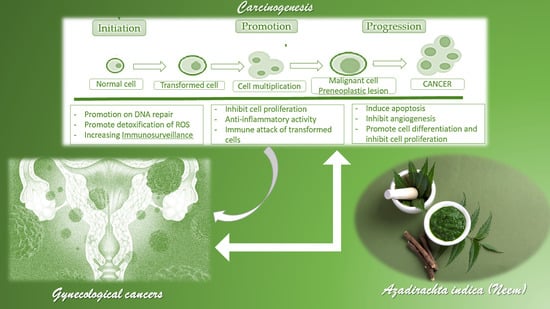
An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers
Abstract
1. Introduction
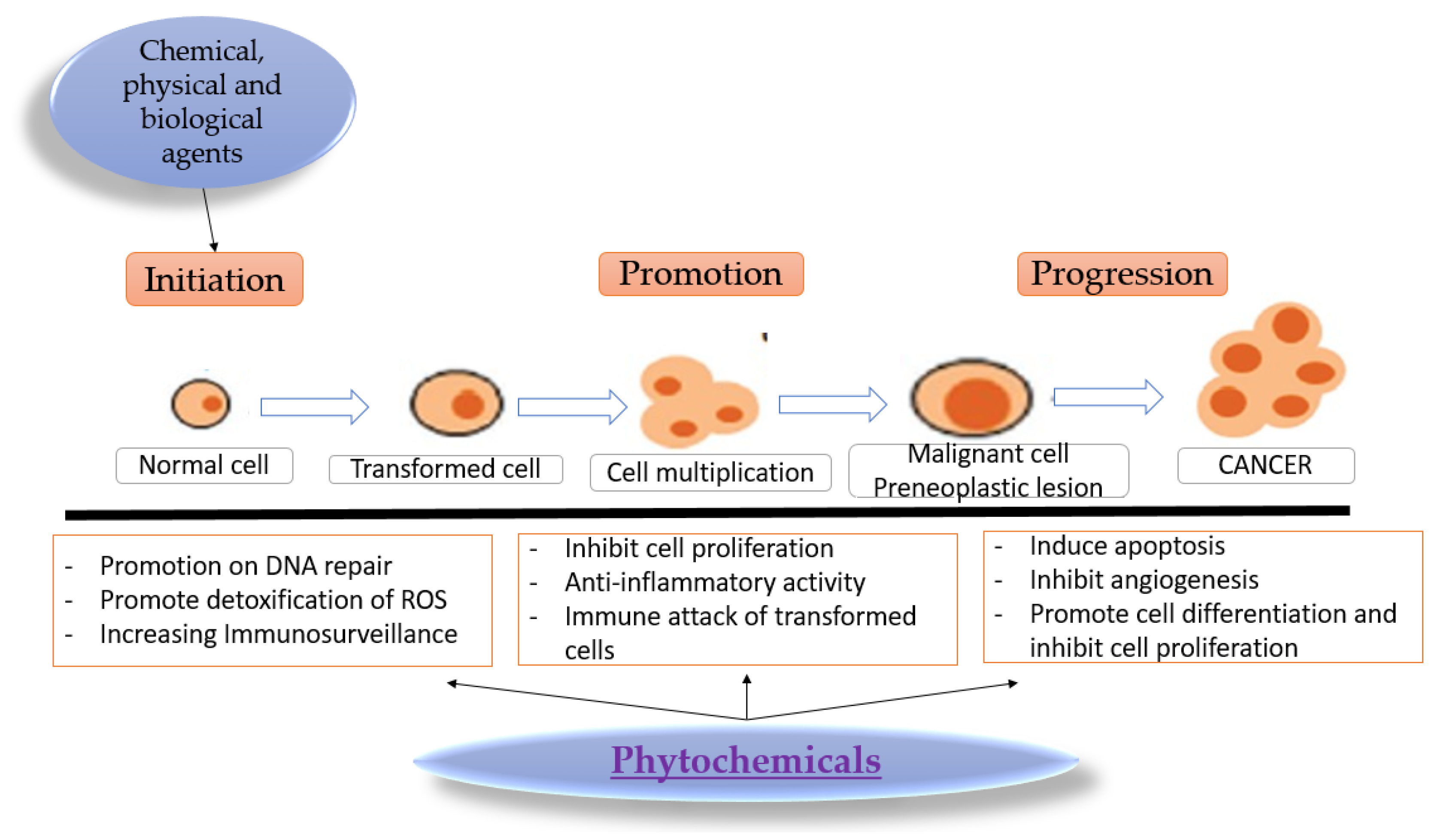
2. Carcinogenesis and Chemopreventive Activity of Phytochemicals
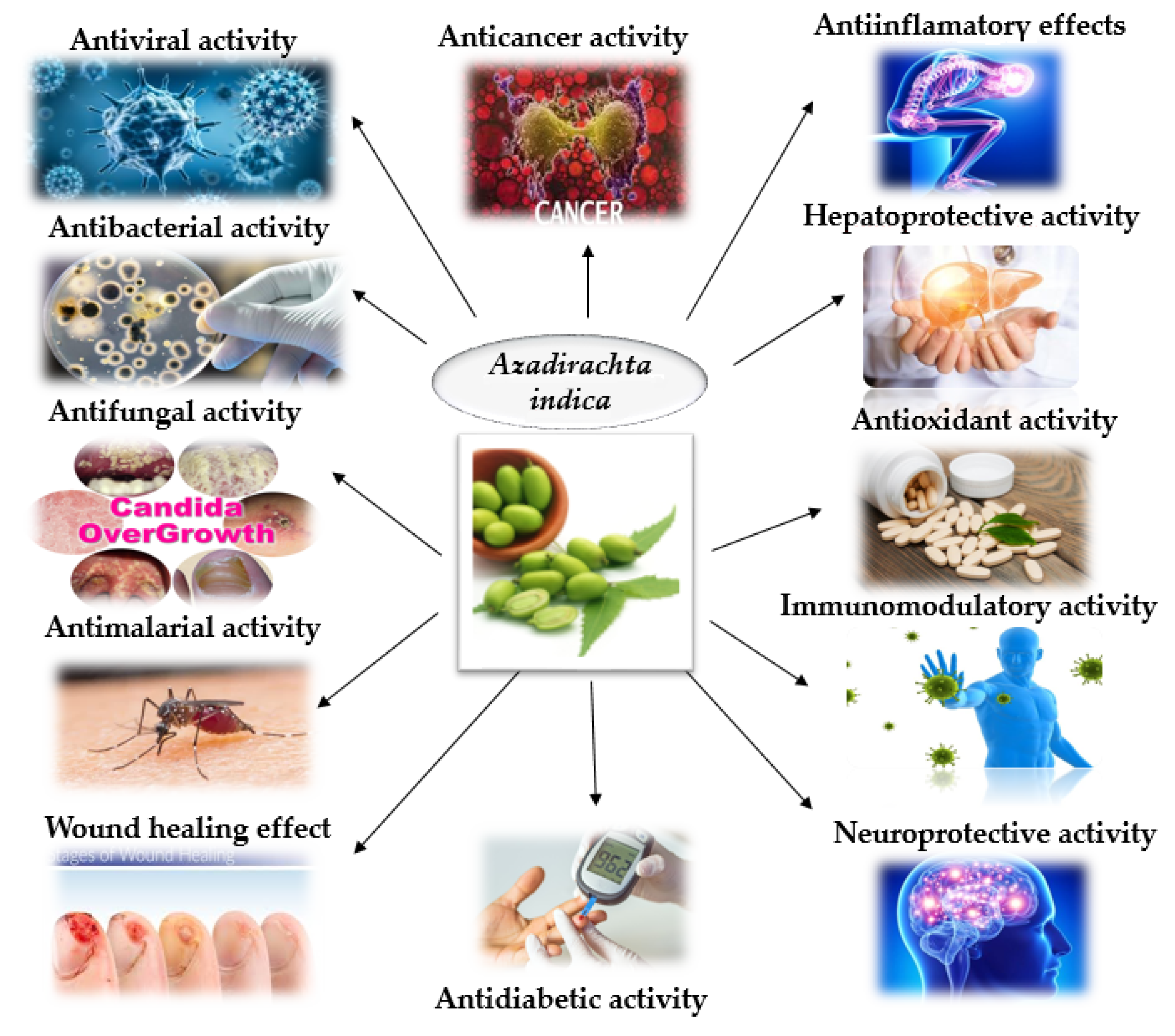
3. Neem plant (A. indica)—Botanical Characteristics and Beneficial Role in Health and Disease Prevention
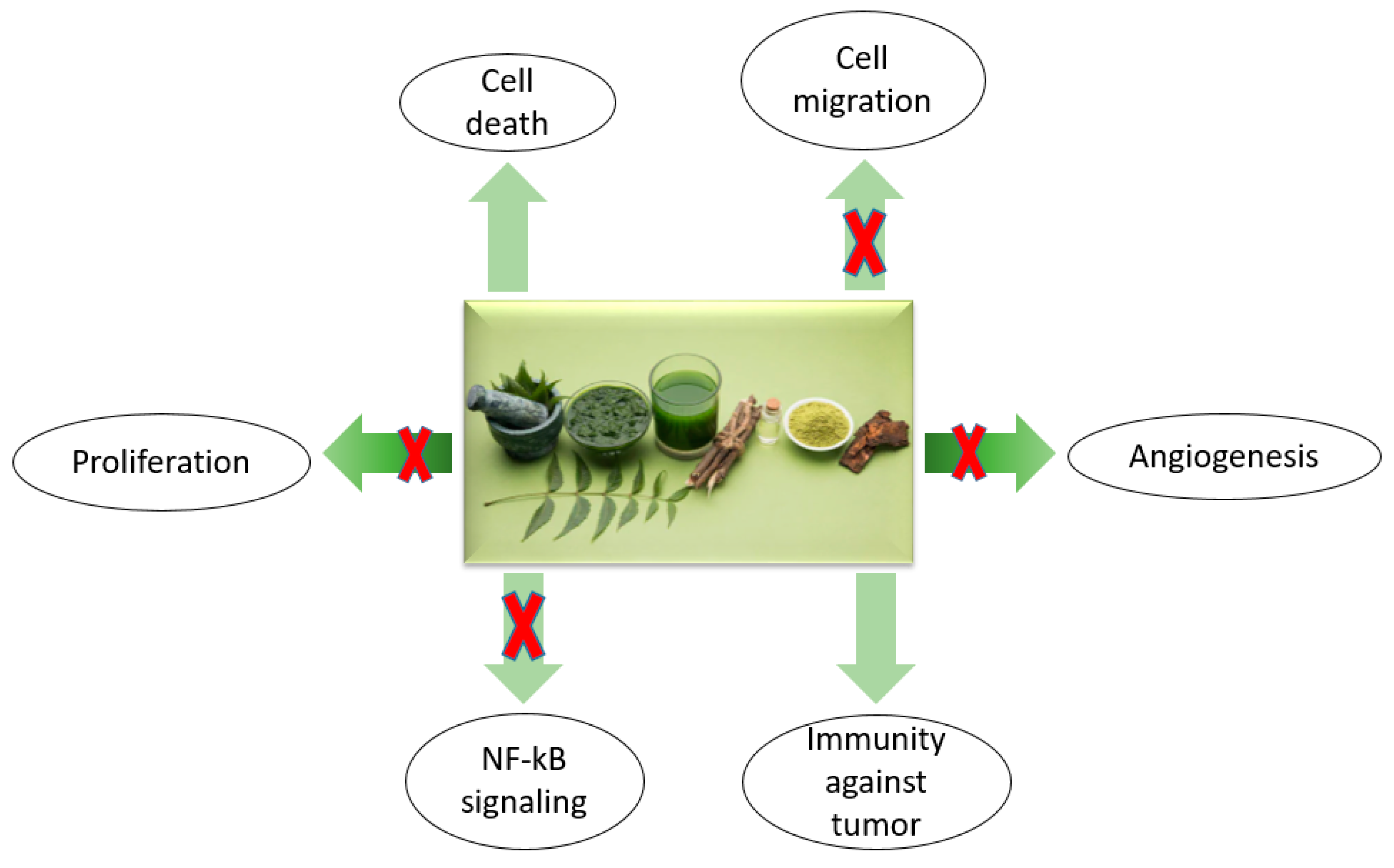
4. Neem Components and Their Effects on Cancer
- azadiradione type 1–15
- gedunin type 16–20
- azadirachtin type 21–24
- nimbin type 25–33
- degraded limonoids 34, 35.
5. Studies of Neem as Anticancer Agents in Gynecological Cancers
5.1. Cervical Cancer
5.2. Ovarian Cancer
5.3. Breast Cancer
6. Beneficial Combination between A. indica Extracts and Classical Anticancer Therapy
7. Toxicity Profile of A. indica
8. Conclusions
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| AMPK | AMP-activated protein kinase |
| Apaf-1 | apoptotic protease activating factor 1 |
| ATM-kinase | protein kinase ataxia-telangiectasia mutated |
| BALB/c | albino, laboratory-bred strain of the house mouse |
| Bax | B cell lymphoma-2 protein associated X protein |
| Bcl-2 | B cell lymphoma-2 protein |
| BTAA | breast tumor associated antigen |
| CAT | chloramphenicol acetyltransferase |
| Cdc37 | Hsp90 co-chaperone encoded by the CDC37 gene |
| Cdk2 | cyclin-dependent kinase 2 |
| CEA | carcinoembryonic antigen |
| CKI p21 | cyclin-dependent kinase inhibitor 1 |
| CTLA4 | cytotoxic T-lymphocyte antigen 4 |
| CuONPs | copper oxide nanoparticles |
| Cyclin D1 | cell cycle regulatory protein |
| CYP 1A1 | gene that encodes a member of the cytochrome P450 superfamily |
| DNA | dezoxiribonuicleic acid |
| EAD | epoxyazadiradione |
| EAF | ethyl acetate fraction |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinases |
| HDAC-2 | histone deacetylase 2 |
| HeLA | human cervical cancer cell line |
| HL60 | human leukemia cell line |
| HPV | human papilloma virus |
| HSF1 | heat shock factor 1 |
| HSP90 | heat shock protein 90 |
| H3K27Ac | modification to DNA packaging protein histone H3 |
| IFN-γ | interferon gamma |
| IGF | insulin-like growth factor |
| JNK | c-Jun N-terminal kinase |
| keap1 | Kelch-like ECH-associated protein 1 |
| LD50 | median lethal dose |
| LSIL | low-grade squamous intraepithelial lesion |
| MAPK1 | mitogen-activated protein kinase 1 |
| MAPKs | mitogen-activated protein kinase |
| MCF-7 | breast cancer cell line estrogen dependent |
| MCL-1 | induced myeloid leukemia cell differentiation protein |
| MDA MB-231 | breast cancer cell line estrogen independent |
| MEX | methanolic extract of neem oil |
| MF | methanolic fraction |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| NOS | nitric oxide syntethase |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| NTERA-2 | human embryonal carcinoma cells |
| OVCAR4 | Cellosaurus cell line |
| p53 | protein 53 |
| PARP | pharmacological inhibitors of the enzyme poly ADP ribose polymerase |
| PI3K | phosphoinositide 3-kinase |
| PP2a | phosphatase 2a |
| PTEN | phosphatase and tensin homolog gene |
| SKOV3 | ovarian cancer cell line |
| SMAD | family of structurally similar proteins that are the main signal transducers for receptors of the transforming growth factor beta superfamily |
| SOD | superoxide dismutase |
| TGF-β1 | transforming growth factor |
| Th1 | T helper 1 |
| TNF α | tumor necrosis factor |
| TNBC | triple negative breast cancer cells |
| ER+ | positive for estrogen receptors |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012 (accessed on 2 October 2018).
- Arbyn, M.; Antoine, J.; Mägi, M.; Smailyte, G.; Stengrevics, A.; Suteu, O.; Valerianova, Z.; Bray, F.; Weiderpass, E. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int. J. Cancer 2011, 128, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Balalau, D.O.; Sima, R.M.; Bacalbasa, N.; Banu, P.; Bălălău, C.; Ples, L.; Stanescu, A.D. High-grade cervical dysplasia in pregnancy–psychological and medical challenges. J. Mind Med. Sci. 2017, 4, 24–30. [Google Scholar] [CrossRef]
- Bosch, F.X.; Manos, M.M.; Munoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shan, K.V. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. J. Nat. Cancer Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Stănescu, A.D.; Pleş, L.; Edu, A.; Olaru, G.O.; Comănescu, A.C.; Potecă, A.G.; Comănescu, M.V. Different patterns of heterogeneity in ovarian carcinoma. Rom. J. Morphol. Embryol. 2015, 56, 1357–1363. [Google Scholar]
- Razi, S.; Ghoncheh, M.; Mohammadian-Hafshejani, A.; Aziznejhad, H.; Mohammadian, M.; Salehiniya, H. The incidence and mortality of ovarian cancer and their relationship with the Human Development Index in Asia. Ecancermedicalscience 2016, 10, 628. [Google Scholar] [CrossRef]
- Ionescu, C.A.; Vlădăreanu, S.; Pleş, L.; Dimitriu, M.C.T.; Furău, G.O.; Vlădescu, T.C.; Calin, A.M.; Oprescu, N.D. Synchronous bilateral primary ovarian carcinoma-case presentation. Rom. J. Morphol. Embryol. 2017, 58, 219–223. [Google Scholar]
- Beral, V. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008, 371, 303–314. [Google Scholar] [CrossRef]
- Poorolajal, J.; Jenabi, E.; Masoumi, S.Z. Body mass index effects on risk of ovarian cancer: A meta- analysis. Asian Pac. J. Cancer Prev. 2014, 15, 7665–7671. [Google Scholar] [CrossRef]
- Riman, T.; Nilsson, S.; Persson, I.R. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstet. Gynecol. Scand. 2004, 83, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Tsilidis, K.K.; Allen, N.E.; Key, T.J.; Dossus, L.; Lukanova, A.; Bakken, K.; Lund, E.; Fournier, A.; Overvad, K.; Hansen, L.; et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2011, 105, 1436–1442. [Google Scholar] [CrossRef]
- Chye, G.L.C.; Yahaya, H. Second Report of the National Cancer Registry, Cancer Incidence in Malaysia 2003; National Cancer Registry: Kuala Lumpur, Malaysia, 2004; Available online: http://www.nilaimc.com/downloads/Malaysia_National_Cancer_Report_2003.pdf (accessed on 2 October 2018).
- Weber, J.; McClure, M. Oncogenes and cancer. Br. Med. J. 1987, 296, 1246–1248. [Google Scholar] [CrossRef]
- Howes, M.J.; Simmonds, M.S. The role of phytochemicals as micronutrients in health and disease. Clin. Nutr. Metab. Care 2014, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agricult. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Nagini, S. Cancer chemoprevention by dietary phytochemicals: Promises and pitfalls. Curr. Pharm. Biotechnol. 2012, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Waladkhani, A.R.; Clemens, M.R. Effect of dietary phytochemicals on cancer development (review). Int. J. Mol. Med. 1998, 1, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Ogbuewu, I.P. Physiological Responses of Rabbits fed Graded Levels of Neem (Azadirachta indica) Leaf meal. Master’s Thesis, Federal University of Technology, Owerri, Nigeria, 2008. [Google Scholar]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between Phytochemicals. The Multi-Target Therapeutic concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Alzohairy, A.M. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid. Based Complement. Alternat. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Al-Bukhari, M.I.; Al-Bukhari, S. The Collection of Authentic Sayings of Prophet Mohammad (Peace Be upon Him), Division 71 on Medicine, 2nd ed.; Hilal Yayinlari: Ankara, Turkey, 1976. [Google Scholar]
- Tiwari, R.; Verma, A.K.; Chakraborty, S.; Dhama, K.; Vir Singh, S. Neem (Azadirachta indica) and its potential for safeguarding health of animals and humans: A review. J. Biol. Sci. 2014, 14, 110–123. [Google Scholar] [CrossRef]
- Brahmachari, G. Neem—An omnipotent plant: A retrospection. Chem. Biol. Chem. 2004, 5, 408–421. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009, 8, 601–620. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alzohairy, M.A.; Khan, M.A.; Aly, S.M. Therapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhatar, H. Green Tea Constituent Epigallocatechin-3-Gallate And Induction Of Apoptosis And Cell Cycle Arrest In Human Carcinoma Cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.U. Basis of carcinogenesis. Health Admin. 2018, 17, 16–24. [Google Scholar]
- Loeb, L.A.; Harris, C.C. Advances in chemical carcinogenesis: A historical review and prospective. Cancer Res. 2008, 68, 6863–6872. [Google Scholar] [CrossRef] [PubMed]
- Land, H.; Parada, L.F.; Weinberg, R.A. Cellular oncogenes and multi-step carcinogenesis. Science 1983, 222, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Upton, A.C.; Jenkins, V.K.; Conklin, J.W. Myeloid leukemia in the mouse. Ann. N. Y. Acad. Sci 1964, 114, 189. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Shen, Z.; Hu, X.; Sun, L.; Zhu, X. Transforming growth factor-β1 in carcinogenesis, progression, and therapy in cervical cancer. Tumor Biol. 2016, 37, 7075–7083. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Archiv. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.; Brosh, R.; Buganim, Y.; Rotter, V. Inactivation of the p53 tumor suppressor gene and activation of the Ras oncogene: Cooperative events in tumorigenesis. Discov. Med. 2010, 9, 448–454. [Google Scholar] [PubMed]
- Roudebush, P.; Davenport, D.J.; Novotny, B.J. The use of nutraceuticals in cancer therapy. Vet. Clin. N. Am. 2004, 34, 249–269. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef]
- See, D.; Mason, S.; Roshan, R. Increased tumor necrosis factor alpha (TNF-alpha) and natural killer cell (NK) function using an integrative approach in late stage cancers. Immunol. Invest. 2002, 31, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Maru, G.B.; Hudlikar, R.R.; Kumar, G.; Gandhi, K.; Mahimkar, M.B. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J. Biol. Chem. 2016, 7, 88–99. [Google Scholar] [CrossRef]
- Harris, C.C. Chemical and physical carcinogenesis: Advances and perspectives for the 1990s. Cancer Res. 1991, 51, 5023s–5044s. [Google Scholar] [PubMed]
- Biswas, K.I.; Chattopadhyay, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of Neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Drabu, S.; Khatr, S.; Babu, S. Neem: Healer of all ailments. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 120–126. [Google Scholar]
- Butterworth, J.H.; Morgan, F.D. Isolation of a substance that suppress feeding in locusts. J. Chem. Soc. Chem. Commun. 1968, 23–24. [Google Scholar] [CrossRef]
- Isman, M.B.; Koul, O.; Luczynski, A.; Kaminski, J. Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 1990, 38, 1406–1411. [Google Scholar] [CrossRef]
- Kaura, S.K.; Gupta, S.K.; Chowdhury, J.B. Morphological and oil content variation in seeds of Azadirachta indica A., Juss. (Neem) from northern and western provenances of India. Plant Foods Hum. Nutr. 1998, 52, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, O.P.; Kumar, V.; Behl, H.M. Variability in neem (Azadirachta indica) with respect to azadirachtin content. J. Agric. Food Chem. 2003, 5, 910–915. [Google Scholar] [CrossRef]
- Uko, O.J.; Kamalu, N.T. The neem tree-uses and potentials. Niger. J. Exp. Appl. Biol. 2001, 2, 223–229. [Google Scholar]
- Lale, N.E.S. Bio-activity and Limitation against wide spread use of neem products for the management of insect pests. Niger. J. Appl. Biol. 2002, 3, 115–125. [Google Scholar]
- Djibril, D.; Mamadou, F.; Gerard, V.; Geuya, M.D.C.; Oumar, S.; Luc, R. Physical characteristics, Chemical composition and Distribution of constituents of the Neem seeds (Azadirachta indica A. Juss) collected in Senegal. Res. J. Chem. Sci. 2015, 5, 52–58. [Google Scholar]
- Djenontin Tindo, S.; Amusant, N.; Dangou, J.; Wotto, D.V.; Avlessi, F.; Dahouénon-Ahoussi, E.; Lozano, P.; Pioch, D.; Sohounhloué, K.C.D. Screening of repellent, termiticidal and preventive activities on wood, of Azadirachta indica and Carapa procera (Meliaceae) seeds oils. ISCA J. Biol. Sci. 2012, 1, 25–29. [Google Scholar]
- Ara, I.; Siddiqui, S.; Faizi, S.; Siddiqui, S. Two new terpenoids from root bark of Azadirachta indica. J. Nat. Prod. 1989, 52, 1209–1213. [Google Scholar] [CrossRef]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. 2005, 149–156. [Google Scholar] [CrossRef]
- Xu, J.; Song, X.; Yin, Z.Q.; Cheng, A.C.; Jia, R.Y.; Deng, Y.X.; Ye, K.C.; Shi, C.F.; Lv, C.; Zhang, W. Antiviral activity and mode of action of extracts from neem seed kernel against duck plague virus in vitro. Poult. Sci. 2012, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Miana, G.A.; Ahmad, S.I. Clinical investigation of hypoglycemic effect of seeds of Azadirachta indica in type 2 (NIDDM) diabetes mellitus. Pak. J. Pharm. Sci. 2006, 19, 322–325. [Google Scholar] [PubMed]
- Paul, R.; Prasad, M.; Sah, N.K. Anticancer biology of Azadirachta indica L (neem): A mini review. Free Radic. Res. 2011, 12, 467–476. [Google Scholar] [CrossRef]
- Sarkar, K.; Bose, A.; Laskar, S.; Choudhuri, S.; Dey, S.; Roychowdhury, P.K.; Baral, R. Antibody response against neem leaf preparation recognizes carcinoembryonic antigen. Int. Immunopharmacol. 2007, 7, 306–312. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Spano, D.; Zollo, M. Tumor microenvironment: A main actor in the metastasis process. Clin. Exp. Metastasis 2012, 29, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Young, C.Y.; Kohli, M.; Karnes, R.J.; Klee, E.W.; Holmes, M.W.; Tindall, D.J.; Donkena, K.V. Antiangiogenic Effects and Therapeutic Targets of Azadirachta indica Leaf Extract in Endothelial Cells. Evid. Based Complement. Alternat. Med. 2012, 2012, 303019. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Berardi, V.; Risuleo, G. Differential cytotoxicity of MEX: A component of neem oil whose action is exerted at the cell membrane level. Molecules 2008, 14, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Kumar, S.; Yadav, N.; Chandra, D. Neem components as potential agents for cancer prevention and treatment. Biochim. Biophys. Acta 2014, 1846, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Thota, N.; Barua, C.C.; Sistla, R. Chemopreventive and therapeutic effects of nimbolide in cancer: The underlying mechanisms. Toxicol. In Vitro 2014, 28, 1026–1035. [Google Scholar] [CrossRef]
- Kashif, M.; Hwang, Y.; Hong, G.; Kim, G. In vitro comparative cytotoxic effect of Nimbolide: A limonoid from Azadirachta indica (Neem tree) on cancer cell lines and normal cell lines through MTT assay. Pak. J. Pharm. Sci. 2017, 30, 967–973. [Google Scholar]
- Uddin, S.J.; Nahar, L.; Shilpi, J.A.; Shoeb, M.; Borkowski, T.; Gibbons, S.; Middleton, M.; Byres, M.; Sarker, S.D. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line. In Vitro Phytother. Res. 2007, 21, 757–761. [Google Scholar] [CrossRef]
- Kamath, S.G.; Chen, N.; Xiong, Y.; Wenham, R.; Apte, S.; Humphrey, M.; Cragun, J.; Lancaster, J.M. Gedunin, a novel natural substance, inhibits ovarian cancer cell proliferation. Int. J. Gynecol. Cancer 2009, 19, 1564–1569. [Google Scholar] [CrossRef]
- Nagini, S. Neem limonoids as anticancer agents: modulation of cancer hallmarks and oncogenic signaling. Enzymes 2014, 36, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Gonzalez, E.; Nandy, S.B.; Arumugam, A.; Camacho, F.; Medel, J.; Alabi, D.; Lakshmanaswamy, R. Gedunin inhibits pancreatic cancer by altering sonic hedgehog signaling pathway. Oncotarget 2017, 8, 10891–10904. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Afshan, F.; Gulzar, T.; Sultana, R.; Naqvi, S.N.; Tariq, R.M. Tetracyclic triterpenoids from the leaves of Azadirachta indica and their insecticidal activities. Chem. Pharm. Bull. 2003, 51, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Ishii, K.; Noto, T.; Takahashi, A.; Tabata, K.; Suzuki, T.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem). J. Nat. Prod. 2011, 74, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Verma, K.K.; Singh, M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int. J. Pharm. Pharm. Sci. 2014, 6, 444–447. [Google Scholar]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Malathi, R.; Rajar, S.S.; Kumar, R.M.; Narasimhan, S.; Ravikumar, K. Epoxyazadiradione. Acta Crystallogr. 2007, E63, o2483–o2485. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Sripriya, P.; Karunagaran, D.; Nagini, S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic. Res. 2010, 44, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Vas, A.; Goala, P.; Gheewala, T.M.; Rizvi, T.A.; Hussain, A. Ethanolic Neem (Azadirachta indica) Leaf Extract Prevents Growth of MCF-7 and HeLa Cells and Potentiates the Therapeutic Index of Cisplatin. J. Oncol. 2014, 2014, 321754. [Google Scholar] [CrossRef]
- Shukla, D.P.; Shah, K.P.; Rawal, R.M.; Jain, N.K. Anticancer and Cytotoxic Potential of Turmeric (Curcuma longa), Neem (Azadirachta indica), Tulasi (Ocimum sanctum) and Ginger (Zingiber officinale) Extracts on HeLa Cell line. Int. J. Life Sci. Sci. Res. 2016, 2, 309–315. [Google Scholar] [CrossRef]
- Soumyabrata, R.; Subhasis, B.; Saptak, B.; Avishek, B.; Smarajit, P.; Parthasarathi, B.; Jaydip, B.; Shyamal, G.; Tathagata, C.; Anamika, B.; et al. Neem leaf glycoprotein overcomes indoleamine 2,3 dioxygenase mediated tolerance in dendritic cells by attenuating hyperactive regulatory T cells in cervical cancer stage IIIB patients. Hum. Immun. 2013, 74, 1015–1023. [Google Scholar] [CrossRef]
- Vasenwala, S.M.; Seth, R.; Haider, N.; Islam, N.; Kan, T.; MAheshwari, V.; Rehman, S. A study on antioxidant and apoptotic effect of Azadirachta indica (neem) in cases of cervical cancer. Arch. Gynecol. Obstet. 2012, 286, 1255–1259. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- Shukla, S.; Bharti, A.C.; Hussain, S.; Mahata, S.; Hedau, S.; Kailash, U.; Kashyap, V.; Bhambhani, S.; Roy, M.; Batra, S.; et al. Elimination of high-risk human papillomavirus type HPV16 infection by ′Praneem′ polyherbal tablet in women with early cervical intraepithelial lesions. J. Cancer Res. Clin. Oncol. 2009, 135, 1701–1709. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer HeLa cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F. Effects of quercetin on proliferation, apoptosis, adhesion and migration and invasion of HeLa cells. Eur. J. Gynecol. Oncol. 2009, 30, 60–64. [Google Scholar]
- Jung, J.H.; Lee, J.O.; Kim, J.H.; Lee, S.K.; You, G.Y.; Park, S.H.; Park, J.M.; Kim, E.K.; Suh, P.G.; An, J.K.; et al. Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J. Cell Physiol. 2010, 223. [Google Scholar] [CrossRef]
- Tharmarajah, L.; Samarakoon, S.R.; Ediriweera, M.K.; Piyathilaka, P.; Tennekoon, K.H.; Senathilake, K.S.; Rajagopalan, U.; Galhena, P.B.; Thabrew, I. In Vitro Anticancer Effect of Gedunin on Human Teratocarcinomal (NTERA-2) Cancer Stem-Like Cells. BioMed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Patwardhan, C.A.; Fauq, A.; Peterson, L.B.; Miller, C.; Blagg, B.S.J.; Chadli, A. Gedunin inactivates the co-chaperone p23 causing cancer cell death by apoptosis. J. Biol. Chem. 2013, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.H.; Chandra Mohan, K.P.V.; Jagannadha Rao, A.; Nagini, S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest. New Drugs 2009, 27, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Shrivastav, T.G.; Chaube, S.K. An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int. J. Appl. Basic. Med. Res. 2013, 3, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chaube, S.K.; Shrivastav, T.G.; Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.K. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 2014, 3, 464. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Wei, X.W.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G.; et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 22. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Panici, P.B.; Ranelletti, F.O.; Ferrandina, G.; de Vincenzo, R.; Piantelli, M.; Masciullo, V.; Bonanno, G.; Isola, G.; Mancuso, S. Quercetin enhances transforming growth factor β1, secretion by human ovarian cancer cells. Int. J. Cancer 1994, 57. [Google Scholar] [CrossRef]
- Shoemaker, M.L.; White, M.C.; Wu, M.; Weir, H.K.; Romieu, I. Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res. Treat. 2018, 169, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Krisnaveni, P.; Ajantha, S.; Narayani, M.; Suherman, J.; Susi, E.; Asmah, R. Confocal microscopy on the effect of Neem leaf extract on MCF-7 breast cancer cell lines. In Proc. Seminar Update on Microscopy and Microanalysis Serdang; UPM Press: Selangor, Malaysia, 2002; pp. 48–49. [Google Scholar]
- Subapriya, R.; Kumaraguruparan, R.; Abraham, S.K.; Nagani, S. Protective effects of ethanolic neem leaf extract on N-Methyl-N-nitro-N-nitrosoguanidine-induced genotoxicity and oxidative stress in mice. Drug Chem. Toxicol. 2004, 27, 15–26. [Google Scholar] [CrossRef]
- Othman, F.; Motalleb, G.; Peng, S.L.; Rahmat, A.; Basri, R.; Pei Pei, C. Effect of Neem Leaf Extract (Azadirachta indica) on c-Myc Oncogene Expression in 4T1 Breast Cancer Cells of BALB/c Mice. Cell J. 2012, 14, 53–60. [Google Scholar]
- Arumugam, A.; Agullo, P.; Boopalan, T.; Nandy, S.; Lopez, R.; Gutierrez, C.; Narayan, M.; Rajkumar, L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol. Ther. 2014, 15, 26–34. [Google Scholar] [CrossRef]
- Elumalai, P.; Gunadharini, D.N.; Senthilkumar, K.; Banudevi, S.; Arunkumar, R.; Benson, C.S.; Sharmila, G.; Arunakaran, J. Ethanolic neem (Azadirachta indica A. Juss) leaf extract induces apoptosis and inhibits the IGF signaling pathway in breast cancer cell lines. Biomed. Prev. Nutr. 2012, 2, 59–68. [Google Scholar] [CrossRef]
- Ahmad, R.; Misra, A.; Trivedi, A.; Khan, M.A. Evaluatioan of in vitro cytotoxic activity of ethanolic extract of Azadirachta indica leaves as a function of pH on human breast cancer cell line MDA-MB 231. J. Basic Clin. Pharm. 2017, 8, S72–S79. [Google Scholar] [CrossRef]
- Mandal-Ghosh, I.; Chattopadhyay, U.; Baral, R. Neem leaf preparation enhances Th1 type immune response and anti-tumor immunity against breast tumor associated antigen. Cancer Immun. 2007, 30, 7–8. [Google Scholar]
- Vinothini, G.; Manikandan, P.; Anandan, R.; Nagini, S. Chemoprevention of rat mammary carcinogenesis by Azadirachta indica leaf fractions: Modulation of hormone status, xenobiotic-metabolizing enzymes, oxidative stress, cell proliferation and apoptosis. Food Chem. Toxicol. 2009, 47, 1852–1863. [Google Scholar] [CrossRef]
- Elumalai, P.; Gunadharini, D.N.; Senthilkumar, K.; Banudevi, S.; Arunkumar, R.; Benson, C.S.; Sharmila, G.; Arunakaran, J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012, 215, 131–142. [Google Scholar] [CrossRef]
- Elumalai, P.; Arunkumar, R.; Benson, C.S.; Sharmila, G.; Arunakaran, J. Nimbolide inhibits IGF-I-mediated PI3K/Akt and MAPK signalling in human breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2014, 32, 476–484. [Google Scholar] [CrossRef]
- Brandt, G.E.; Schmidt, M.D.; Prisinzano, T.E.; Blagg, B.S.J. Gedunin, a Novel Hsp90 Inhibitor: Semisynthesis of Derivatives and Preliminary Structure−Activity Relationships. J. Med. Chem. 2008, 51, 6495–6502. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, J.Y.; Lee, J.Y.; Kang, C.M.; Kwon, H.J.; Yoo, Y.D.; Kim, T.W.; Lee, Y.S.; Lee, S.J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Bae, S.M.; Ahn, W.S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res. 2008, 31, 1281. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H. Neoadjuvant chemotherapy for cervical cancer. Exp. Opin. Pharmacother. 2003, 4, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Charfare, H.; Limongelli, S.; Purushotham, A.D. Neoadjuvant chemotherapy in brast cancer. BJS 2005, 92, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vicenzo, R.; Bonano, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rummi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.E.A.; Othman, M.S.; Aref, A.M. Azadirachta indica Attenuates Cisplatin-Induced Nephrotoxicity and Oxidative Stress. Biol. Med. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Shareef, M.; Akhatar, M.D. Neem (Azadirachta indica) and its potential for safeguarding health, prevention and treatment of diseases. Matrix Sci. Med. 2018, 2, 4–8. [Google Scholar] [CrossRef]
- Lin, C.; Yu, Y.; Zhao, H.G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radioter. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef]
- Boeke, S.J.; Boersma, M.G.; Alink, G.M.; Van Loon, J.J.A.; Van Huis, A.; Dicke, M.; Rietjens, I.M.C.M. Safety evaluation of neem (Azadirachta indica) derived pesticides. J. Ethnopharmacol. 2004, 94, 25–41. [Google Scholar] [CrossRef]
- Okpanyi, S.N.; Ezeukwu, G.C. Anti-inflammatory and antipyretic activities of Azadirachta indica. Planta Med. 1981, 41, 34–39. [Google Scholar] [CrossRef]
- Kupradinun, P.; Tepsuwan, A.; Tanthasri, N.; Meesiripan, N.; Tunsakul, S.; Tompat, W.; Jarratwisarutporn, Y.; Kusamran, W.R. Toxicity testing of flowers of neem tree. Thai J. Vet. Med. 2010, 40, 47–55. [Google Scholar]
- Dorababu, M.; Joshi, M.C.; Bhawani, G.; Kumar, M.M.; Chaturvedi, A.; Goel, R.K. Effect of aqueous extract of neem (Azadirachta indica) leaves on offensive and diffensive gastric mucosal factors in rats. Indian J. Physiol. Pharmacol. 2006, 50, 241–249. [Google Scholar] [PubMed]
- Tarboush, F.M.A.; Ashamoui, H.M.E. Toxic and teratogenic effects of Azadirachtin of Neemix-4.5 on fetuses and pups of SWR/J mice. Eyg. J. Hosp. Med. 2004, 15, 30–39. [Google Scholar]
- Srivastava, M.K.; Raizada, R.B. Lack of toxic effects of technical azadirachtin during postnatal development of rats. Food Chem. Toxicol. 2007, 45, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Glinsukon, T.; Somjaree, P.; Piyachaturawat, P.; Thebtaranonth, Y. Acute toxicity of nimbolide and nimbic acid in mice, rats and hamsters. Toxicol. Lett. 1986, 30, 159–166. [Google Scholar] [CrossRef]
- Singh, T.; Saini, H.S.; Ranganayakulu, S.V.; Ravi, S.; Sudhakar, K. Molecular Docking Assisted Isolation of Azadirachtin-A., from Seeds of Azadirachta indica Extract against Cervical Cancer. Ind. J. Sci. Technol. 2017, 10. [Google Scholar] [CrossRef]
- Pooladanda, V.; Bandi, S.; Mondi, S.R.; Gottumukkala, K.M.; Godugu, C. Nimbolide epigenetically regulates autophagy and apoptosis in breast cancer. Toxicol. In Vitro 2018, 51, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Sahabjada, R.A.; Misra, A. Effect of alkaline pH on cytotoxicity profile of neem (Azadirachta indica) ethanolic extract against breast cancer cell line MDA-MB-231. Eur. J. Integr. Med. 2018, 24, 1–7. [Google Scholar] [CrossRef]
- Kumar, D.; Haldar, S.; Gorain, M.; Kumar, S.; Mulani, F.A.; Yadav, A.S.; Miele, L.; Thulasiram, H.V.; Kundu, G.C. Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway. BMC Cancer 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Manna, S.; Chattopadhyay, S.; Mondal, D.; Chattopadhyay, D.; Raj, A.; Das, S.; Bag, B.G.; Roy, S. Azadirachta indica leaves mediated green synthesized cooper oxide nanoparticles induce apoptosis through activation of TNF-α and caspase signaling pathway against cancer cells. J. Saudi Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Baral, R.; Chattopadhyay, U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004, 4, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Barik, S.; Sarkar, K.; Bose, A.; Baral, R.; Laskar, S. Chemical investigation of neem leaf glycoprotein used as immunoprophylactic agents for tumor growth restriction. Int. J. Pharm. Pharm. Sci. 2015, 7, 195–199. [Google Scholar]
- Banerjee, S.; Ghosh, T.; Barik, S.; Das, A.; Ghosh, S.; Bhuniya, A.; Bose, A.; Baral, R. Neem leaf glycoprotein prophylaxis transduces immune dependent stop signal for tumor angiogenic switch within tumor microenvironment. PLoS ONE 2014, 9, e110040. [Google Scholar] [CrossRef] [PubMed]
| Number | Neem Constituent/Extract | Cell Type | Function | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| 1 | Azadirachtin | HeLa | Cell apoptosis | Enhances CKI p21 expression, decreases cyclin B and cyclin D1 levels, both leading to G0/G1 cell cycle arrest | [85] |
| Cell cycle interruption | Induces the modulation of Bcl-2 protein family upon the exposure | ||||
| HeLa | Cell apoptosis and tumor volume reduction | Interacts with cyclin E, causes phosphorylation of the same, prevents the G1/S phase protein expression | [131] | ||
| 2 | Nimbolide | HeLa | Cell apoptosis | Induces the modulation of Bcl-2 protein family | [85] |
| Cell cycle interruption | Induces the expression of CKI p21, decreases cyclin B and cyclin D1 level, both leading to G0/G1 cell cycle arrest | ||||
| BeWo | Cells apoptosis and disruption of BeWo cell cycle progression | Decreases Bcl-2/Bax report and increases the expression of Apaf-1 and caspase-3 | [97] | ||
| MDA-MB-231, MCF-7 | Inhibits cell proliferation (IC50 values of 1.97 ± 0.24 and 5.04 ± 0.25 μM), induces autophagy | Reduces Bcl-2, induces Bax and caspases protein expression with modulation of HDAC-2 and H3K27Ac expression. Autophagy signal is induced by increasing Beclin 1, LC3B, and decreasing p62 and mTOR expression | [132] | ||
| MCF-7, MDA-MB-231 | Cell apoptosis | Induces the cleavage of pro-caspase-3, pro-caspase-8, and PARP; modulation of the IGF signaling molecules | [111] | ||
| 3 | Gedunin | SKOV3, OVCAR4, and OVCAR8 | Inhibits cell proliferation | Up to 80% decrease in cell proliferation | [74] |
| NTERA-2 | Inhibits cell proliferation | Induces the inhibition of Hsp90, cochaperone Cdc37, and HSP proteins (AKT, ErbB2, and HSF1) and upregulation of Bax and p53 | [95] | ||
| SKOV3, OVCAR4, and OVCAR8 | Cell apoptosis | Caspase-7-mediated cleavage of the cochaperone p23 | [96] | ||
| MCF-7 and SkBr3 | Inhibits cell proliferation | Inhibition of Hsp90 | [113] | ||
| 4 | Quercetin | HeLa | Cell apoptosis | Induces G2/M phase interruption during the cellular cycle and mitochondrial apoptosis through a mechanism dependent of p53; also induces modulation of NF-κB family members | [92] |
| HeLa | Antimetastatic function | Inhibits adhesion, migration, and invasion of tumor cells | [93] | ||
| HeLa | Antioxidant effect | Increases AMPK phosphorylation to downstream acetyl-coA carboxylase. Activates EGFR by suppressing PP2a and SHP-2, and induces the tyrosine phosphorylation of Cbl by increasing the interaction between EGFR and Cbl | [94] | ||
| OVCA 433 | Cell-growth-inhibitory activity | Modulation of transforming growth factor β1 (TGF-β1) production | [101] | ||
| MCF-7 | Cell-growth-inhibitory activity | Inhibition of cell cycle progression and subsequent G2 arrest | [114] | ||
| MDA-MB-231 | Cell apoptosis | Activation of caspase-3, caspase-8, and caspase-9, increasing the abundance of Bax protein and decreasing the level of antiapoptotic protein Bcl-2 | [115] | ||
| 5 | Ethanolic/methanolic/aqueous neem leaf extract | HeLa | Cell apoptosis | Modulation of the expression of bax, cyclin D1, and cytochrome P450 monooxygenases (CYP 1A1 and CYP 1A2) | [87] |
| HeLa | Cell apoptosis | Intrinsic: cytochrome c, Bcl-2 proteins Extrinsic: death receptors | [86] | ||
| Rats oocytes | Granulosa cell apoptosis | Increases p53, Bax, and p53 expression, decreases Bcl2 expression, increases cytochrome c concentration, and induces DNA fragmentation | [99] | ||
| MCF-7 and MDA-MB-231 | Cell apoptosis and cell-growth-inhibitory activity | Decreases the protein expression of insulin-like growth factor (IGF) signaling molecules IGF-1R, Ras, Raf, p-Erk, p-Akt, and cyclin D1. | [107] | ||
| MDA-MB-231 | Cell-growth-inhibitory activity, antioxidant activity | Decreases the growth of cancer cells at a concentration of 1600 μg/mL and pH 8.6. Alkaline pH increases the cytotoxic potential of neem | [133] | ||
| MCF-7 | Immunomodulatory effect | Induces Th1 immune response as evidence of the secretion of IFN-gamma and increases the production of IgG2a antibody in immunized mice | [109] | ||
| 6 | Neem leaf glycoprotein | cervical cancer stage IIIB cells | Relieves tumor immune suppression | Inhibits the induction of indoleamine 2,3 dioxygenase, limits the expression CTLA4 on Tregs, and induces normal maturation of dendritic cells | [88] |
| 7 | Epoxyazadiradione | TNBC MDA-MB-231 and ER+ MCF-7 breast cancer cells | Cell apoptosis, antimetastatic, and antiangiogenic | Inhibits the expression of proangiogenic and prometastatic genes Cox2, OPN, VEGF, and MMP-9. Attenuates PI3K/Akt-mediated AP-1 activation | [134] |
| 8 | Copper oxide nanoparticles of neem (CuONPs) | MCF-7 and HeLa | Cell apoptosis | Decreases proinflammatory cytokine level and proapoptotic protein expression, generates ROS inside the cancer cells, and induces DNA fragmentation | [135] |
| Neem Extract | Animal Model | Administration Protocol | Mechanism of Action | Results | Reference |
|---|---|---|---|---|---|
| Breast Cancer | |||||
| Ethanolic leaf extract | BALB/c female mice with 4T1 induced breast cancer | Intratumoral injections of 500mg/kg of the neem extract every 48 h for 4 weeks after the tumor developed | Suppression of c-Myc oncogene expression | [105] | |
| Ethanolic leaf extract | Sprague Dawley female rats with NMU-induced carcinogenesis | 4 mg/kg (p.o) daily, 4 weeks | Increases caspase expression and p53, Bax, and Bad proteins and decreases MAPK1, Bcl-2, cyclin D1, and Cdk 2 activity | Suppressed tumor progression | [106] |
| Aqueous leaf extract | Swiss mice and Balb/c mice after BTAA-induced carcinogenesis | 1 unit/week, 4 weeks | Decreases Th1 immunity cells and Il-10 and increases NK cells and IFN-gamma | Enhanced immune response to tumor vaccine | [109] |
| Methanolic leaf extract and ethyl acetate leaf fraction | Sprague Dawley female rats with DMBA-induced carcinogenesis | 1–10 mg/kg (p.o), three times/week, for 12 weeks | Increases apoptosis through increased Bcl-2, NF-κB, and estradiol expression and decreases SOD, CAT, and caspase-3 activity | Suppressed tumor progression | [108] |
| Aqueous leaf extract | Swiss female mice with Ehrlich carcinoma | 1 unit/week (p.o.), 4 weeks | Immunomodulation | Suppressed tumor growth | [136] |
| Ethanolic leaf extract | Balb/c female mice with 4T1 xenograft | 250.5 mg/kg twice per day for 4 weeks | Suppression of c-Myc oncogene expression | [105] | |
| Leaf glycoprotein | Swiss female mice with Ehrlich carcinoma | 0.25 mg daily for 4 weeks | Reduced tumoral volume | [137] | |
| Leaf glycoprotein | Swiss female mice with Ehrlich carcinoma | 25 µg (s.c.) one time per week, 4 weeks | Suppression of VEGF and VEGFR2 expression | Normalized angiogenesis and suppressed tumor growth | [138] |
| Cervical Cancer | |||||
| Mixture of neem limonoids and other components | Patients with LSIL and HPV 16 infection | Intravaginal application of praneem tablet or placebo for 30 days, excluding menstrual period | Elimination of HPV DNA | Improved cytological abnormalities and clinical symptoms, eliminated HPV infection in 60% of cases | [91] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, M.A.; Bălan, A.; Anastasiu, C.V.; Dimienescu, O.G.; Neculoiu, C.D.; Gavriș, C. An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers. Int. J. Mol. Sci. 2018, 19, 3898. https://doi.org/10.3390/ijms19123898
Moga MA, Bălan A, Anastasiu CV, Dimienescu OG, Neculoiu CD, Gavriș C. An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers. International Journal of Molecular Sciences. 2018; 19(12):3898. https://doi.org/10.3390/ijms19123898
Chicago/Turabian StyleMoga, Marius Alexandru, Andreea Bălan, Costin Vlad Anastasiu, Oana Gabriela Dimienescu, Carmen Daniela Neculoiu, and Claudia Gavriș. 2018. "An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers" International Journal of Molecular Sciences 19, no. 12: 3898. https://doi.org/10.3390/ijms19123898
APA StyleMoga, M. A., Bălan, A., Anastasiu, C. V., Dimienescu, O. G., Neculoiu, C. D., & Gavriș, C. (2018). An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers. International Journal of Molecular Sciences, 19(12), 3898. https://doi.org/10.3390/ijms19123898