Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice
Abstract
1. Introduction
2. Results
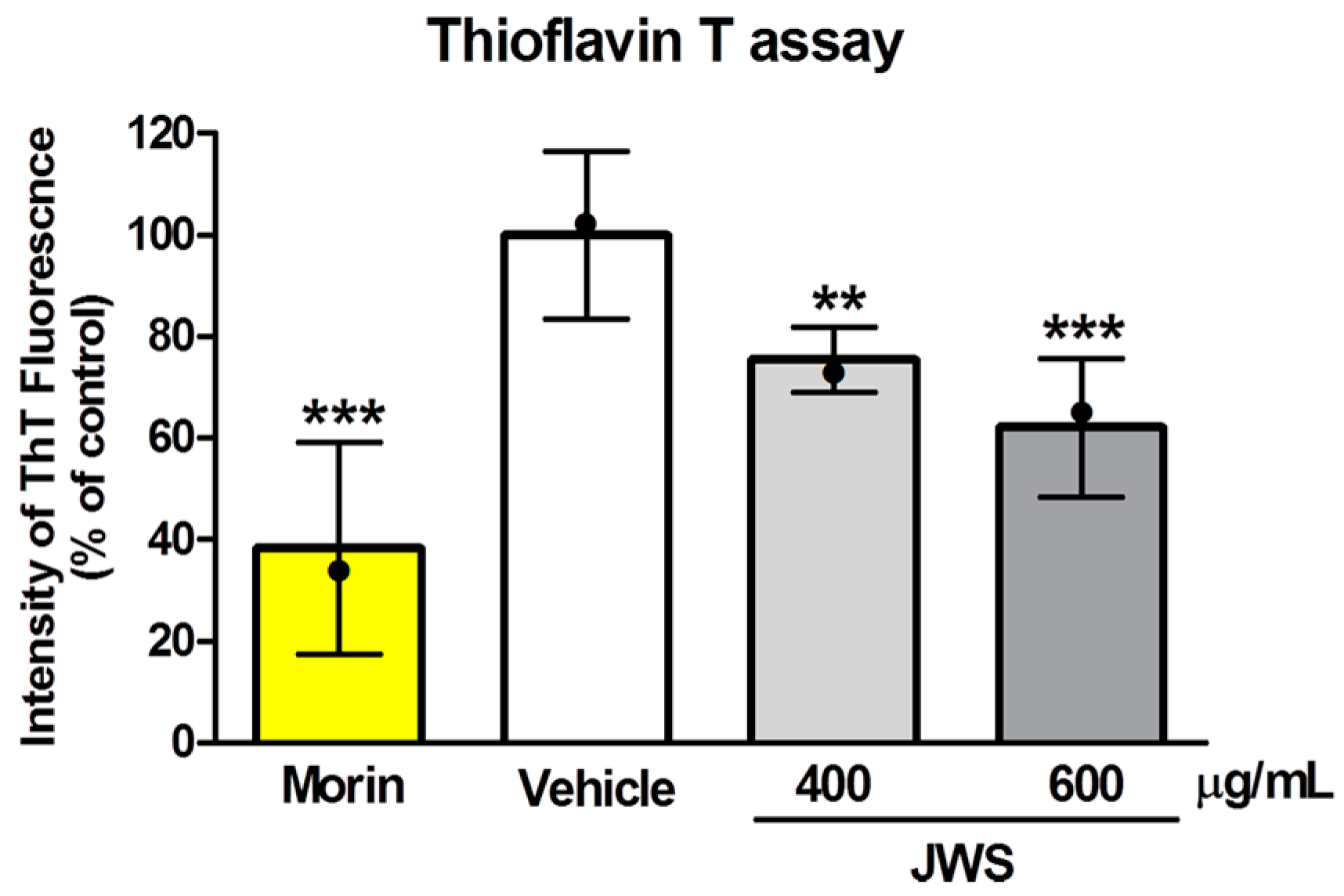
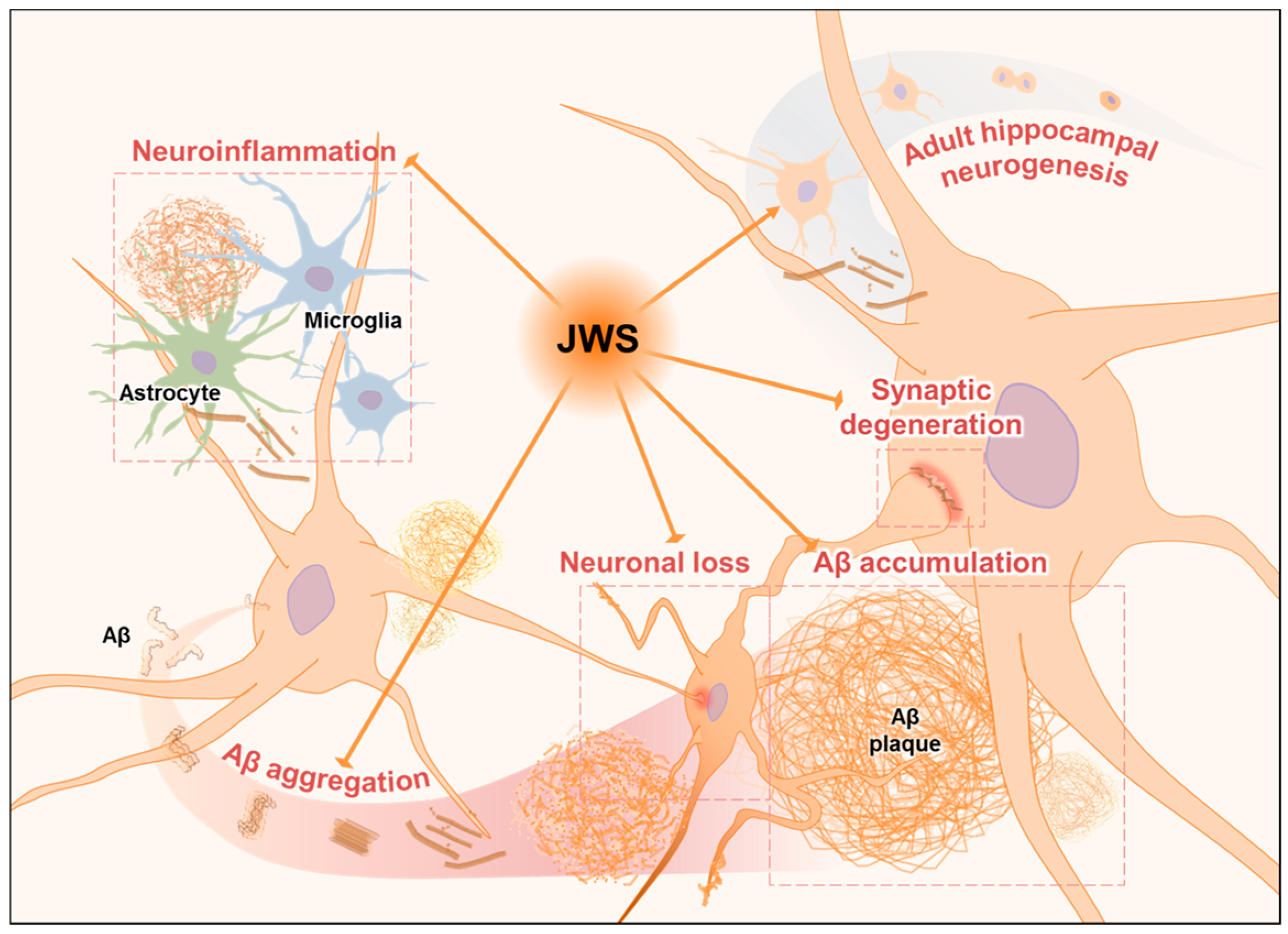
2.1. Inhibitory Effects of JWS on Aβ Aggregation In Vitro
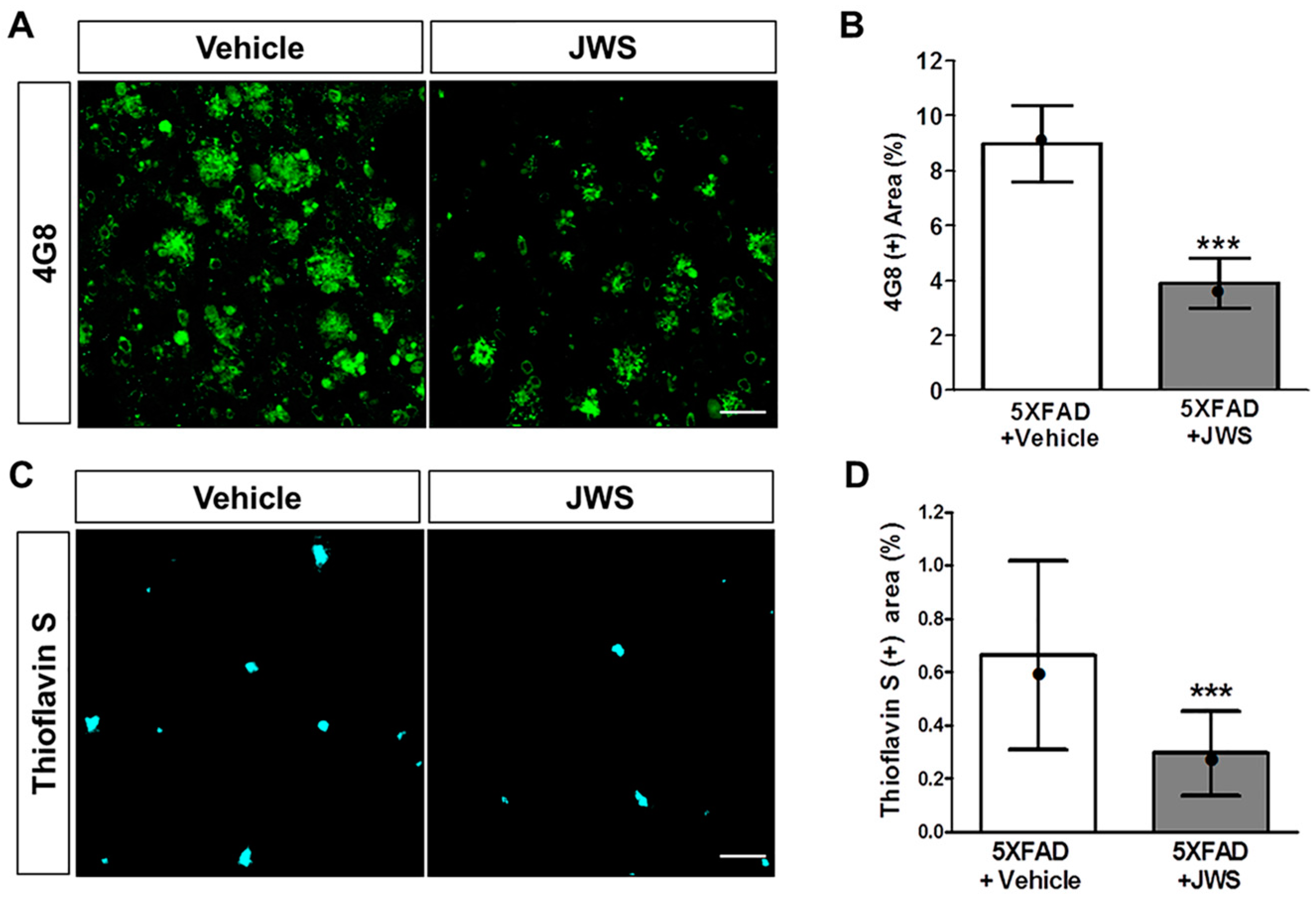
2.2. Inhibitory Effects of JWS on Aβ Accumulation in the Cerebral Cortex of 5XFAD Mice
2.3. Inhibitory Effects of JWS on Neuroinflammation in the Cerebral Cortex of 5XFAD Mice
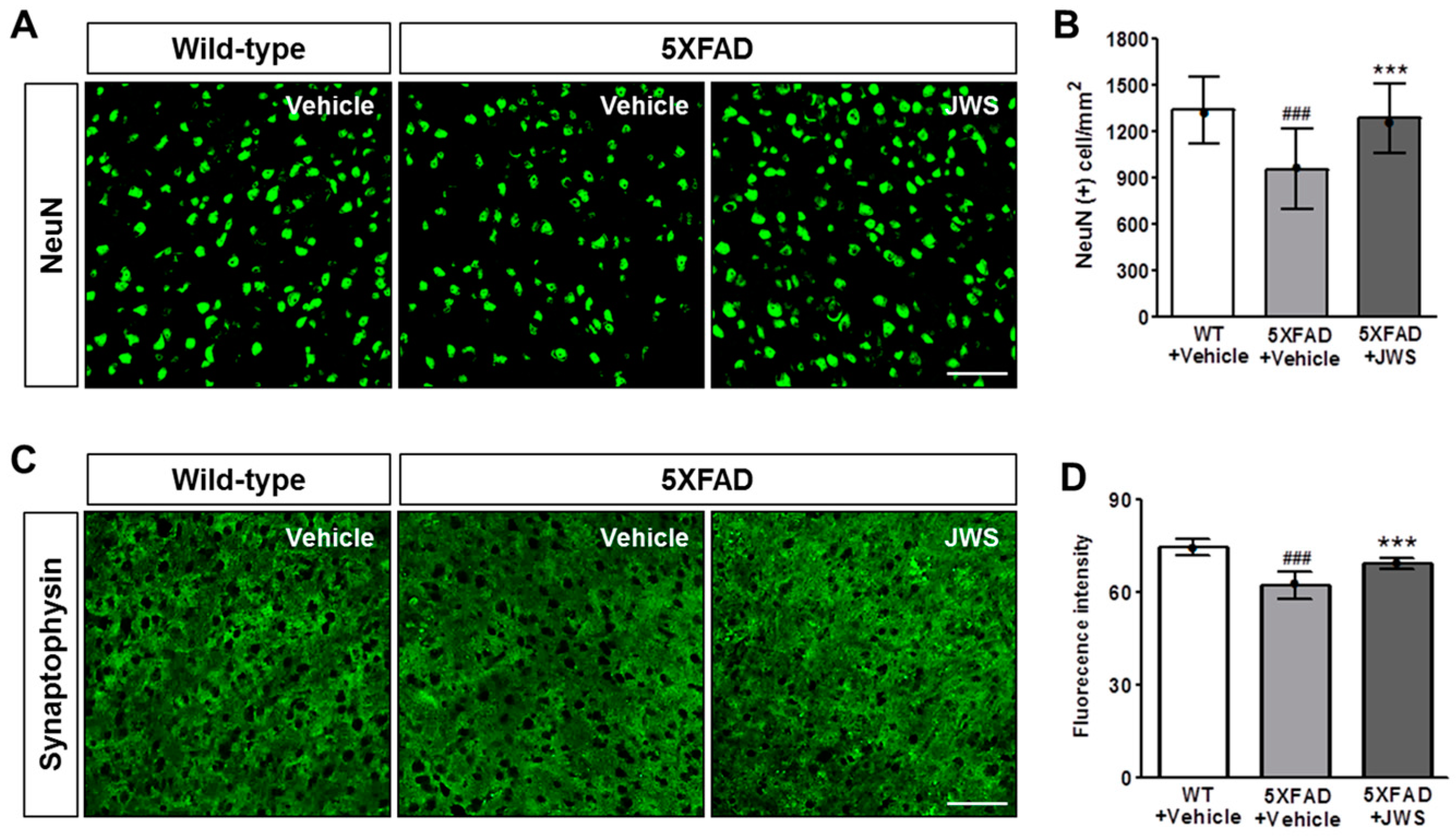
2.4. Inhibitory Effects of JWS on Neurodegeneration in the Cerebral Cortex of 5XFAD Mice
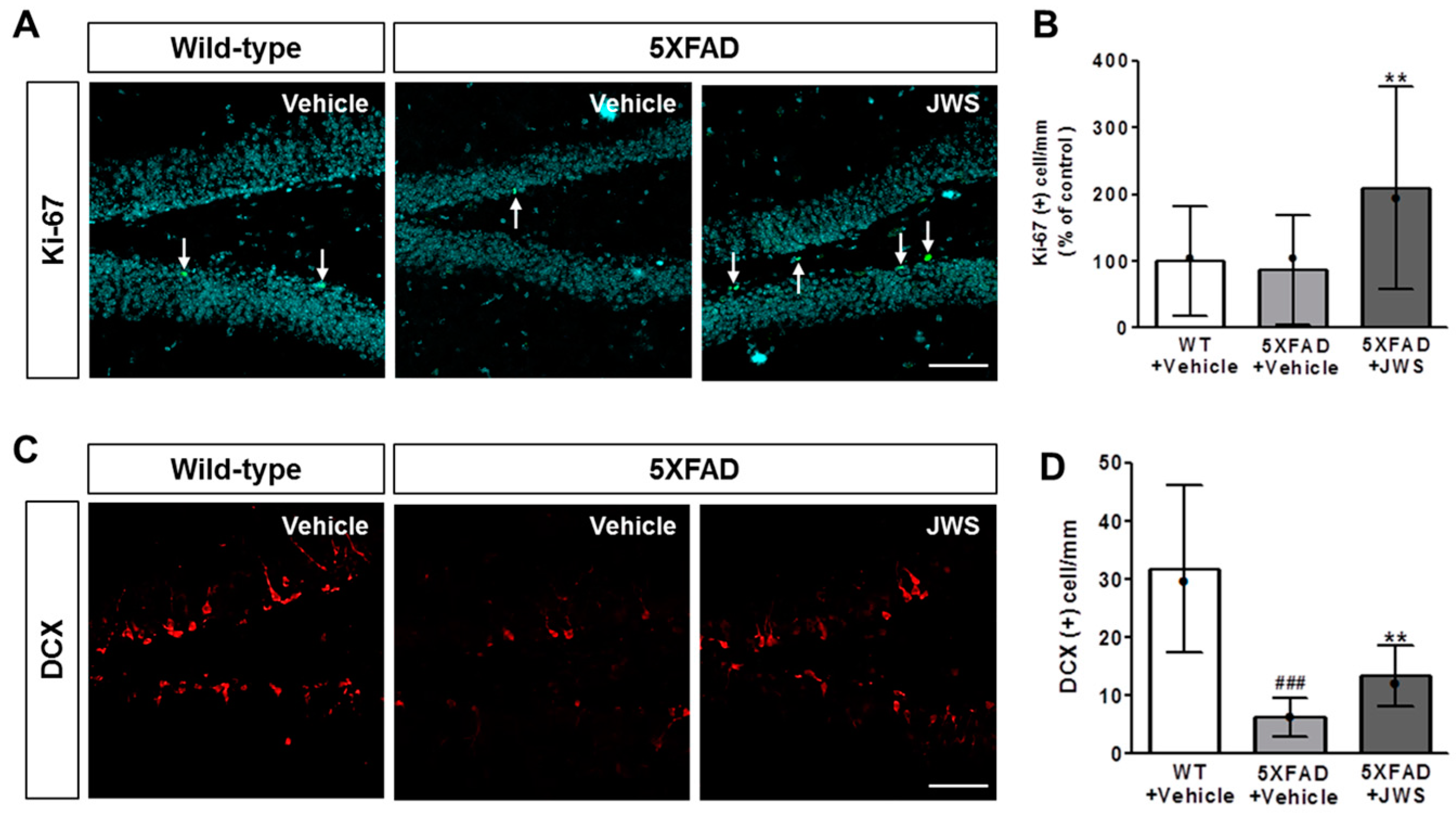
2.5. Inhibitory Effects of JWS on Impairment of Adult Hippocampal Neurogenesis in 5XFAD Mice
3. Discussion
4. Materials and Methods
4.1. Animals and JWS Administration
4.2. Brain Tissue Preparation
4.3. ThT Assay
4.4. Thioflavin S (ThS) Staining
4.5. Immunofluorescence Labeling and Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wirths, O.; Multhaup, G.; Bayer, T.A. A modified beta-amyloid hypothesis: Intraneuronal accumulation of the beta-amyloid peptide—The first step of a fatal cascade. J. Neurochem. 2004, 91, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Alonso Adel, C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Weggen, S.; Beher, D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimer’s Res. Ther. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.; Feightner, J.W.; Garcia, A.; Hsiung, G.Y.; MacKnight, C.; Sadovnick, A.D. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ 2008, 178, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Choi, J.G.; Moon, M.; Kim, H.G.; Lee, W.; Bak, H.R.; Sung, H.; Park, C.H.; Kim, S.Y.; Oh, M.S. An Optimized Combination of Ginger and Peony Root Effectively Inhibits Amyloid-beta Accumulation and Amyloid-beta-Mediated Pathology in AbetaPP/PS1 Double-Transgenic Mice. J. Alzheimers Dis. 2016, 50, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.R.; Dubey, A.K.; Masters, C.L.; Martins, R.N.; Macreadie, I.G. Abeta aggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2009, 13, 412–421. [Google Scholar] [CrossRef]
- Han, S.H.; Mook-Jung, I. Diverse molecular targets for therapeutic strategies in Alzheimer’s disease. J. Korean Med. Sci. 2014, 29, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Franchi, C.; Lucca, U.; Tettamanti, M.; Riva, E.; Fortino, I.; Bortolotti, A.; Merlino, L.; Pasina, L.; Nobili, A. Cholinesterase inhibitor use in Alzheimer’s disease: The EPIFARM-Elderly Project. Pharmacoepidemiol. Drug Saf. 2011, 20, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanokashira, D.; Mamada, N.; Yamamoto, F.; Taniguchi, K.; Tamaoka, A.; Lakshmana, M.K.; Araki, W. The neurotoxicity of amyloid beta-protein oligomers is reversible in a primary neuron model. Mol. Brain 2017, 10, 4. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, M.; Prots, I.; Winner, B. Adult hippocampal neurogenesis in Parkinson’s disease: Impact on neuronal survival and plasticity. Neural Plast. 2014, 2014, 454696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nallar, L.; Aranguiz, F.C.; Abbott, A.C.; Slater, P.G.; Inestrosa, N.C. Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Birth Defects Res. C Embryo Today Rev. 2010, 90, 284–296. [Google Scholar] [CrossRef]
- Suarez-Pereira, I.; Carrion, A.M. Updating stored memory requires adult hippocampal neurogenesis. Sci. Rep. 2015, 5, 13993. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, H.J.; Kim, H.T.; Whang, W.W. An experimental study of driental medicine on cure for dementia: The effect of Jowiseungcheongtang and Hyungbangjihwangtang on cure for aged rats. J. Orient. Neuropsychiatr. 1998, 9, 19–35. [Google Scholar]
- Oh, S.-W.; Kim, B.-W. Effects of jowiseungcheung-tang extract on the lipid metabolism, anti-oxidation and inflammatory reflex high fat diet obese rats. J. Internal Korean Med. 2013, 34, 1–13. [Google Scholar]
- Ryu, J.M.; Kim, J.W.; Chi, S.E.; Kim, E.J.; Park, E.H.; Hwang, U.W. The effects of jowiseungchungtang versus fluoxetine in the chronic mild stress model of depression in rats. J. Orient. Neuropsychiatr. 2004, 15, 27–41. [Google Scholar]
- Ansari, A.; Bose, S.; Yadav, M.K.; Wang, J.H.; Song, Y.K.; Ko, S.G.; Kim, H. CST, an Herbal Formula, Exerts Anti-Obesity Effects through Brain-Gut-Adipose Tissue Axis Modulation in High-Fat Diet Fed Mice. Molecules 2016, 21, 1522. [Google Scholar] [CrossRef]
- Joo, Y.W.; Jong, W.K.; Whang, W.W.; Hyun, T.K.; Soon, K.P. An Experimental Study on the Effects of Jowiseungchungtang on Learning and Memory of Rats in the Radial–Arm Maze. J. Orient. Neuropsychiatr. 1997, 8, 69–79. [Google Scholar]
- Whang, W.W.; Park, S.-K.; Lee, U.-S.; Kim, H.-T. The effects of Jowiseungchungtang on Learning and Memory of AD Rats using Morris water maze and Radial arm maze paradigm. J. KyungHee Orient. Med. Coll. 1998, 21, 479–501. [Google Scholar]
- Yoo, K.Y.; Park, O.K.; Hwang, I.K.; Li, H.; Ryu, S.Y.; Kang, I.J.; Yi, J.S.; Bae, Y.S.; Park, J.; Kim, Y.S.; et al. Induction of cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus by aqueous extract from Platycodon grandiflorum in middle-aged mice. Neurosci. Lett. 2008, 444, 97–101. [Google Scholar] [CrossRef]
- Seong Gak Jeon, E.J.S.; Lee, D.; Park, J.; Nam, Y.; Kim, J.; Moon, M. Traditional Oriental Medicines and Alzheimer’s Disease. Aging Dis. 2018. [Google Scholar] [CrossRef]
- Vassar, P.S.; Culling, C.F. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959, 68, 487–498. [Google Scholar] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Choi, J.G.; Nam, D.W.; Hong, H.S.; Choi, Y.J.; Oh, M.S.; Mook-Jung, I. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-beta1-42 oligomer-injected mice. J. Alzheimers Dis. 2011, 23, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Hong, H.S.; Nam, D.W.; Baik, S.H.; Song, H.; Kook, S.Y.; Kim, Y.S.; Lee, J.; Mook-Jung, I. Intracellular amyloid-beta accumulation in calcium-binding protein-deficient neurons leads to amyloid-beta plaque formation in animal model of Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 615–628. [Google Scholar] [CrossRef]
- Cotman, C.W.; Su, J.H. Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol. (Zurich, Switzerland) 1996, 6, 493–506. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Tian, J.; Shi, J.; Zhang, X.; Wang, Y. Herbal therapy: A new pathway for the treatment of Alzheimer’s disease. Alzheimer’s Res. Ther. 2010, 2, 30. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.W.; Kim, H.T.; Chung, K.C.; Whang, W.W. A Study of Jowiseungchungtang in patients with mild Dementia of Alzheimer type. J. Orient. Neuropsychiatr. 2003, 14, 17–26. [Google Scholar]
- Kim, B.G.; Kim, J.W.; Kim, H.T.; Chung, K.C.; Whang, W.W. The effects on Jowiseungchungtang of patients with early DAT using auditory ERP and K-DRS. J. Orient. Neuropsychiatr. 2003, 14, 43–59. [Google Scholar]
- Fujiwara, H.; Takayama, S.; Iwasaki, K.; Tabuchi, M.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kudo, Y.; Kase, Y.; Arai, H.; et al. Yokukansan, a traditional Japanese medicine, ameliorates memory disturbance and abnormal social interaction with anti-aggregation effect of cerebral amyloid beta proteins in amyloid precursor protein transgenic mice. Neuroscience 2011, 180, 305–313. [Google Scholar] [CrossRef]
- Chen, H.-L.; Guan, F. Effect of Huanglian Jiedu Decoction on pitavastatin treatment of Alzheimer’s disease. J. Hainan Med. Univ. 2016, 22, 71–74. [Google Scholar]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.C.; Wang, K.F.; Chen, X.Y. Abeta peptide secretion is reduced by Radix Polygalae-induced autophagy via activation of the AMPK/mTOR pathway. Mol. Med. Rep. 2015, 12, 2771–2776. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Abeta1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar]
- Choi, S.I.; Go, J.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Jung, Y.J.; Hwang, D.Y. Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer’s disease in Tg2576 mice. Lab. Anim. Res. 2013, 29, 212–220. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Kimura, R.; Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef]
- Wirths, O.; Bayer, T.A. Intraneuronal Abeta accumulation and neurodegeneration: Lessons from transgenic models. Life Sci. 2012, 91, 1148–1152. [Google Scholar] [CrossRef]
- Park, E.-K.; Shim, E.-S.; Jung, H.-S.; Sohn, N.-W.; Sohn, Y.-J. Effects of Chongmyung-tang, Polygalae Radix and Acori Graminei Rhizoma on Aβ Toxicity and Memory Dysfunction in Mice. J. Int. Korean Med. 2008, 29, 608–620. [Google Scholar]
- Tian, S.M.; Ma, Y.X.; Sun, L.Z.; Tan, L.; Liu, J.; Li, G.Y. Effects of different fractions of Acori graminei rhizoma extracts on learning and memory abilities in Aβ-induced Alzheimer disease mice. Chin. J. Pathophysiol. 2012, 28, 159–162. [Google Scholar]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar]
- Braskie, M.N.; Thompson, P.M. Understanding cognitive deficits in Alzheimer’s disease based on neuroimaging findings. Trends Cogn. Sci. 2013, 17, 510–516. [Google Scholar] [CrossRef]
- Moon, M.; Cha, M.Y.; Mook-Jung, I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimers Dis. 2014, 41, 233–241. [Google Scholar] [CrossRef]
- Zhen, J.; Qian, Y.; Fu, J.; Su, R.; An, H.; Wang, W.; Zheng, Y.; Wang, X. Deep Brain Magnetic Stimulation Promotes Neurogenesis and Restores Cholinergic Activity in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neural Circ. 2017, 11, 48. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, B.T. Effects of α-asarone on Proliferation and Differentiation of Neural Progenitor Cells. Korean J. Phys. Anthropol. 2018, 31, 41–49. [Google Scholar] [CrossRef]
- Yang, S.H.; Jeng, C.J.; Chen, C.H.; Chen, Y.; Chen, Y.C.; Wang, S.M. Schisandrin enhances dendrite outgrowth and synaptogenesis in primary cultured hippocampal neurons. J. Sci. Food Agric. 2011, 91, 694–702. [Google Scholar] [CrossRef]
- Sun, Y.X.; Cong, Y.L.; Liu, Y.; Jin, B.; Si, L.; Wang, A.B.; Cai, H.; Che, G.Y.; Tang, B.; Wang, C.F.; et al. Schisandrin A and B affect subventricular zone neurogenesis in mouse. Eur. J. Pharmacol. 2014, 740, 552–559. [Google Scholar] [CrossRef]
- Ryota, A.; Hironori, F.; Kinzo, M.; Kazufumi, T.; Takeshi, Y. Polygalae radix extract ameliorates behavioral and neuromorphological abnormalities in chronic corticosterone-treated mice. Tradit. Kampo Med. 2018. [Google Scholar] [CrossRef]
- Zheng, H.; Fridkin, M.; Youdim, M. From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals (Basel, Switzerland) 2014, 7, 113–135. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, A.; Sharma, A. Changing Paradigm from one Target one Ligand Towards Multi-target Directed Ligand Design for Key Drug Targets of Alzheimer Disease: An Important Role of In Silico Methods in Multi-target Directed Ligands Design. Curr. Neuropharmacol. 2018, 16, 726–739. [Google Scholar] [CrossRef]
- Jung, T.Y. Single Oral Dose Toxicity Test of Choweseuncheng-tang, a Polyherbal Formula in ICR Mice. J. Physiol. Pathol. Korean Med. 2014, 28, 53–58. [Google Scholar] [CrossRef]

| Constituents | Amount |
|---|---|
| Coicis Semen | 8.0 g |
| Castaneae Semen | 8.0 g |
| Raphani Semen | 6.0 g |
| Longanae Arillus | 6.0 g |
| Liriopis Tuber | 4.0 g |
| Platycodi Radix | 4.0 g |
| Acori Gramineri Rhizoma | 4.0 g |
| Thujae Semen | 4.0 g |
| Zizyphi Semen | 4.0 g |
| Massa Medicata Fermentata | 4.0 g |
| Ephedrae Herba | 3.0 g |
| Schisandrae Fructus | 3.0 g |
| Amomi Semen | 3.0 g |
| Polygalae Radix | 3.0 g |
| Total amount | 64.0 g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.J.; Jeong, Y.-o.; Jeon, S.G.; Kim, S.; Lee, S.-k.; Nam, Y.; Park, Y.H.; Kim, D.; Lee, Y.S.; Choi, H.S.; et al. Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice. Int. J. Mol. Sci. 2018, 19, 4026. https://doi.org/10.3390/ijms19124026
Shin SJ, Jeong Y-o, Jeon SG, Kim S, Lee S-k, Nam Y, Park YH, Kim D, Lee YS, Choi HS, et al. Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice. International Journal of Molecular Sciences. 2018; 19(12):4026. https://doi.org/10.3390/ijms19124026
Chicago/Turabian StyleShin, Soo Jung, Yu-on Jeong, Seong Gak Jeon, Sujin Kim, Seong-kyung Lee, Yunkwon Nam, Yong Ho Park, Dabi Kim, Youn Seok Lee, Hong Seok Choi, and et al. 2018. "Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice" International Journal of Molecular Sciences 19, no. 12: 4026. https://doi.org/10.3390/ijms19124026
APA StyleShin, S. J., Jeong, Y.-o., Jeon, S. G., Kim, S., Lee, S.-k., Nam, Y., Park, Y. H., Kim, D., Lee, Y. S., Choi, H. S., Kim, J.-i., Kim, J.-J., & Moon, M. (2018). Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice. International Journal of Molecular Sciences, 19(12), 4026. https://doi.org/10.3390/ijms19124026




