nucleus
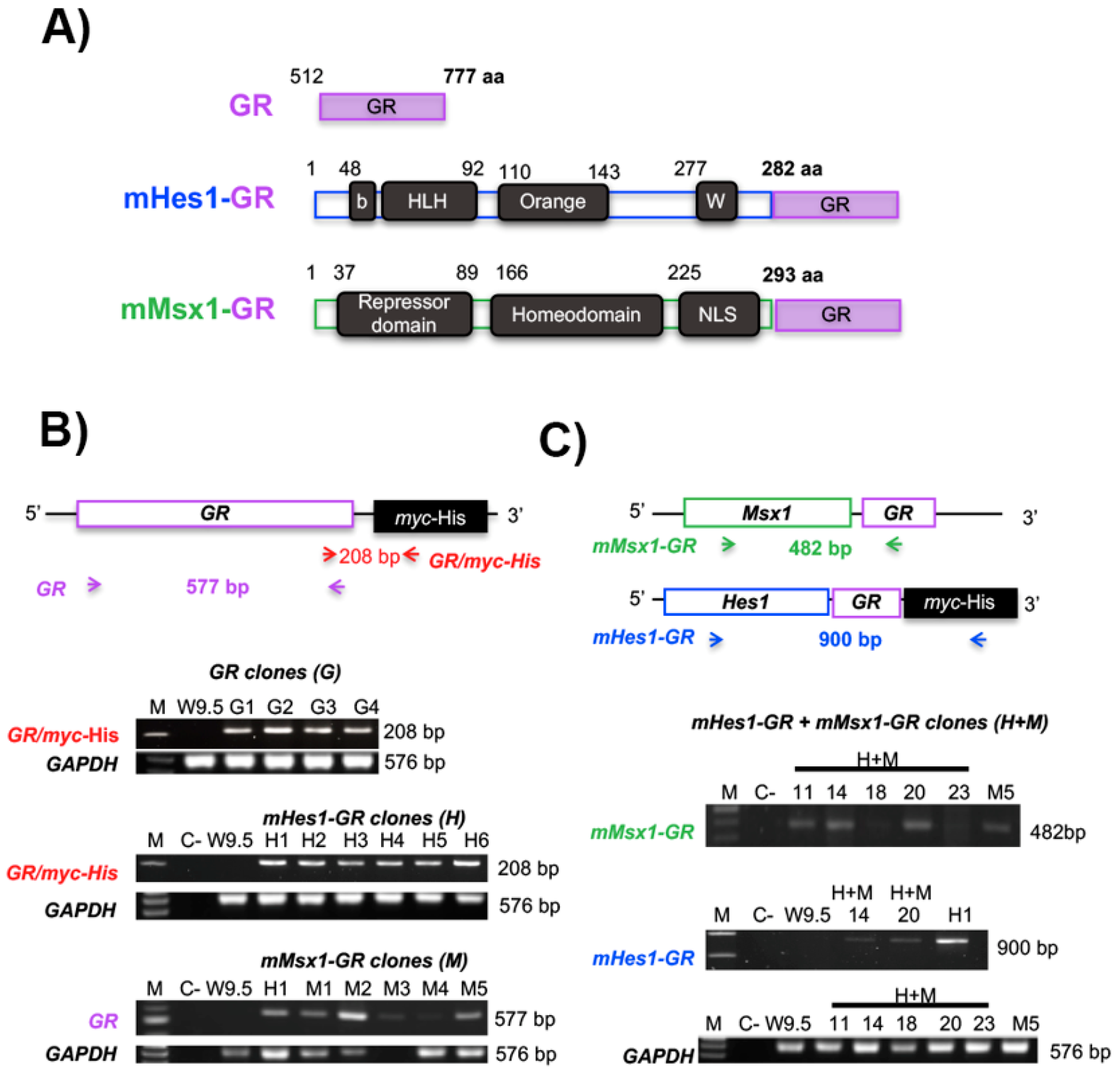
2.3. Generation of Transgenic Stable mESC Lines
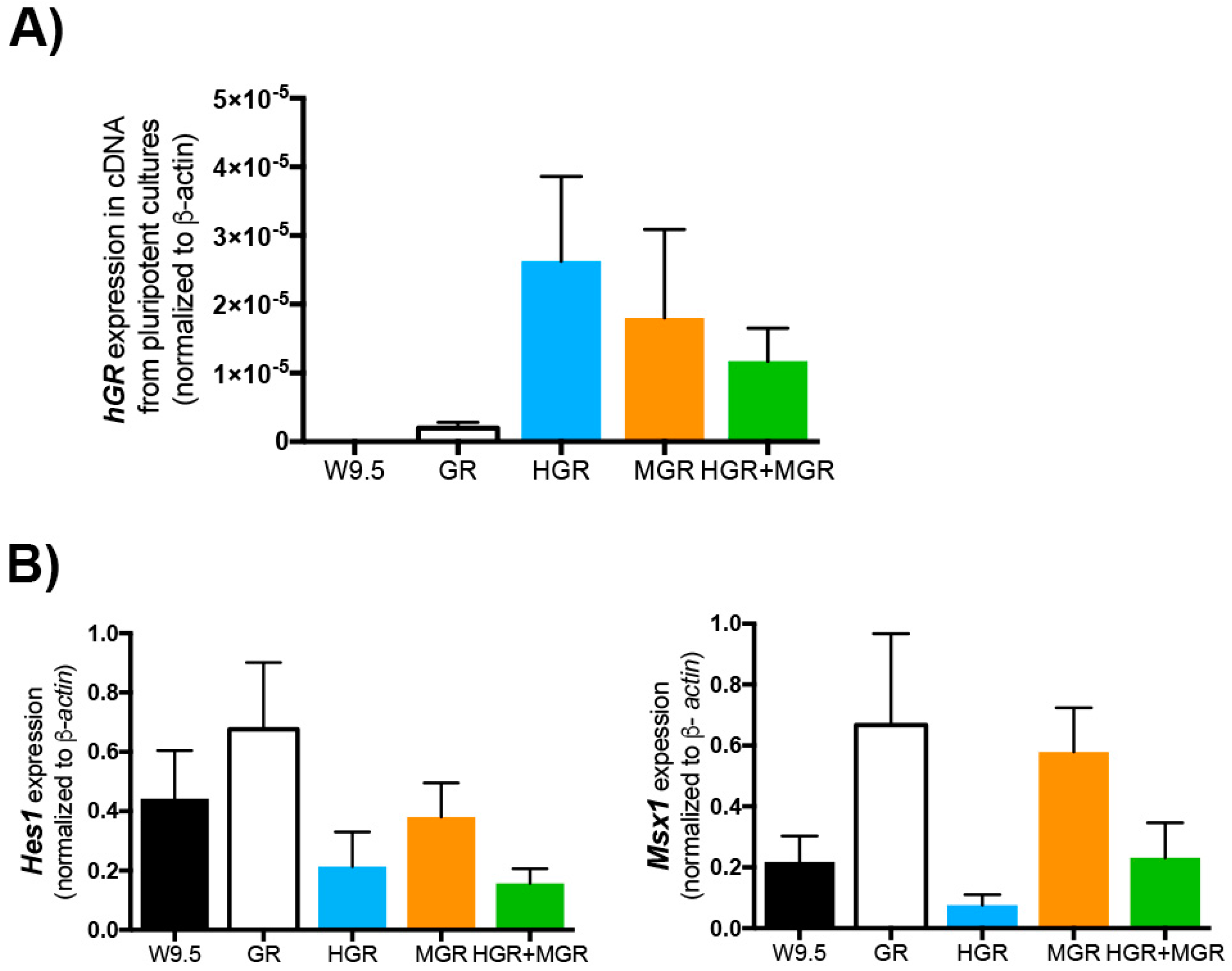
2.4. Expression of the Human Glucocorticoid Receptor, Hes1 and Msx1 are Similar in the Transgenic Cell Lines before Differentiation.
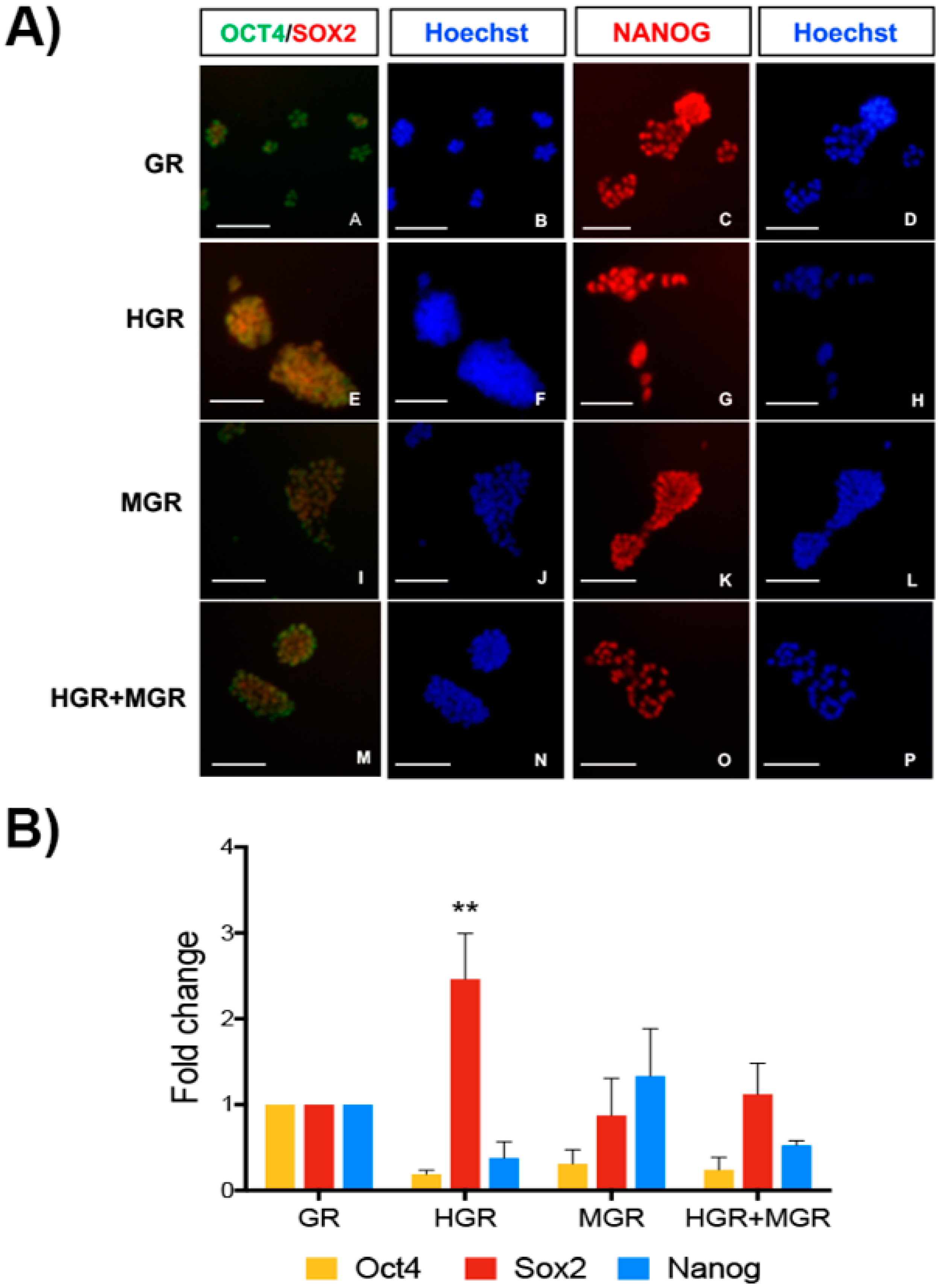
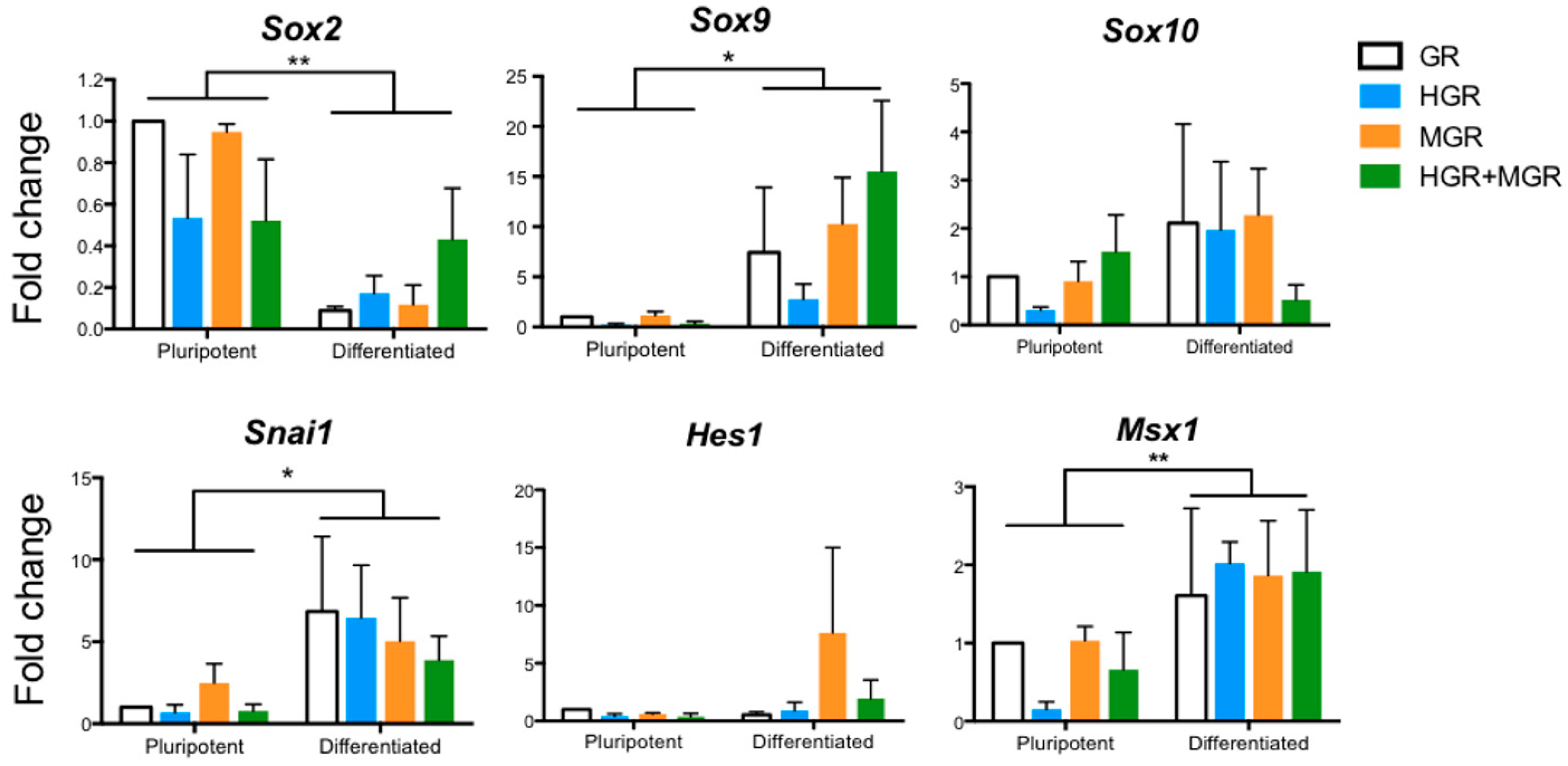
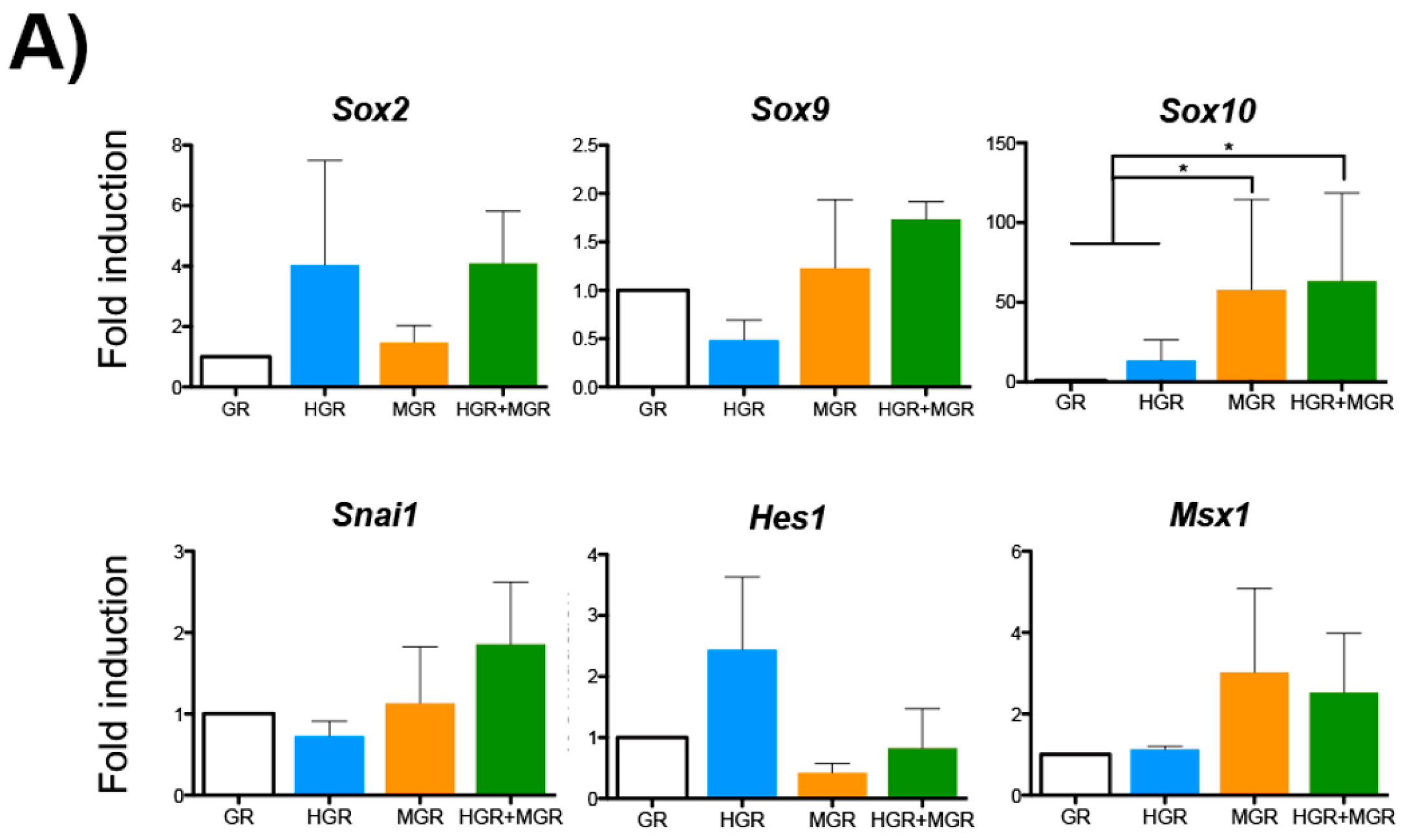
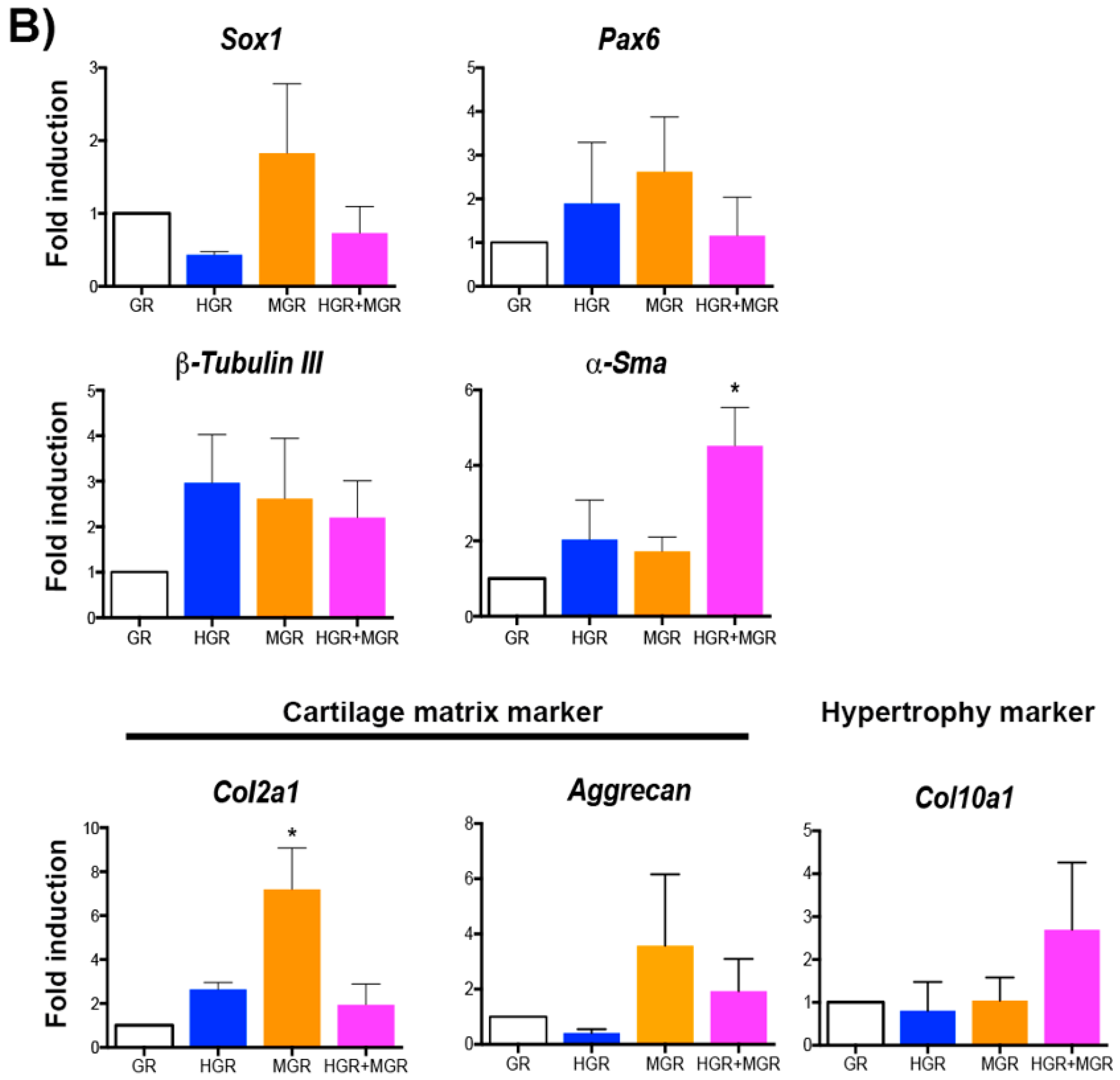
2.5. Neural and NC Marker Expression in Transgenic Pluripotent and Differentiated Cells
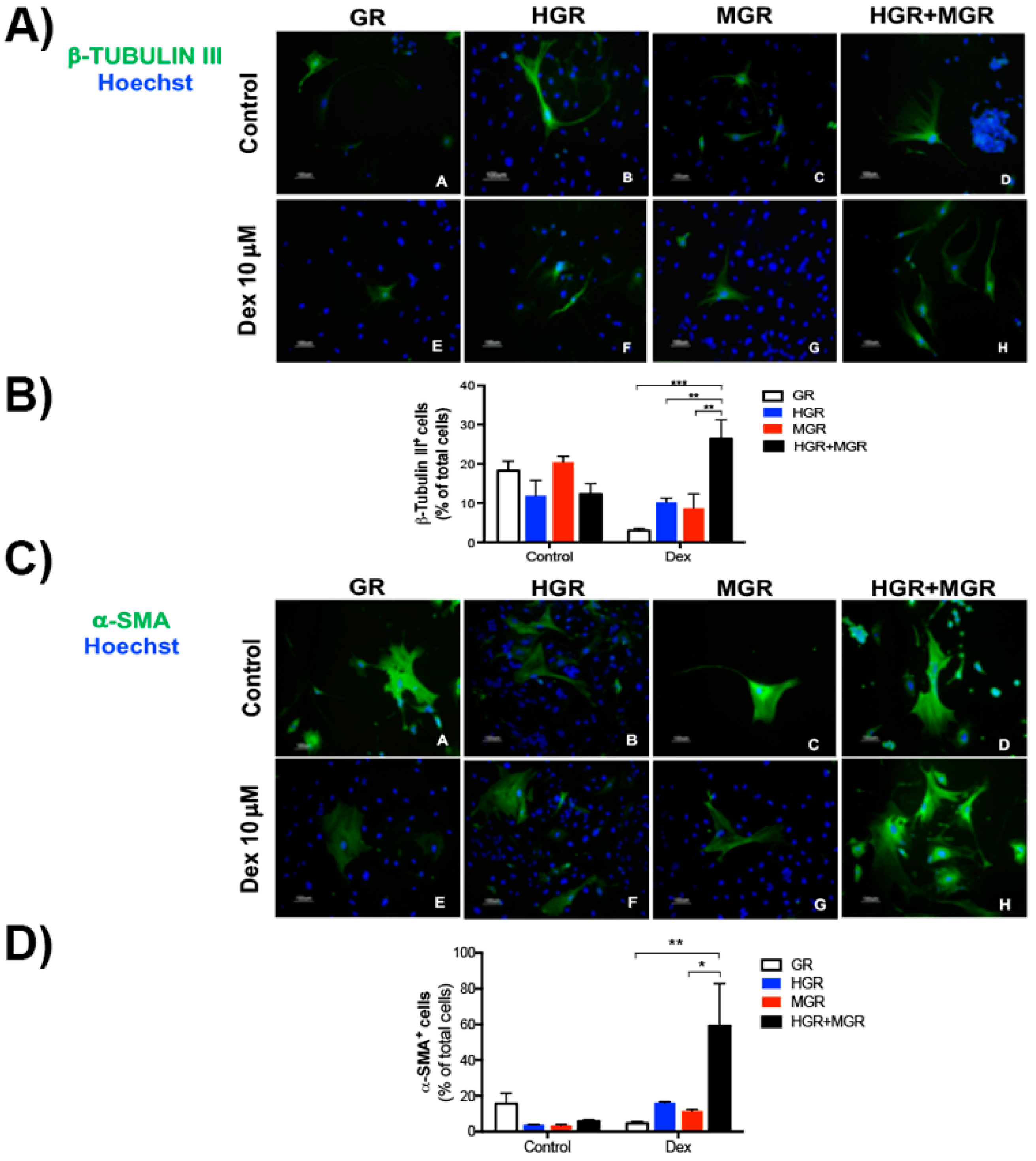
2.6. Activation of HES-1 and MSX-1 Increased the Number of β-TUBULIN III- and α-SMA-positive Cells
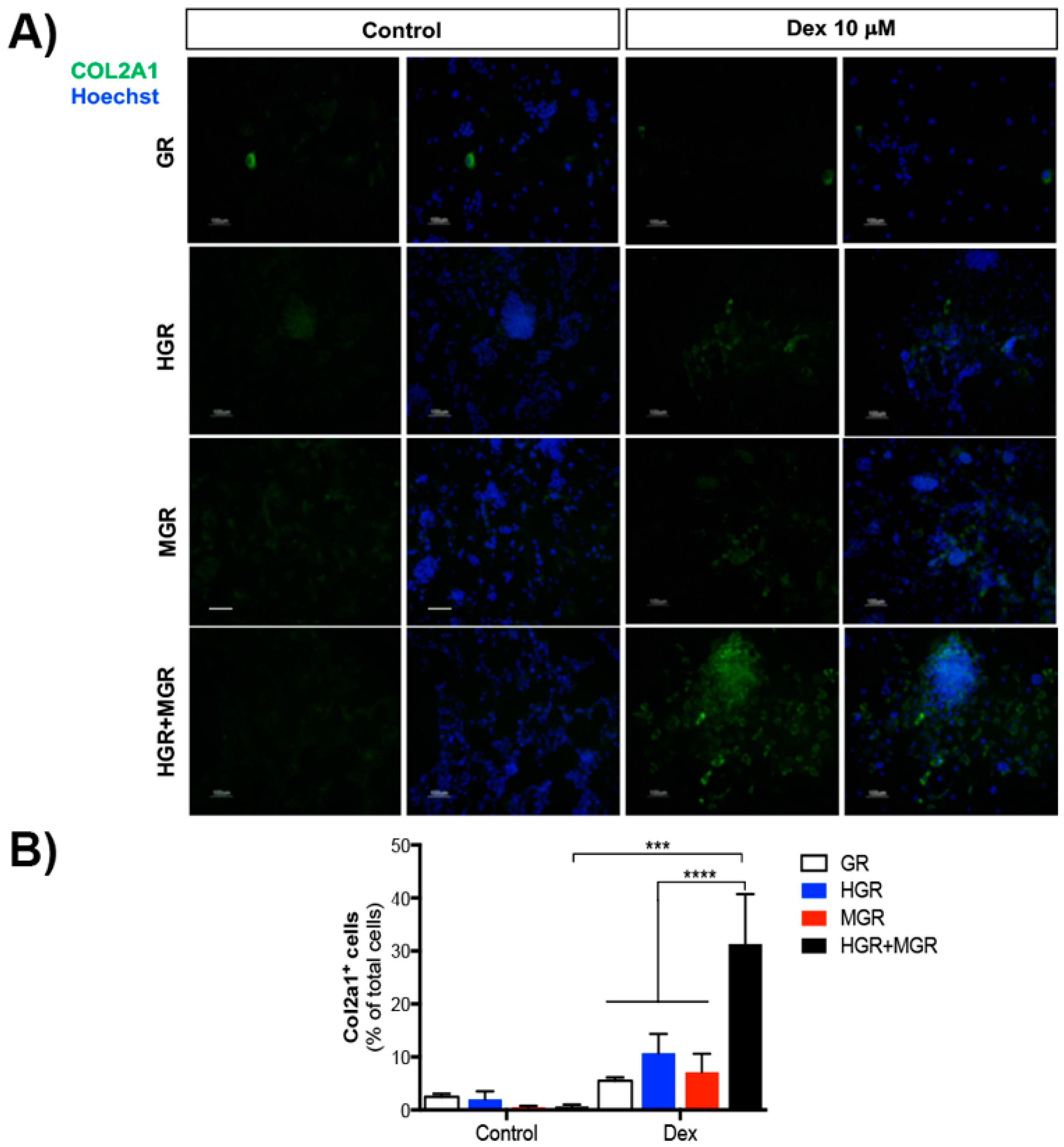
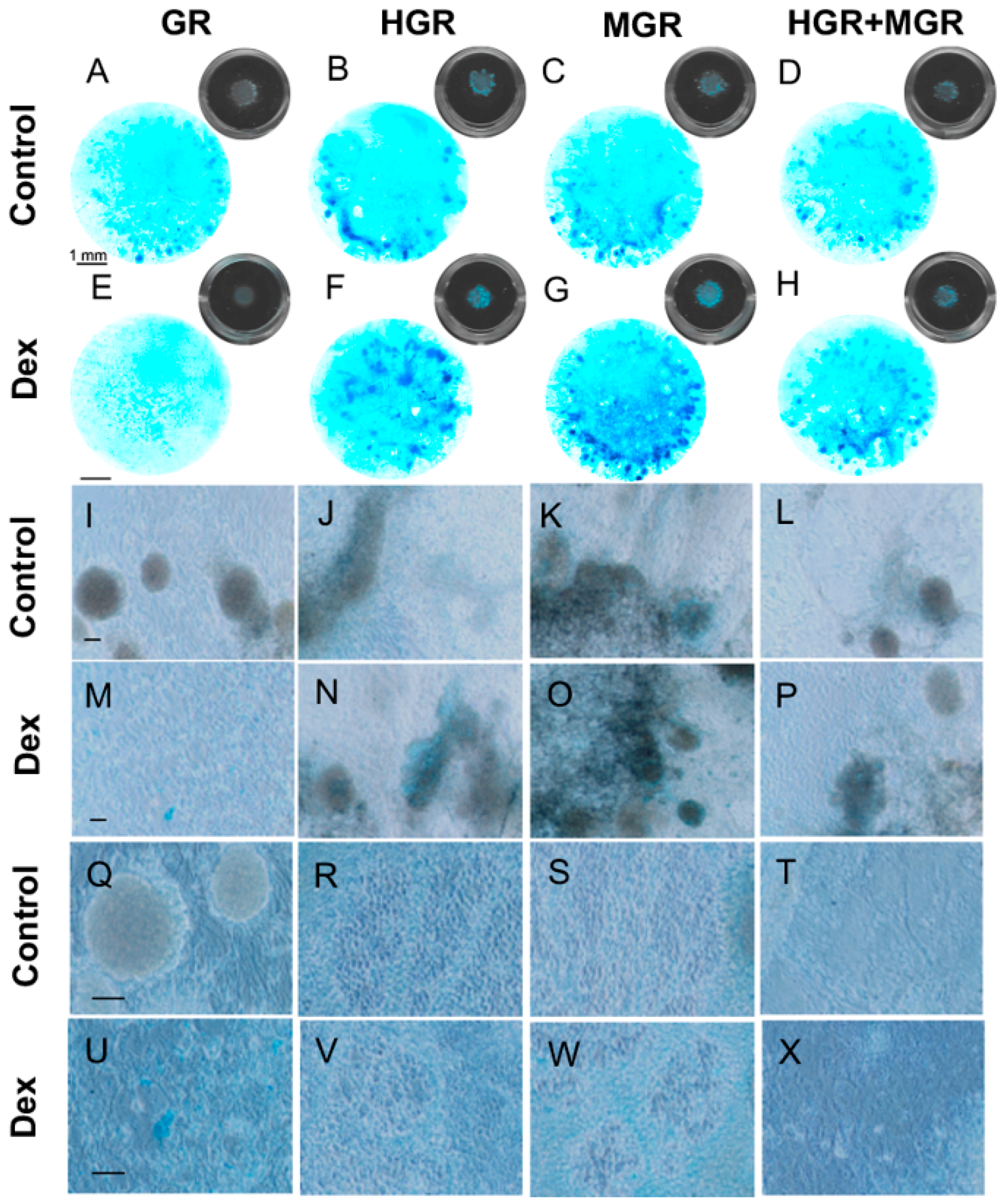
2.7. Activation of MGR and Double-transgenic Cells Increases Chondrocyte Differentiation.
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. NIH-3T3 Cells
4.1.2. ESCs
4.1.3. PA6 Cells
4.2. Generation of cDNA Constructs and the Inducible System
4.3. Nuclear Translocation after Dexamethasone Stimulation
4.4. Generation of Stable mESC Lines
4.5. Genotyping of the Resistant Clones
4.6. Neural Crest Differentiation
4.7. Immunofluorescence Staining
4.8. RNA Extraction and RT-PCR
4.9. qPCR
4.10. Micromass Culture
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP | Bone morphogenetic protein |
| COL2A | Collagen type II alpha 1 chain |
| Dex | Dexamethasone |
| ESC | Embryonic stem cells |
| FBS | Fetal bovine serum |
| FGF | Fibroblast growth factor |
| GR | Glucocorticoid binding domain |
| HES-1 | Hairy and enhancer of split 1 |
| HGR | Hes1-GR |
| LIF | Leukemia inhibitory factor |
| MSX-1 | Muscle-segment homeobox 1-like |
| MGR | Msx1-GR |
| NC | Neural crest |
| NIH-3T3 | Mouse embryo fibroblast cell line |
| α-SMA | α-Smooth muscle actin |
References
- Vega-Lopez, G.A.; Cerrizuela, S.; Tribulo, C.; Aybar, M.J. Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vega-Lopez, G.A.; Cerrizuela, S.; Aybar, M.J. Trunk neural crest cells: Formation, migration and beyond. Int. J. Dev. Biol. 2017, 61, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Simões-Costa, M.; Bronner, M.E. Insights into neural crest development and evolution from genomic analysis. Genome Res. 2013, 23, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.E.; LeDouarin, N.M. Development and evolution of the neural crest: An overview. Dev. Biol. 2012, 366, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mayor, R.; Theveneau, E. The neural crest. Development 2013, 140, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Mizuseki, K.; Sakamoto, T.; Watanabe, K.; Muguruma, K.; Ikeya, M.; Nishiyama, A.; Arakawa, A.; Suemori, H.; Nakatsuji, N.; Kawasaki, H.; et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5828–5833. [Google Scholar] [CrossRef] [PubMed]
- Basch, M.L.; Bronner-Fraser, M.; García-Castro, M.I. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 2006, 441, 218–222. [Google Scholar] [CrossRef]
- Hong, C.-S.; Park, B.-Y.; Saint-Jeannet, J.-P. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 2008, 135, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Aybar, M.J.; Mayor, R. Early induction of neural crest cells: Lessons learned from frog, fish and chick. Curr. Opin. Genet. Dev. 2002, 12, 452–458. [Google Scholar] [CrossRef]
- Aybar, M.J.; Nieto, M.A.; Mayor, R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 2003, 130, 483–494. [Google Scholar] [CrossRef]
- Aybar, M.J.; Glavic, A.; Mayor, R. Extracellular signals, cell interactions and transcription factors involved in the induction of the neural crest cells. Biol. Res. 2002, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Barembaum, M.; Bronner-Fraser, M. Early steps in neural crest specification. Semin. Cell Dev. Biol. 2005, 16, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Liem, K.F.; Tremml, G.; Roelink, H.; Jessell, T.M. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 1995, 82, 969–979. [Google Scholar] [CrossRef]
- Selleck, M.A.; García-Castro, M.I.; Artinger, K.B.; Bronner-Fraser, M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development 1998, 125, 4919–4930. [Google Scholar] [PubMed]
- Streit, A.; Stern, C.D. Establishment and maintenance of the border of the neural plate in the chick: Involvement of FGF and BMP activity. Mech. Dev. 1999, 82, 51–66. [Google Scholar] [CrossRef]
- Vega-López, G.A.; Bonano, M.; Tríbulo, C.; Fernández, J.P.; Agüero, T.H.; Aybar, M.J. Functional analysis of Hairy genes in Xenopus neural crest initial specification and cell migration. Dev. Dyn. 2015, 244, 988–1013. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Mizuseki, K.; Nishikawa, S.; Kaneko, S.; Kuwana, Y.; Nakanishi, S.; Nishikawa, S.I.; Sasai, Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000, 28, 31–40. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Nichols, J.; Gierl, M.S.; Faial, T.; Smith, A. Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos. Development 2010, 137, 693–704. [Google Scholar] [CrossRef]
- Aihara, Y.; Hayashi, Y.; Hirata, M.; Ariki, N.; Shibata, S.; Nagoshi, N.; Nakanishi, M.; Ohnuma, K.; Warashina, M.; Michiue, T.; et al. Induction of neural crest cells from mouse embryonic stem cells in a serum-free monolayer culture. Int. J. Dev. Biol. 2010, 54, 1287–1294. [Google Scholar] [CrossRef]
- Huang, M.; Miller, M.L.; McHenry, L.K.; Zheng, T.; Zhen, Q.; Ilkhanizadeh, S.; Conklin, B.R.; Bronner, M.E.; Weiss, W.A. Generating trunk neural crest from human pluripotent stem cells. Sci. Rep. 2016, 6, 19727. [Google Scholar] [CrossRef]
- Buitrago-Delgado, E.; Nordin, K.; Rao, A.; Geary, L.; LaBonne, C. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 2015, 348, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Murdoch, B.; Salem, A.F.; Prasad, M.S.; Gomez, G.A.; Garcia-Castro, M.I. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 2016, 143, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of neural crest cell development in vivo. Dev. Dyn. 2012, 241, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Noisa, P.; Lund, C.; Kanduri, K.; Lund, R.; La, H. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J. Cell Sci. 2014, 127, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Osumi, N.; Wakamatsu, Y. Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development 2002, 129, 863–873. [Google Scholar] [PubMed]
- Glavic, A.; Silva, F.; Aybar, M.J.; Bastidas, F.; Mayor, R. Interplay between Notch signaling and the homeoprotein Xiro1 is required for neural crest induction in Xenopus embryos. Development 2003, 131, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Ang, S.L.; Shiota, K.; Nakanishi, S.; Kageyama, R.; Guillemot, F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995, 9, 3136–3148. [Google Scholar] [CrossRef]
- Kageyama, R.; Ohtsuka, T.; Kobayashi, T. The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development 2007, 134, 1243–1251. [Google Scholar] [CrossRef]
- Tribulo, C.; Aybar, M.J.; Nguyen, V.H.; Mullins, M.C.; Mayor, R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 2003, 130, 6441–6452. [Google Scholar] [CrossRef]
- Ichiro, S.; Richard, M. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature 1994, 7, 362–369. [Google Scholar]
- Ishii, M. Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development 2005, 132, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Mori-Akiyama, Y.; Akiyama, H.; Rowitch, D.H.; de Crombrugghe, B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA 2003, 100, 9360–9365. [Google Scholar] [CrossRef] [PubMed]
- Craft, A.M.; Ahmed, N.; Rockel, J.S.; Baht, G.S.; Alman, B.A.; Kandel, R.A.; Grigoriadis, A.E.; Keller, G.M. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 2013, 140, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Monroy, J.; Montero, J.A.; Gañan, Y.; Macias, D.; Garcia-Porrero, J.A.; Hurle, J.M. Comparative analysis of the expression and regulation of Wnt5a, Fz4, and Frzb1 during digit formation and in micromass cultures. Dev. Dyn. 2002. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, T.; Lim, C.; Hayward, P.; Muñoz-Descalzo, S.; Nichols, J.; Garcia-Ojalvo, J.; Arias, A.M. Regulated fluctuations in Nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Tong, I.L.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.P.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.R.; Turner, D.L.; Weintraub, H.; Parkhurst, S.M. Specificity for the hairy/enhancer of split basic helix-loop-helix (bHLH) proteins maps outside the bHLH domain and suggests two separable modes of transcriptional repression. Mol. Cell. Biol. 1995, 15, 6923–6931. [Google Scholar] [CrossRef]
- Taelman, V.; Van Wayenbergh, R.; Sölter, M.; Pichon, B.; Pieler, T.; Christophe, D.; Bellefroid, E.J. Sequences downstream of the bHLH domain of the Xenopus hairy-related transcription factor-1 act as an extended dimerization domain that contributes to the selection of the partners. Dev. Biol. 2004, 276, 47–63. [Google Scholar] [CrossRef]
- Seon, A.K.; Jea, H.S.; Kim, J. The conserved WRPW motif of Hes6 mediates proteasomal degradation. Biochem. Biophys. Res. Commun. 2005, 332, 33–36. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Levine, E.M.; Rao, M.S. Hes1 but not Hes5 regulates an astrocyte versus oligodendrocyte fate choice in glial restricted precursors. Dev. Dyn. 2003, 226, 675–689. [Google Scholar] [CrossRef]
- Nichane, M.; Ren, X.; Souopgui, J.; Bellefroid, E.J. Hairy2 functions through both DNA-binding and non DNA-binding mechanisms at the neural plate border in Xenopus. Dev. Biol. 2008, 322, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.Q.; Ai, W.B.; Hu, Q.T.; Zhang, Q.J.; Wan, L.Y.; Wang, X.L.; Liu, C.B.; Wu, J.F. Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-beta/BMP signaling. World J. Gastroenterol. 2015, 21, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, L.; Lin, N.; Pan, W.; Hu, K.; Xu, R. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci. 2011, 89, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Sasai, Y.; Kageyama, R.; Tagawa, Y.; Shigemoto, R.; Nakanishi, S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992, 6, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, Z.; Li, X.; Jiang, Y.; Tsou, D.A.; Li, S. Derivation of Smooth Muscle Cells with Neural Crest Origin from Human Induced Pluripotent Stem Cells. Cells Tissues Organs 2012, 195, 5–14. [Google Scholar] [CrossRef]
- Robert, B.; Sassoon, D.; Jacq, B.; Gehring, W.; Buckingham, M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989, 8, 91–100. [Google Scholar] [CrossRef]
- Han, J.; Ishii, M.; Bringas, P.; Maas, R.L.; Maxson, R.E.; Chai, Y. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech. Dev. 2007, 124, 729–745. [Google Scholar] [CrossRef]
- Cheung, M.; Briscoe, J. Neural crest development is regulated by the transcription factor Sox9. Development 2003, 130, 5681–5693. [Google Scholar] [CrossRef]
- Bergwerff, M.; Verberne, M.E.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Neural crest cell contribution to the developing circulatory system implications for vascular morphology? Circ. Res. 1998, 82, 221–231. [Google Scholar] [CrossRef]
- Nakamura, T.; Colbert, M.C.; Robbins, J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 2006, 98, 1547–1554. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Jessell, T.M. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 1998, 125, 5055–5067. [Google Scholar]
- McKeown, S.J.; Lee, V.M.; Bronner-Fraser, M.; Newgreen, D.F.; Farlie, P.G. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev. Dyn. 2005, 233, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; White, P.M.; Zock, C.; Anderson, D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 1999, 96, 737–749. [Google Scholar] [CrossRef]
- Zhao, H.; Bringas, P.; Chai, Y. An in vitro model for characterizing the post-migratory cranial neural crest cells of the first branchial arch. Dev. Dyn. 2006. [Google Scholar] [CrossRef]
- Kerosuo, L.; Nie, S.; Bajpai, R.; Bronner, M.E. Crestospheres: Long-term maintenance of multipotent, premigratory neural crest stem cells. Stem Cell Rep. 2015. [Google Scholar] [CrossRef]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; De Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Meech, R.; Edelman, D.B.; Jones, F.S.; Makarenkova, H.P. The homeobox transcription factor Barx2 regulates chondrogenesis during limb development. Development 2005, 132, 2135–2146. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Y.; Li, F.; Qiao, L.; Wang, Q.; Li, N.; Borgia, J.A.; Deng, Y.; Lei, G.; Zheng, Q. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Wang, Y.H.; Ivanisevic, A.M.; Upholt, W.B.; Rodgers, B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev. Dyn. 2002. [Google Scholar] [CrossRef] [PubMed]
- Ashique, A.M.; Fu, K.; Richman, J.M. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int. J. Dev. Biol. 2002. [Google Scholar] [CrossRef]
- Ying, Q.-L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kolm, P.J.; Sive, H.L. Efficient hormone-inducible protein function in Xenopus laevis. Dev. Biol. 1995, 171, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Hollenberg, A.N. Regulation of chorionic gonadotropin gene expression. Endocr. Rev. 1993, 14, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Monroy, J.; De León, L.D. Differential effects of transforming growth factors β1, β2, β3 and β5 on chondrogenesis in mouse limb bud mesenchymal cells. Int. J. Dev. Biol. 1997, 41, 91–102. [Google Scholar] [PubMed]
- Curchoe, C.L.; Maurer, J.; Mckeown, S.J.; Cattarossi, G.; Cimadamore, F.; Nilbratt, M.; Snyder, E.Y.; Bronner-Fraser, M.; Terskikh, A.V. Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]

| Plasmid Name | NCBI Accession Number and Name | Vector Backbone | Reference |
|---|---|---|---|
| hGR-pcDNA3.1-myc-His | HQ692822, Homo sapiens glucocorticoid nuclear receptor mRNA | pcDNA3.1(+)/myc-His A™ Invitrogen | Current work |
| mHes1-hGR-pcDNA3.1-myc-His | BC051428, Mus musculus hairy and enhancer of split 1, mRNA | hGR-pcDNA3.1-myc-His (Current work) | Current work |
| mMsx1-hGR-pcDNA3.1-Hygro | BC016426, Mus musculus homeobox 1, msh-like 1, mRNA | hGR-pcDNA3.1-myc-His (Current work) | Current work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Maldonado, K.; Vega-López, G.; Caballero-Chacón, S.; Aybar, M.J.; Velasco, I. Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives. Int. J. Mol. Sci. 2018, 19, 4025. https://doi.org/10.3390/ijms19124025
Méndez-Maldonado K, Vega-López G, Caballero-Chacón S, Aybar MJ, Velasco I. Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives. International Journal of Molecular Sciences. 2018; 19(12):4025. https://doi.org/10.3390/ijms19124025
Chicago/Turabian StyleMéndez-Maldonado, Karla, Guillermo Vega-López, Sara Caballero-Chacón, Manuel J. Aybar, and Iván Velasco. 2018. "Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives" International Journal of Molecular Sciences 19, no. 12: 4025. https://doi.org/10.3390/ijms19124025
APA StyleMéndez-Maldonado, K., Vega-López, G., Caballero-Chacón, S., Aybar, M. J., & Velasco, I. (2018). Activation of Hes1 and Msx1 in Transgenic Mouse Embryonic Stem Cells Increases Differentiation into Neural Crest Derivatives. International Journal of Molecular Sciences, 19(12), 4025. https://doi.org/10.3390/ijms19124025







