Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450
Abstract
1. Introduction
2. Results
2.1. Bacterial Coexpression of CPR Mutants and CYP
2.2. CYP-Activities When Combined with the Three CPR Hinge Domain Mutant
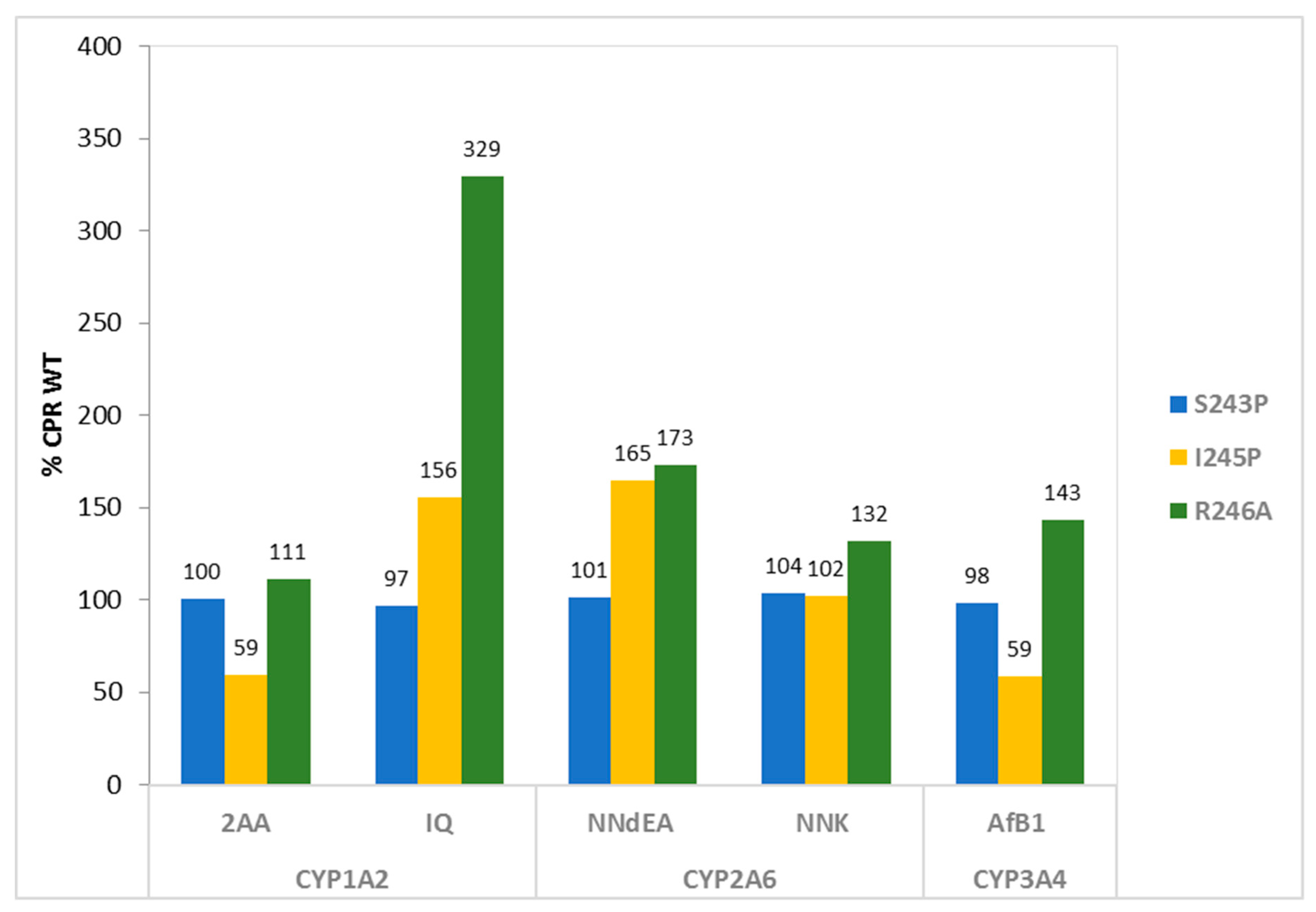
2.2.1. Whole-Cell Bioactivation Assays
2.2.2. Membrane Preparations
CYP-Enzyme Kinetic Analysis
Ionic Strength Effect on CYP:CPR Interaction
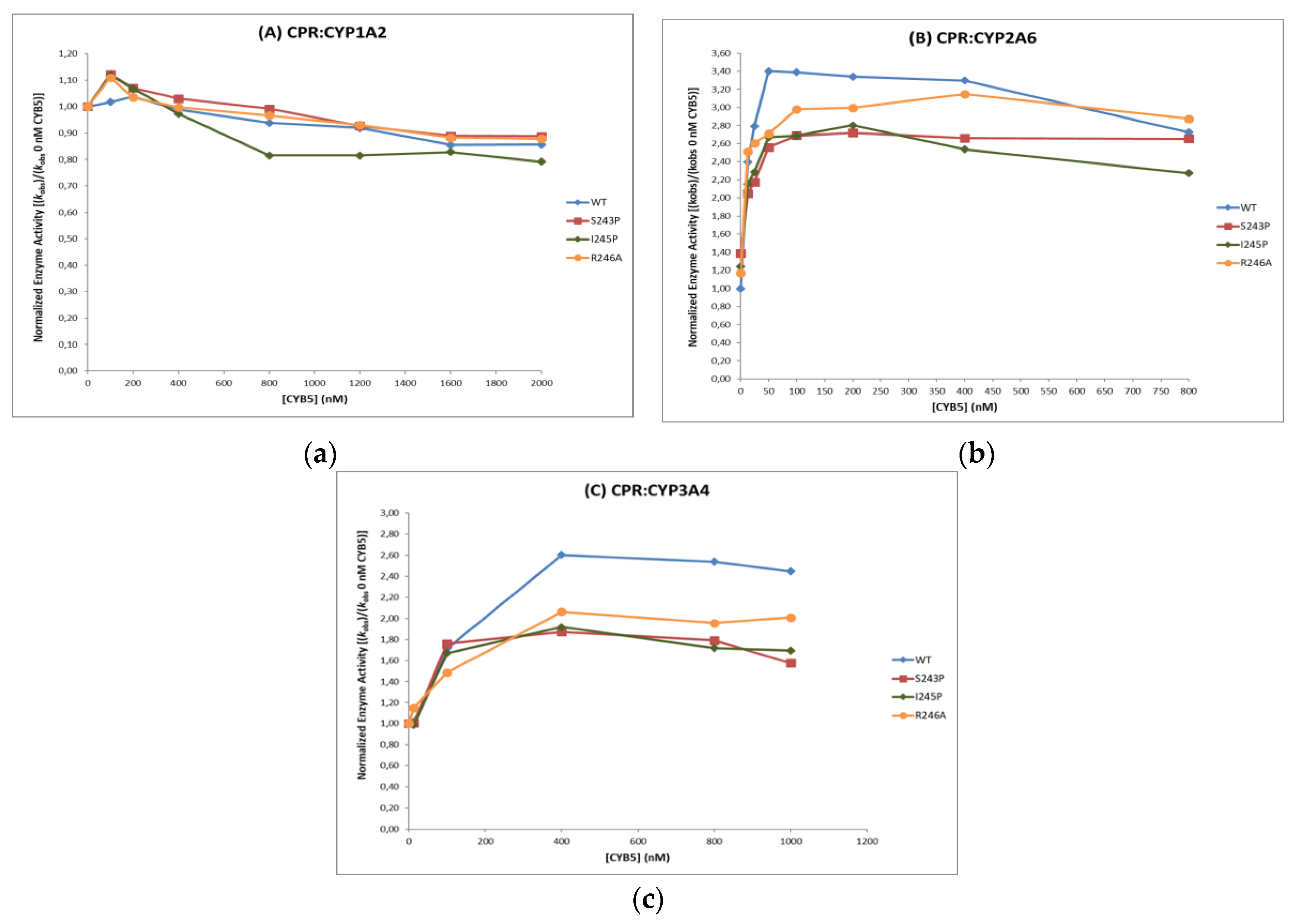
CYB5 Effect on Activity of CYP1A2, 2A6 and 3A4
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Bacterial Expression of Human CYB5 and Purification
4.3. Bacterial Co-Expression of Human CPR Mutants and CYPs
4.4. Whole-Cell Mutagenicity Assays
4.5. Membrane Preparation and Characterization
4.6. CYP-Enzyme Assays
4.7. Ionic Strength Effect
4.8. CYP Activity Titration with CYB5
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2AA | 2-Aminoanthracene |
| ACN | Acetonitrile |
| AfB1 | Aflatoxin B1 |
| CPR | NADPH-cytochrome P450 reductase |
| CYB5 | Cytochrome b5 |
| CYP | Microsomal cytochrome P450 |
| DBF | Dibenzylfluorescein |
| DMSO | Dimethyl sulfoxide |
| EROD | Ethoxyresorufin O-deethylation |
| ET | Electron transfer |
| EthR | Ethoxyresorufin |
| FAD | Flavin adenine dinucleotide |
| FMN | Flavin adenine dinucleotide |
| IPTG | Isopropyl β-d-thiogalactoside |
| IQ | 2-Amino-3-methylimidazo(4,5-f)quinoline |
| NADP+/NADPH | Nicotinamide adenine dinucleotide phosphate |
| NNdEA | N-nitrosodiethylamine |
| NNK | 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone |
References
- Yasukochi, Y.; Masters, B.S. Some properties of a detergent-solubilized NADPH-cytochrome c (cytochrome P-450) reductase purified by biospecific affinity chromatography. J. Biol. Chem. 1976, 251, 5337–5344. [Google Scholar] [PubMed]
- Rendic, S.; Guengerich, F.P. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed.; De Montellano, P.R.O., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 523–785. [Google Scholar]
- Pandey, A.V.; Flück, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef] [PubMed]
- Vergéres, G.; Waskell, L. Cytochrome b5, its functions, structure and membrane topology. Biochimie 1995, 77, 604–620. [Google Scholar] [CrossRef]
- Bart, A.G.; Scott, E.E. Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes. J. Biol. Chem. 2017, 292, 20818–20833. [Google Scholar] [CrossRef] [PubMed]
- Murataliev, M.B.; Feyereisen, R.; Walker, F.A. Electron transfer by diflavin reductases. Biochim. Biophys. Acta 2004, 1698, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.; Kim, J.J. Three-dimensional structure of NADPH-cytochrome P450reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef] [PubMed]
- Grunau, A.; Geraki, K.; Grossmann, J.G.; Gutierrez, A. Conformational dynamics and the energetics of protein ligand interactions: role of interdomain loop in human cytochrome P450 reductase. Biochemistry 2007, 46, 8244–8255. [Google Scholar] [CrossRef]
- Ellis, J.; Gutierrez, A.; Barsukov, I.L.; Huang, W.C.; Grossmann, J.G.; Roberts, G.C. Domain motion in cytochrome P450 reductase: Conformational equilibria revealed by NMR and small angle xray scattering. J. Biol. Chem. 2009, 284, 36628–36637. [Google Scholar] [CrossRef]
- Paine, M.J.I.; Scrutton, N.S.; Munro, A.W.; Gutierrez, A.; Roberts, G.C.K.; Wolf, C.R. Electron transfer partners of cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 3th ed.; De Montellano, P.R.O., Ed.; Springer International Publishing: Basel, Switzerland, 2005; pp. 115–148. [Google Scholar]
- Xia, C.; Hamdane, D.; Shen, A.L.; Choi, V.; Kasper, C.B.; Pearl, N.M.; Zhang, H.; Im, S.C.; Waskell, L.; Kim, J.J. Conformational changes of NADPH-cytochrome P450 oxidoreductase are essential for catalysis and cofactor binding. J. Biol. Chem. 2011, 286, 16246–16260. [Google Scholar] [CrossRef]
- Vincent, B.; Morellet, N.; Fatemi, F.; Aigrain, L.; Truan, G.; Guittet, E.; Lescop, E. The closed and compact domain organization of the 70-kDa human cytochrome P450 reductase in its oxidized state as revealed by NMR. J. Mol. Biol. 2012, 420, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, D.; Xia, C.; Im, S.C.; Zhang, H.; Kim, J.; Waskell, L. Structure and function of an NADPH cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J. Biol. Chem. 2009, 284, 11374–11384. [Google Scholar] [CrossRef] [PubMed]
- Aigrain, L.; Pompon, D.; Truan, G.; Morera, S. Cloning, purification, crystallization and preliminary Xray analysis of a chimeric NADPH cytochrome P450 reductase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65 Pt 3, 210–212. [Google Scholar] [CrossRef]
- Huang, W.C.; Ellis, J.; Moody, P.C.; Raven, E.L.; Roberts, G.C. Redox linked domain movements in the catalytic cycle of cytochrome p450 reductase. Structure 2013, 21, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Frances, O.; Fatemi, F.; Pompon, D.; Guittet, E.; Sizun, C.; Pérez, J.; Lescop, E.; Truan, G. A Well-Balanced Preexisting Equilibrium Governs Electron Flux Efficiency of a Multidomain Diflavin Reductase. Biophys. J. 2015, 108, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, A.; Oostenbrink, C. Molecular dynamics simulations give insight into the conformational change, complex formation, and electron transfer pathway for cytochrome p450 reductase. Protein Sci. 2013, 22, 1183–1195. [Google Scholar] [CrossRef]
- Aigrain, L.; Pompon, D.; Truan, G. Role of the interface between the FMN and FAD domains in the control of redox potential and electronic transfer of NADPH-cytochrome P450 reductase. Biochem. J. 2011, 435, 197–206. [Google Scholar] [CrossRef]
- Kranendonk, M.; Marohnic, C.C.; Panda, S.P.; Duarte, M.P.; Oliveira, J.S.; Masters, B.S.; Rueff, J. Impairment of human CYP1A2mediated xenobiotic metabolism by Antley Bixler syndrome variants of cytochrome P450 oxidoreductase. Arch. Biochem. Biophys. 2008, 475, 93–99. [Google Scholar] [CrossRef]
- Miller, W.L.; Agrawal, V.; Sandee, D.; Tee, M.K.; Huang, N.; Choi, J.H.; Morrissey, K.; Giacomini, K.M. Consequences of POR mutations and polymorphisms. Mol. Cell. Endocrinol. 2011, 336, 174–179. [Google Scholar] [CrossRef]
- Moutinho, D.; Marohnic, C.C.; Panda, S.P.; Rueff, J.; Masters, B.S.; Kranendonk, M. Altered human CYP3A4 activity caused by Antley Bixler syndrome related variants of NADPH cytochrome P450 oxidoreductase measured in a robust in vitro system. Drug Metab. Dispos. 2012, 40, 754–760. [Google Scholar] [CrossRef]
- Marohnic, C.C.; Panda, S.P.; McCammon, K.; Rueff, J.; Masters, B.S.; Kranendonk, M. Human cytochrome P450 oxidoreductase deficiency caused by the Y181D mutation: Molecular consequences and rescue of defect. Drug Metab. Dispos. 2010, 38, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Campelo, D.; Lautier, T.; Urban, P.; Esteves, F.; Bozonnet, S.; Truan, G.; Kranendonk, M. The Hinge Segment of Human NADPH-Cytochrome P450 Reductase in Conformational Switching: The Critical Role of Ionic Strength. Front. Pharmacol. 2017, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Kasper, C.B. Role of acidic residues in the interaction of NADPH-cytochrome P450 oxidoreductase with cytochrome P450 and cytochrome c. J. Biol. Chem. 1995, 270, 27475–27480. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Palma, B.B.; Gilep, A.A.; Laires, A.; Oliveira, J.S.; Usanov, S.A.; Rueff, J.; Kranendonk, M. The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6 and 2E1: A new cell expression system to study cytochrome P450 mediated biotransformation. Mutagenesis 2005, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- McCammon, K.M.; Panda, S.; Xia, C.; Kim, J.J.; Moutinho, D.; Kranendonk, M.; Auchus, R.J.; Lafer, E.M.; Ghosh, D.; Martasek, P.; et al. Instability of the Human Cytochrome P450 Reductase A287P Variant Is the Major Contributor to Its Antley-Bixler Syndrome-like Phenotype. J. Biol. Chem. 2016, 291, 20487–20502. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Palma, B.B.; Laires, A.; Oliveira, J.S.; Rueff, J.; Kranendonk, M. Escherichia coli BTC, a human cytochrome P450 competent tester strain with a high sensitivity towards alkylating agents: Involvement of alkyltransferases in the repair of DNA damage induced by aromatic amines. Mutagenesis 2005, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Palma, B.B.; Silva, E.S.M.; Urban, P.; Rueff, J.; Kranendonk, M. Functional characterization of eight human CYP1A2 variants: The role of cytochrome b5. Pharmacogenet. Genomics 2013, 23, 41–52. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; von Moltke, L.L.; Court, M.H.; Harmatz, J.S.; Crespi, C.L.; Greenblatt, D.J. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab. Dispos. 2000, 28, 1493–1504. [Google Scholar]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.L.; Perkins, J.D.; Thummel, K.E. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 1552–1562. [Google Scholar]
- Hlavica, P.; Schulze, J.; Lewis, D.F. Functional interaction of cytochrome P450 with its redox partners: A critical assessment and update of the topology of predicted contact regions. J. Inorg. Biochem. 2003, 96, 279–297. [Google Scholar] [CrossRef]
- Kandel, S.E.; Lampe, J.N. Role of protein-protein interactions in cytochrome P450-mediated drug metabolism and toxicity. Chem. Res. Toxicol. 2014, 27, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Ahn, T.; Guengerich, F.P. Conformational change and activation of cytochrome P450 2B1 induced by salt and phospholipid. Arch. Biochem. Biophys. 1998, 356, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Song, M.; Ahn, T.; Kim, H. Conformational change of cytochrome P450 1A2 induced by sodium chloride. J. Biol. Chem. 1996, 271, 31312–31316. [Google Scholar] [CrossRef] [PubMed]
- Voznesensky, A.I.; Schenkman, J.B. The cytochrome P450 2B4-NADPH cytochrome P450 reductase electron transfer complex is not formed by charge-pairing. J. Biol. Chem. 1992, 267, 14669–14676. [Google Scholar] [PubMed]
- Voznesensky, A.I.; Schenkman, J.B. Quantitative analyses of electrostatic interactions between NADPH-cytochrome P450 reductase and cytochrome P450 enzymes. J. Biol. Chem. 1994, 269, 15724–15731. [Google Scholar] [PubMed]
- Waskell, L.; Kim, J.J.P. Electron Transfer Partners of Cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed.; De Montellano, P.R.O., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 33–68. [Google Scholar]
- Im, S.C.; Waskell, L. The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b(5). Arch. Biochem. Biophys. 2011, 507, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, R.; Im, S.C.; Waskell, L.; Ramamoorthy, A. Effects of membrane mimetics on cytochrome P450-cytochrome b5 interactions characterized by NMR spectroscopy. J. Biol. Chem. 2015, 290, 12705–12718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Le Clair, S.V.; Huang, R.; Ahuja, S.; Im, S.C.; Waskell, L.; Ramamoorthy, A. Insights into the role of substrates on the interaction between cytochrome b5 and cytochrome P450 2B4 by NMR. Sci. Rep. 2015, 5, 8392. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Cao, C.; Waterman, M.R. An additional electrostatic interaction between adrenodoxin and P450c27 (CYP27A1) results in tighter binding than between adrenodoxin and p450scc (CYP11A1). J. Biol. Chem. 1999, 274, 2045–2052. [Google Scholar] [CrossRef]
- Nunez, M.; Guittet, E.; Pompon, D.; van Heijenoort, C.; Truan, G. NMR structure note: Oxidized microsomal human cytochrome b5. J. Biomol. NMR 2010, 47, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.; Carreira, F.; Theisen, P.; Laires, A.; Fisher, C.W.; Rueff, J.; Estabrook, R.W.; Vermeulen, N.P. Escherichia coli MTC, a human NADPH P450 reductase competent mutagenicity tester strain for the expression of human cytochrome P450 isoforms 1A1, 1A2, 2A6, 3A4, or 3A5: Catalytic activities and mutagenicity studies. Mutat. Res. 1999, 441, 73–83. [Google Scholar] [CrossRef]
- Bauer, S.; Shiloach, J. Maximal exponential growth rate and yield of E. coli obtainable in a bench-scale fermentor. Biotechnol. Bioeng. 1974, 16, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Palma, B.B.; Silva, E.S.M.; Vosmeer, C.R.; Lastdrager, J.; Rueff, J.; Vermeulen, N.P.; Kranendonk, M. Functional characterization of eight human cytochrome P450 1A2 gene variants by recombinant protein expression. Pharmacogenom. J. 2010, 10, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.; Mesquita, P.; Laires, A.; Vermeulen, N.P.; Rueff, J. Expression of human cytochrome P450 1A2 in Escherichia coli: A system for biotransformation and genotoxicity studies of chemical carcinogens. Mutagenesis 1998, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]


| CYP Isoform | CPR Form | Whole-Cells | Membrane Fractions | ||
|---|---|---|---|---|---|
| CYP 1 | CYP 1 | CPR 1 | CPR:CYP Ratios | ||
| (nM) | (pmol/mg Protein) | ||||
| CYP1A2 | WT | 109 ± 4 | 54 ± 1 | 4.1 ± 1.5 | 1:13 |
| S243P | 241 ± 4 | 73 ± 4 | 7.7 ± 0.2 | 1:9 | |
| I245P | 206 ± 11 | 102 ± 1 | 6.1 ± 0.5 | 1:17 | |
| R246A | 176 ± 3 | 91 ± 2 | 5.4 ± 0.2 | 1:17 | |
| CYP2A6 | WT | 130 ± 2 | 139 ± 1 | 10.5 ± 1.3 | 1:13 |
| S243P | 98 ± 1 | 106 ± 3 | 11.2 ± 0.5 | 1:9 | |
| I245P | 96 ± 7 | 102 ± 1 | 9.5 ± 0.9 | 1:11 | |
| R246A | 98 ± 2 | 146 ± 1 | 10.6 ± 1.5 | 1:14 | |
| CYP3A4 | WT | 105 ± 2 | 83 ± 3 | 19.8 ± 0.2 | 1:4 |
| S243P | 122 ± 3 | 77 ± 2 | 22.5 ± 2.6 | 1:3 | |
| I245P | 128 ± 3 | 78 ± 2 | 18.3 ± 0.7 | 1:4 | |
| R246A | 143 ± 5 | 78 ± 1 | 21.4 ± 0.3 | 1:4 | |
| CYP Isoform | Mutagen | CPR Form | |||
|---|---|---|---|---|---|
| WT | S243P | I245P | R246A | ||
| CYP1A2 | 2AA 1 | 5643 ± 271 | 5666 ± 177 | 3339 ± 145 | 6274 ± 106 |
| IQ 1 | 335 ± 8 | 323 ± 7 | 521 ± 104 | 1103 ± 253 | |
| CYP2A6 | NNdEA 2 | 537 ± 14 | 543 ± 21 | 885 ± 122 | 929 ± 118 |
| NNK 2 | 770 ± 121 | 799 ± 110 | 788 ± 57 | 1014 ± 29 | |
| CYP3A4 | AfB1 1 | 1129 ± 97 | 1109 ± 115 | 661 ± 185 | 1616 ± 163 |
| CYP Isoform | CPR Form | kcat1 | KM1 | Efficiency |
|---|---|---|---|---|
| (Product Formed pmol·min−1·pmol−1 CYP) | (µM) | (kcat/KM) (% WT) | ||
| CYP1A2 | WT | 0.62 ± 0.02 | 1.94 ± 0.16 | 0.32 (1.00) |
| S243P | 0.63 ± 0.01 | 1.23 ± 0.05 | 0.51 (1.59) | |
| I245P | 0.40 ± 0.01 | 0.89 ± 0.04 | 0.46 (1.44) | |
| R246A | 0.43 ± 0.01 | 0.74 ± 0.06 | 0.59 (1.84) | |
| CYP2A6 | WT | 1.37 ± 0.07 | 1.99 ± 0.34 | 0.69 (1.00) |
| S243P | 1.17 ± 0.08 | 2.03 ± 0.40 | 0.58 (0.84) | |
| I245P | 1.20 ± 0.09 | 1.78 ± 0.39 | 0.67 (0.97) | |
| R246A | 1.53 ± 0.08 | 1.98 ± 0.33 | 0.77 (1.12) | |
| CYP3A4 | WT | 2.53 ± 0.16 | 3.75 ± 0.55 | 0.67 (1.00) |
| S243P | 1.83 ± 0.08 | 3.07 ± 0.34 | 0.60 (0.88) | |
| I245P | 1.89 ± 0.13 | 2.85 ± 0.50 | 0.66 (0.98) | |
| R246A | 2.18 ± 0.22 | 3.82 ± 0.87 | 0.57 (0.85) |
| CYP Isoform | CPR Form | Maximal kobs 1 | CYB5 Stimulus 2 | CYB5:CPR Ratio 3 |
|---|---|---|---|---|
| (%) | ||||
| CYP1A2 | WT | 1.04 ± 0.01 | 100 | 163 |
| S243P | 1.11 ± 0.02 | 107 | 118 | |
| I245P | 1.12 ± 0.04 | 108 | 208 | |
| R246A | 1.12 ± 0.02 | 108 | 211 | |
| CYP2A6 | WT | 3.40 ± 0.04 | 100 | 26 |
| S243P | 2.72 ± 0.05 | 80 | 106 | |
| I245P | 2.69 ± 0.04 | 79 | 43 | |
| R246A | 3.15 ± 0.04 | 93 | 211 | |
| CYP3A4 | WT | 2.60 ± 0.01 | 100 | 66 |
| S243P | 1.87 ± 0.02 | 72 | 55 | |
| I245P | 1.92 ± 0.01 | 74 | 68 | |
| R246A | 2.06 ± 0.02 | 79 | 67 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campelo, D.; Esteves, F.; Brito Palma, B.; Costa Gomes, B.; Rueff, J.; Lautier, T.; Urban, P.; Truan, G.; Kranendonk, M. Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. Int. J. Mol. Sci. 2018, 19, 3914. https://doi.org/10.3390/ijms19123914
Campelo D, Esteves F, Brito Palma B, Costa Gomes B, Rueff J, Lautier T, Urban P, Truan G, Kranendonk M. Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. International Journal of Molecular Sciences. 2018; 19(12):3914. https://doi.org/10.3390/ijms19123914
Chicago/Turabian StyleCampelo, Diana, Francisco Esteves, Bernardo Brito Palma, Bruno Costa Gomes, José Rueff, Thomas Lautier, Philippe Urban, Gilles Truan, and Michel Kranendonk. 2018. "Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450" International Journal of Molecular Sciences 19, no. 12: 3914. https://doi.org/10.3390/ijms19123914
APA StyleCampelo, D., Esteves, F., Brito Palma, B., Costa Gomes, B., Rueff, J., Lautier, T., Urban, P., Truan, G., & Kranendonk, M. (2018). Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. International Journal of Molecular Sciences, 19(12), 3914. https://doi.org/10.3390/ijms19123914









