Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity
Abstract
1. Introduction
2. Results
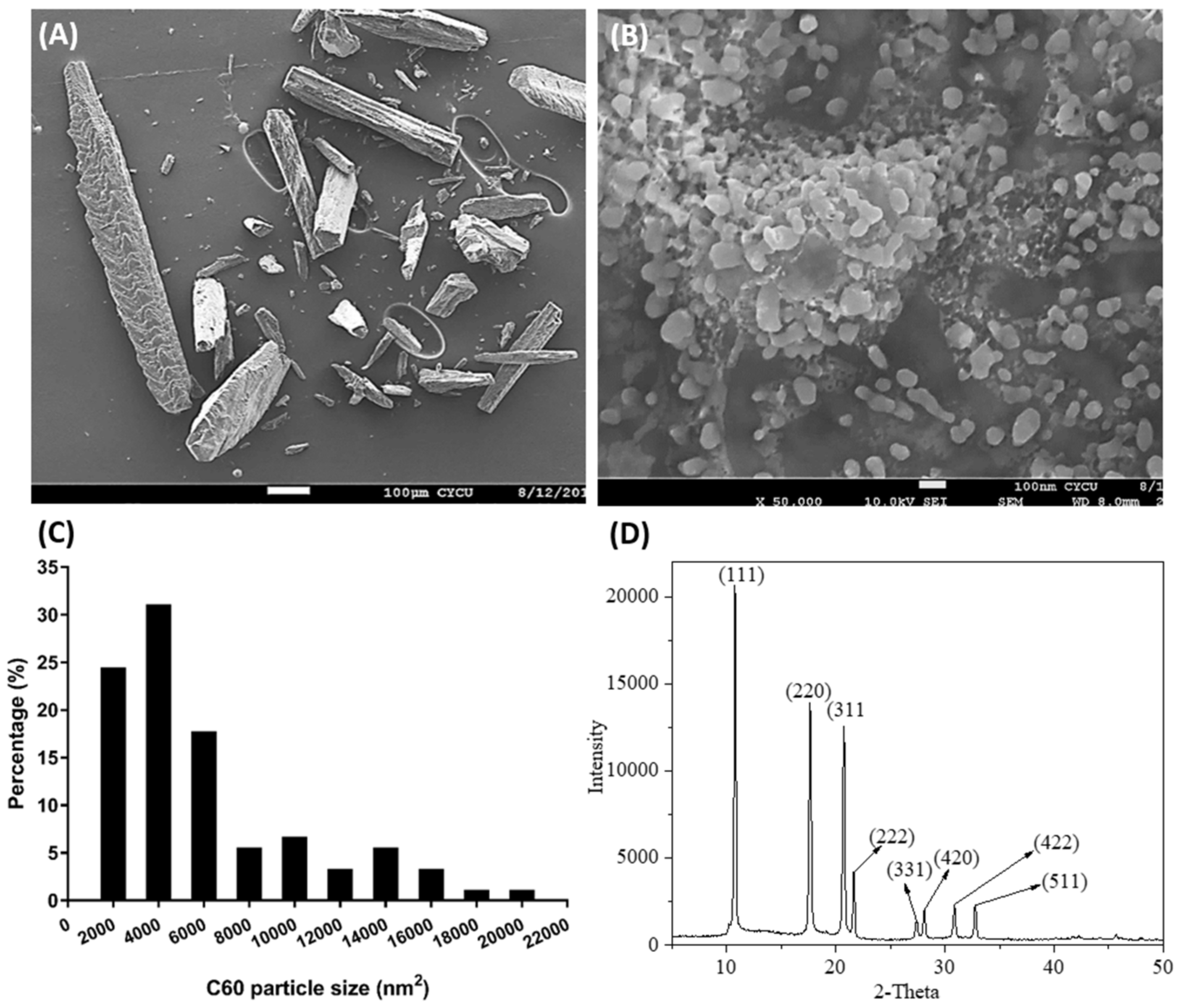
2.1. Determination of C60 NPs Size Distribution
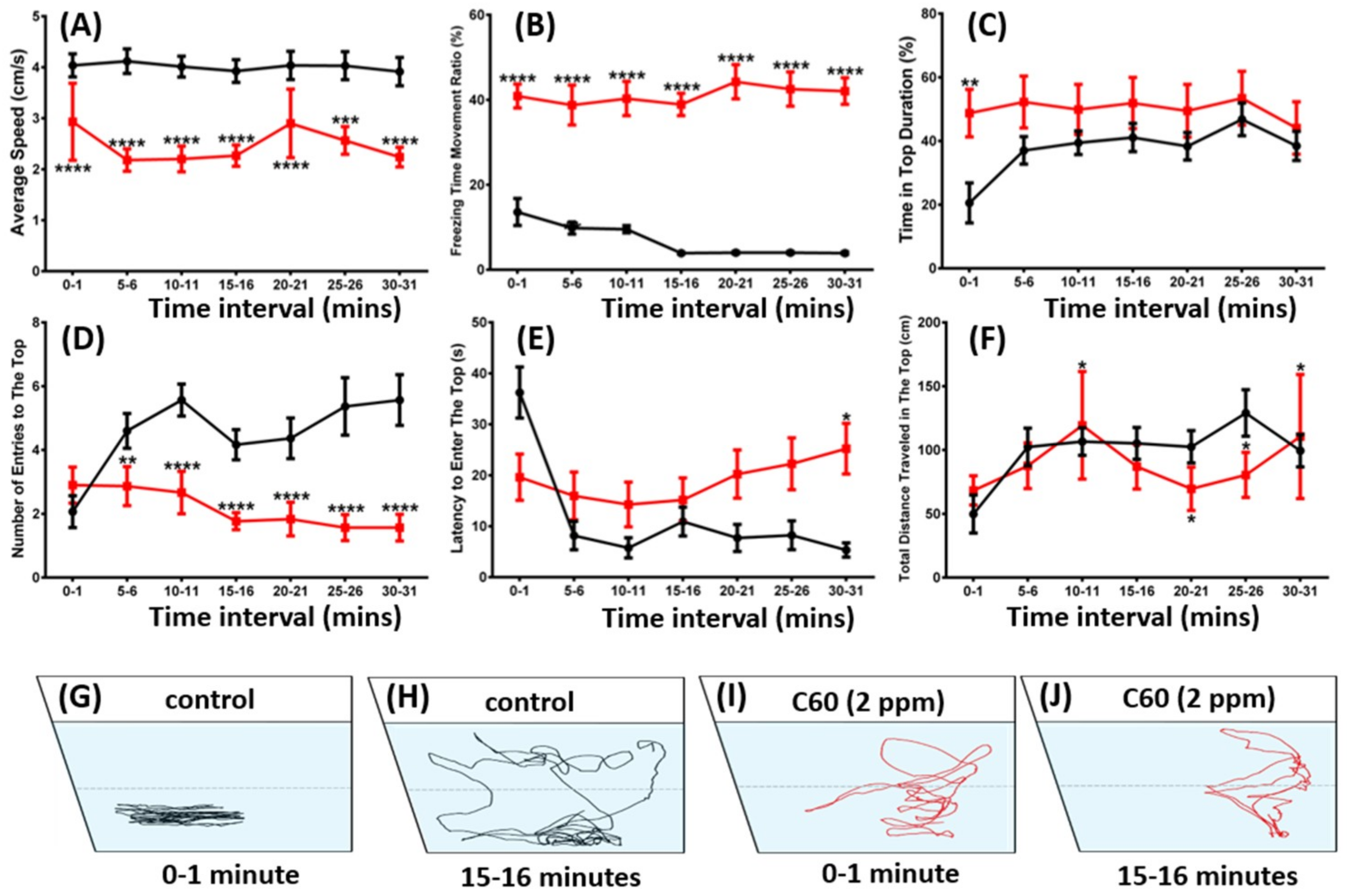
2.2. C60 NPs Exposure Reduced Zebrafish Exploration in New Environments and Locomotor Activity
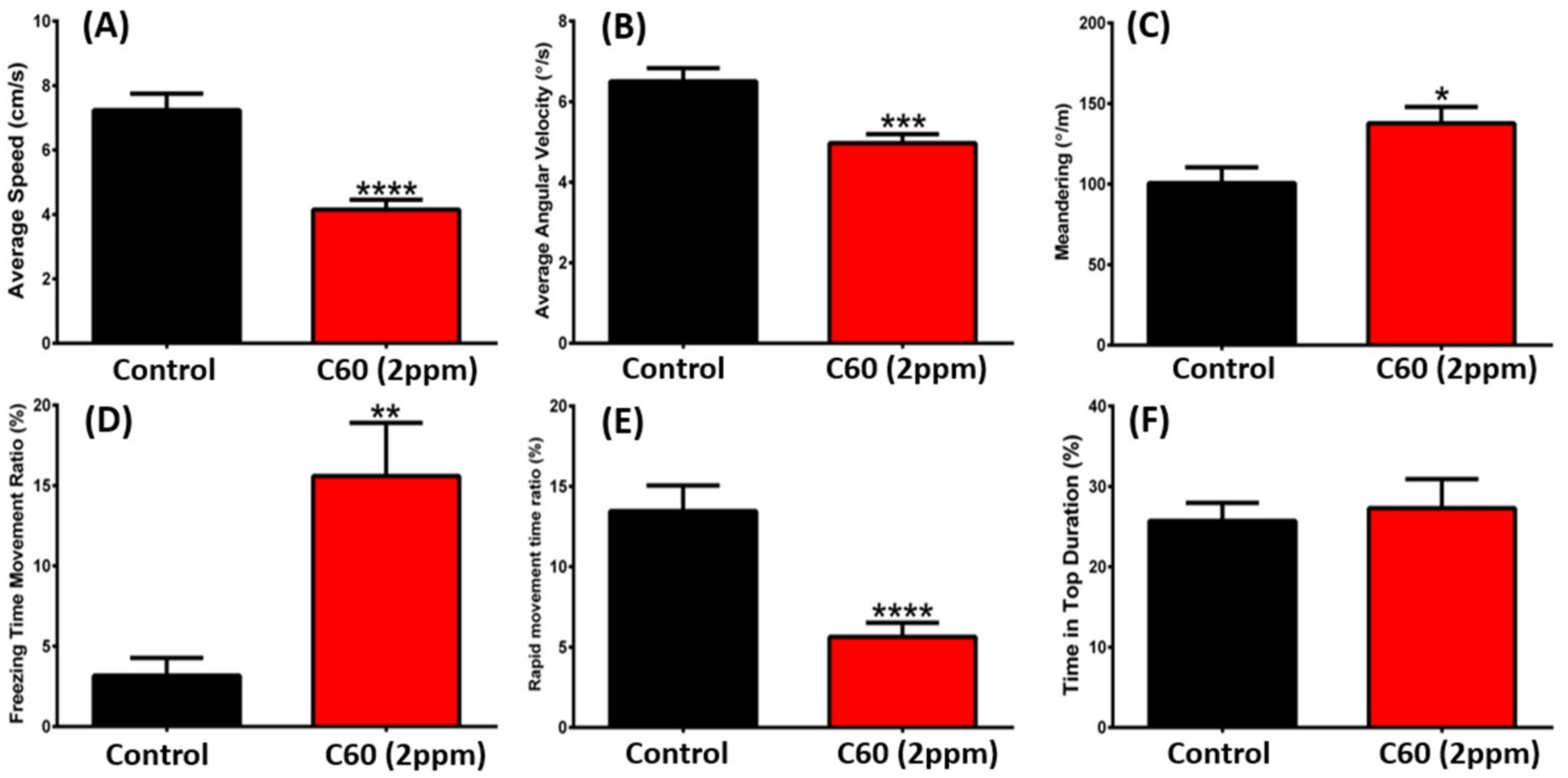
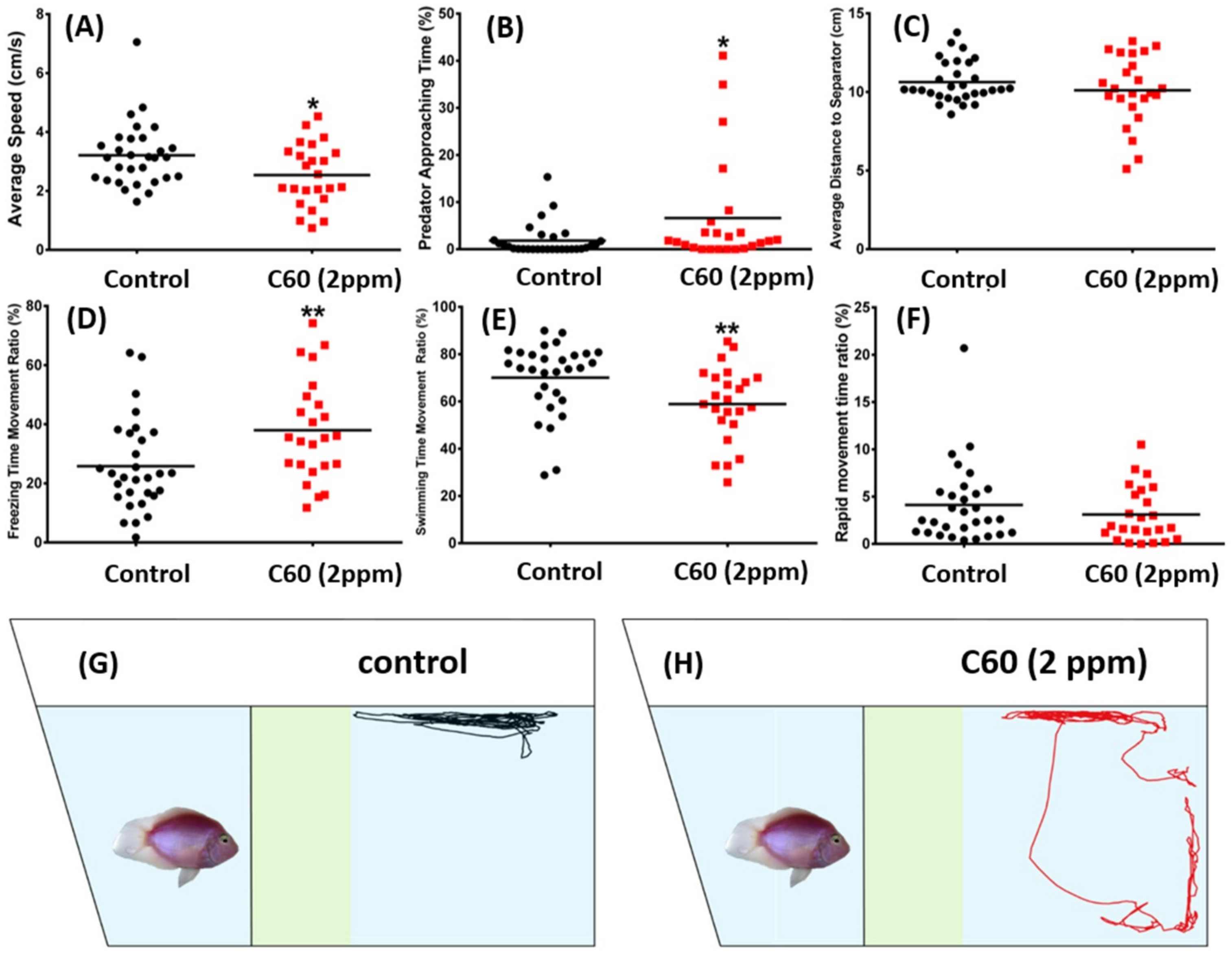
2.3. C60 NPs Exposure Reduced Aggression but Not Fear to Predator
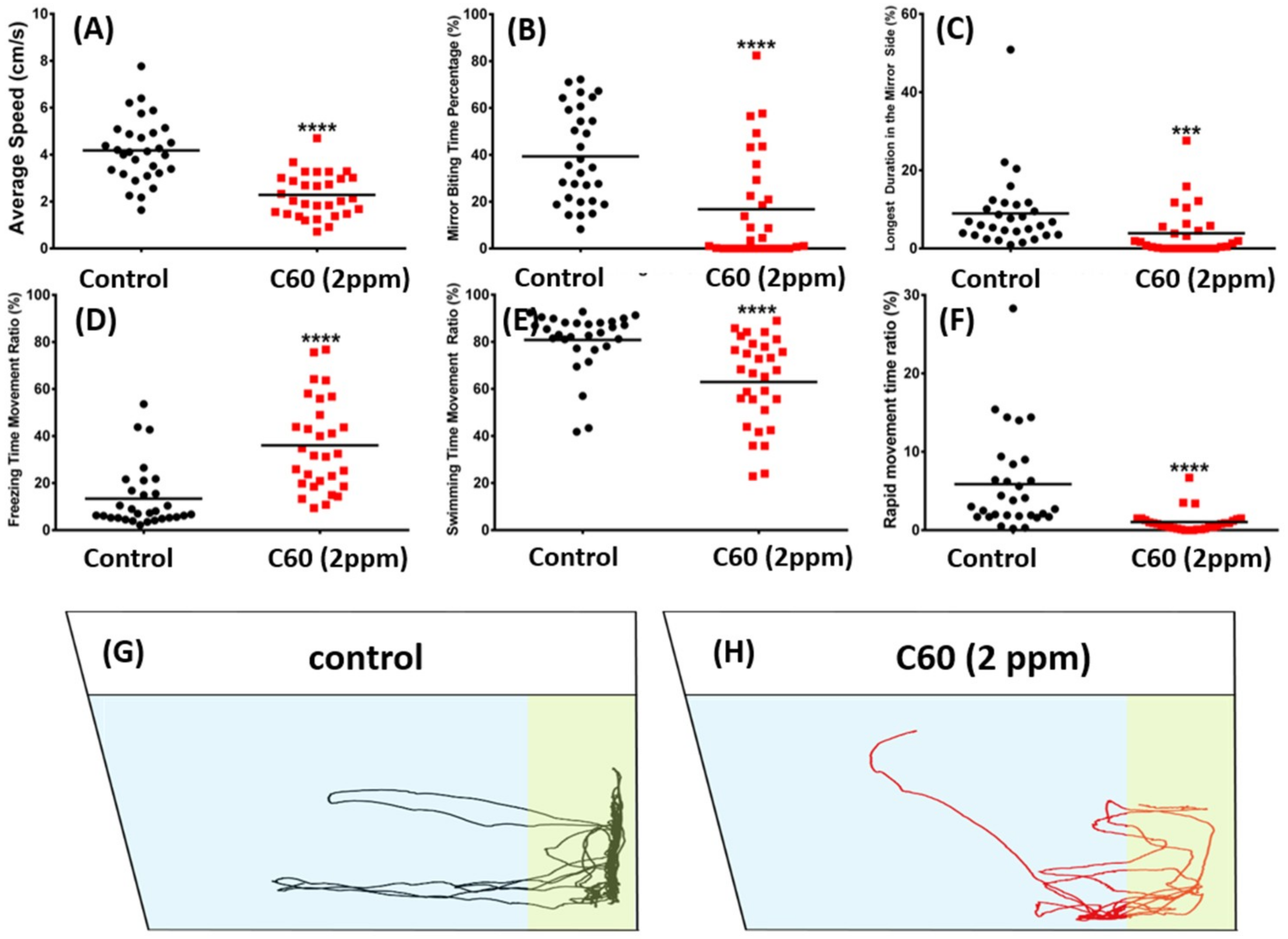
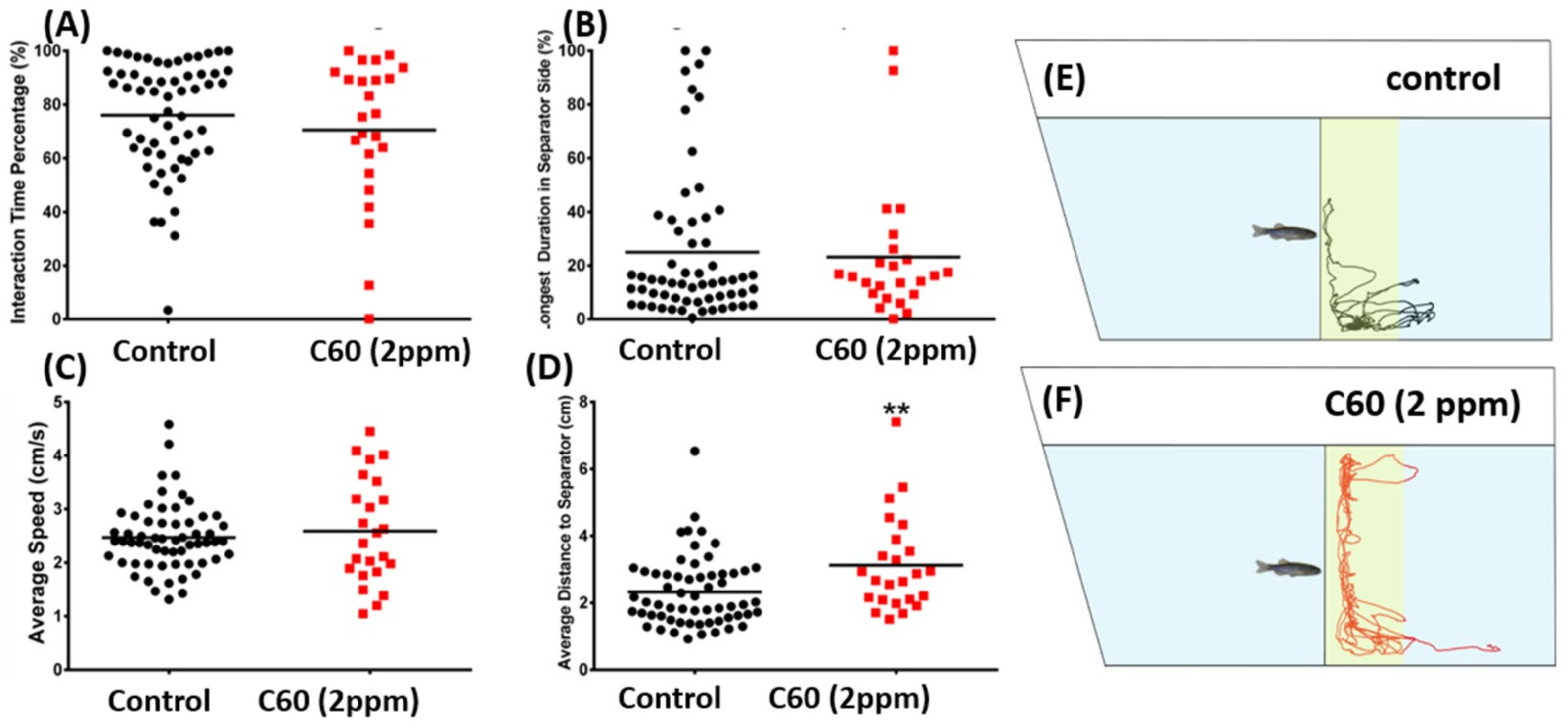
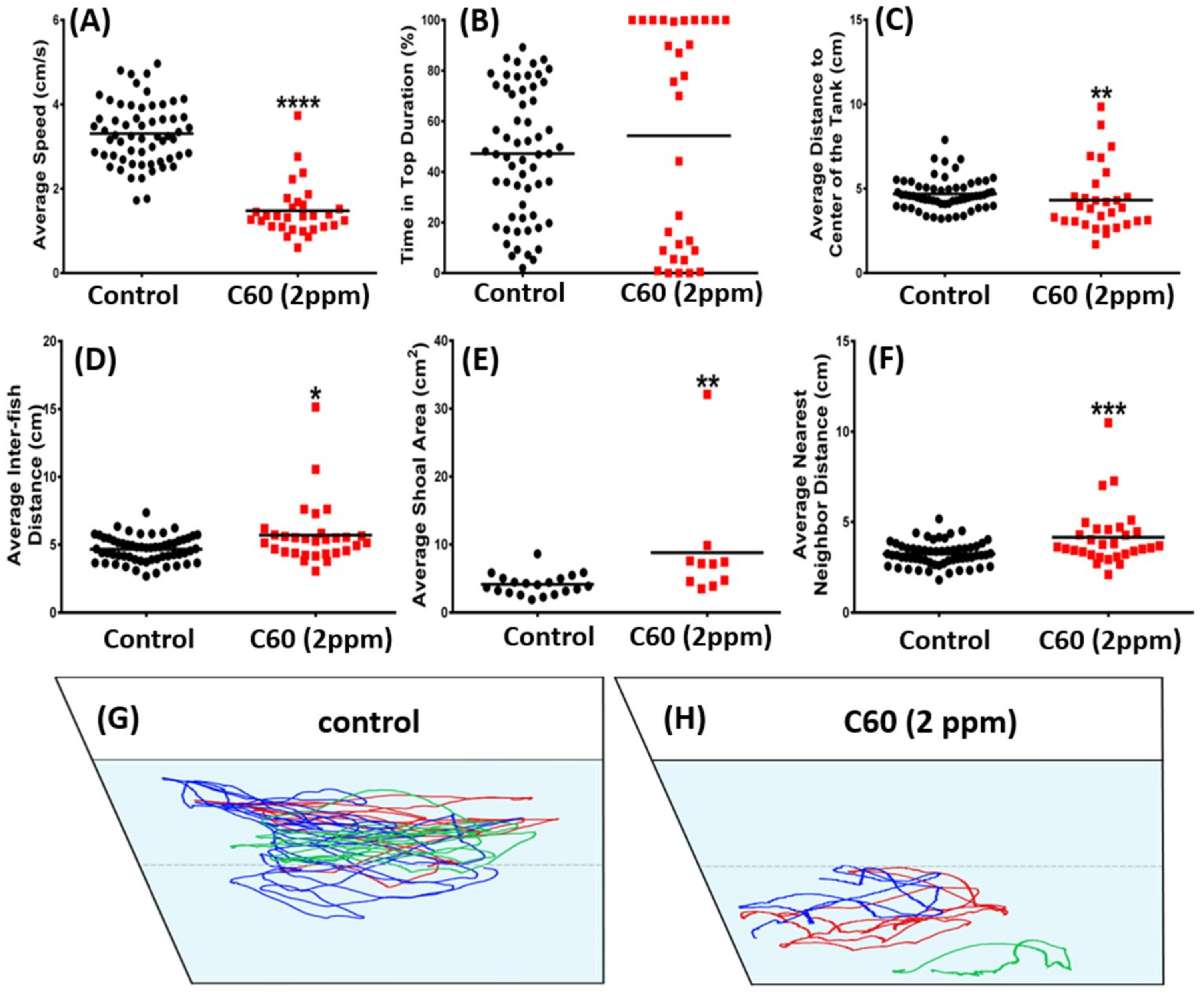
2.4. C60 NPs Exposure Had No Effect on Social Interaction but Can Reduce Shoaling Behavior
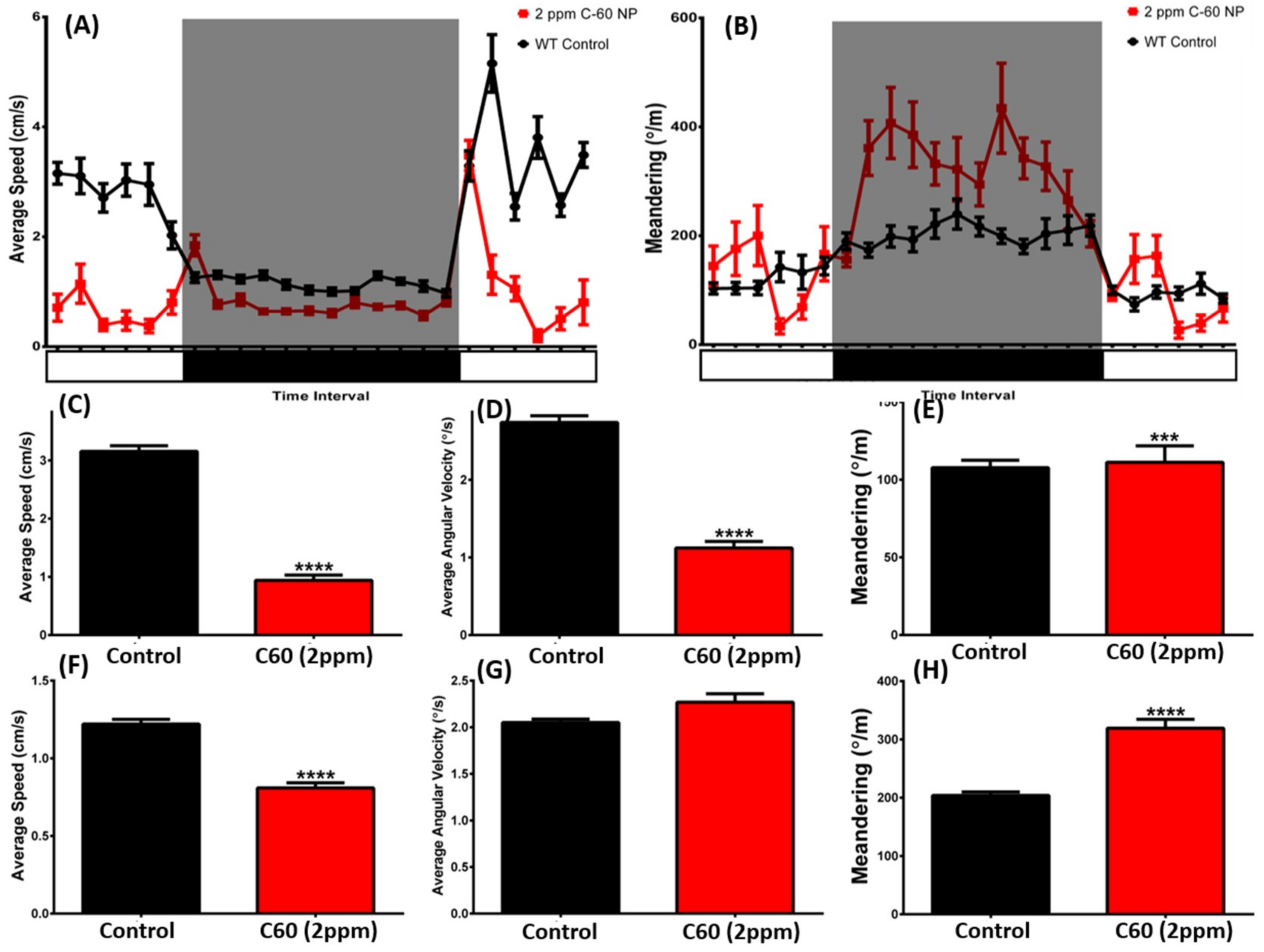
2.5. C60 NPs Exposure Induces the Dysregulation of Circadian Rhythm
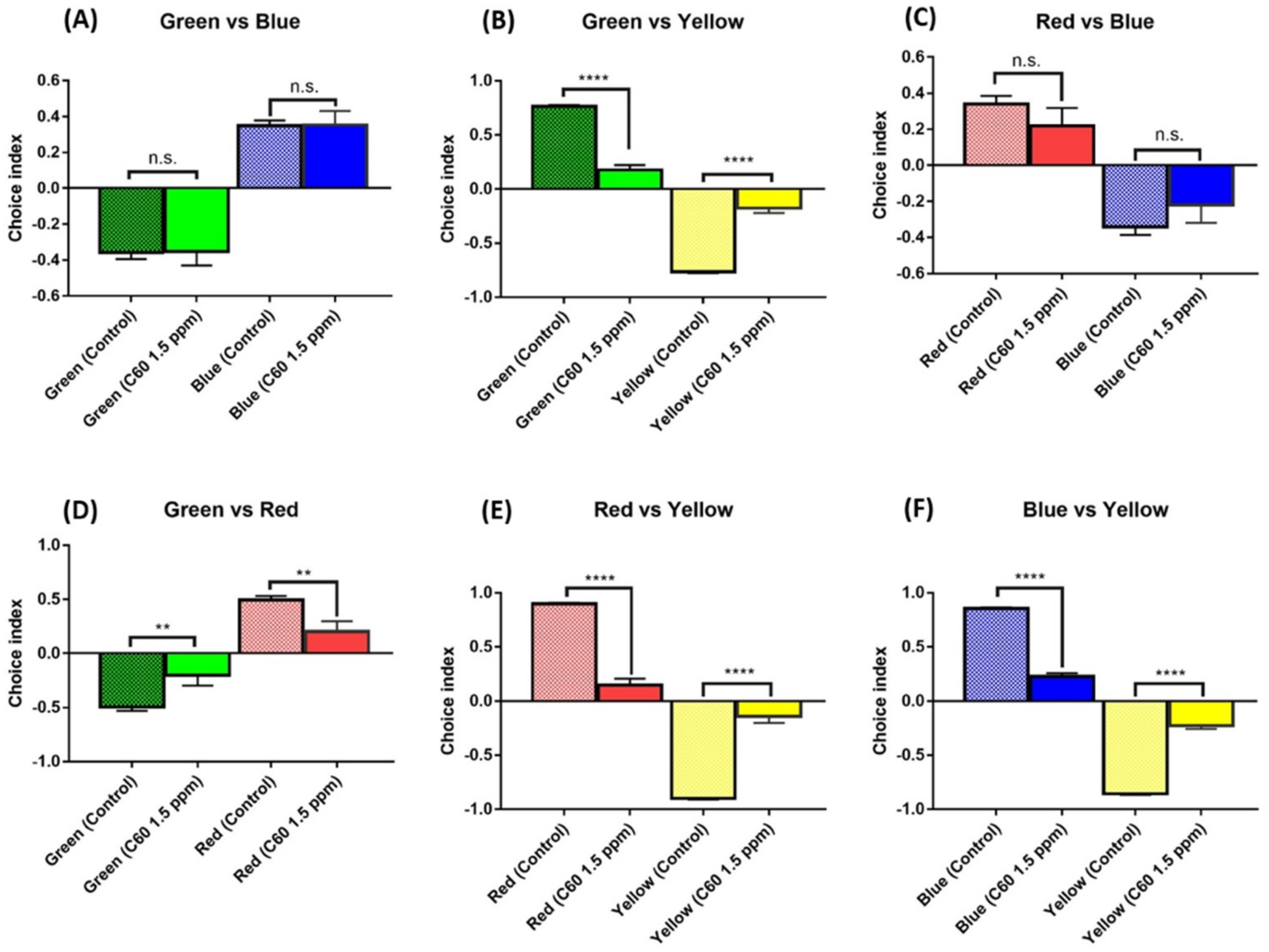
2.6. C60 NPs Exposure Reduced the Color Preference Index
2.7. Impact of C60 NPs on Oxidative Stress and Lipid Peroxidation Markers
2.8. Effect of C60 NPs on Antioxidant Enzymes
2.9. Effect of C60 NPs on Stress and Inflammatory Markers
2.10. C60 NPs Induced Creatine Kinase Activity and Hypoxia Signaling
2.11. C60 NPs Induced Behavioral Abnormalities are Linked to Changes in Acetylcholine, Melatonin, and GABA Contents
3. Discussion
4. Materials and Methods
4.1. C60 NPs Suspension
4.2. Morphological and Structural Characterization
4.3. Animal Ethics
4.4. Zebrafish Exposed to Fullerene Nanoparticle
4.5. Behavior Tests
4.6. Circadian Rhythm Test
4.7. Video Tracking and Data Analysis
4.8. Total Protein Extraction from Tissues
4.9. Determination of Oxidative, Anti-Oxidative Capacity, Lipid Peroxidation, DNA Damage, Stress Hormone, and Inflammation Markers
4.10. Statistical Analysis
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Alon, N.; Miroshnikov, Y.; Perkas, N.; Nissan, I.; Gedanken, A.; Shefi, O. Substrates coated with silver nanoparticles as a neuronal regenerative material. Int. J. Nanomed. 2014, 9, 23. [Google Scholar]
- Garcia-Alegria, E.; Iluit, M.; Stefanska, M.; Silva, C.; Heeg, S.; Kimber, S.J.; Kouskoff, V.; Lacaud, G.; Vijayaraghavan, A.; Batta, K. Graphene oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci. Rep. 2016, 6, 25917. [Google Scholar] [CrossRef]
- Iswarya, V.; Sharma, V.; Chandrasekaran, N.; Mukherjee, A. Impact of tetracycline on the toxic effects of titanium dioxide (TiO2) nanoparticles towards the freshwater algal species, scenedesmus obliquus. Aquat. Toxicol. 2017, 193, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Liu, Y. Potential impacts of silver nanoparticles on bacteria in the aquatic environment. J. Environ. Manag. 2017, 191, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Sood, P.; Kim, K.C.; Jang, S.S. Electrochemical properties of boron-doped fullerene derivatives for lithium-ion battery applications. ChemPhysChem 2018, 19, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, A.; Omidvar, A. Fullerene-based materials for solar cell applications: Design of novel acceptors for efficient polymer solar cells–a dft study. Phys. Chem. Chem. Phys. 2015, 17, 22367–22376. [Google Scholar] [CrossRef] [PubMed]
- Ogami, A.; Morimoto, Y.; Myojo, T.; Oyabu, T. Biological effect of fullerene (C60) to lung by inhalation or instillation. J. UOEH 2012, 34, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Gamo, M.; Nakanishi, J. Fullerene C60: Inhalation hazard assessment and derivation of a period-limited acceptable exposure level. Toxicol. Sci. 2011, 123, 576–589. [Google Scholar] [CrossRef]
- Barron, A.R. [60] fullerene-peptides: Bio-nano conjugates with structural and chemical diversity. J. Enzyme Inhib. Med. Chem. 2016, 31, 164–176. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Vereshchaka, I.V.; Maznychenko, A.V.; Bulgakova, N.V.; Gonchar, O.O.; Kyzyma, O.A.; Ritter, U.; Scharff, P.; Tomiak, T.; Nozdrenko, D.M. C60 fullerene as promising therapeutic agent for correcting and preventing skeletal muscle fatigue. J. Nanobiotechnol. 2017, 15, 8. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, C.; He, J.; Hu, Z.; Guan, W.-C.; Liu, S.-H. Protective effect of reduced glutathione C60 derivative against hydrogen peroxide-induced apoptosis in hek 293t cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Iohara, D.; Umezaki, Y.; Anraku, M.; Uekama, K.; Hirayama, F. In vitro and in vivo evaluation of hydrophilic C60 (oh) 10/2-hydroxypropyl-β-cyclodextrin nanoparticles as an antioxidant. J. Pharm. Sci. 2016, 105, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Sayers, B.C.; Germolec, D.R.; Walker, N.J.; Shipkowski, K.A.; Stout, M.D.; Cesta, M.F.; Roycroft, J.H.; White, K.L.; Baker, G.L.; Dill, J.A. Respiratory toxicity and immunotoxicity evaluations of microparticle and nanoparticle C60 fullerene aggregates in mice and rats following nose-only inhalation for 13 weeks. Nanotoxicology 2016, 10, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.L.; Gupta, A.; Clark, M.L.; Valenzuela, B.R.; Staska, L.M.; Harbo, S.J.; Pierce, J.T.; Dill, J.A. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol. Sci. 2007, 101, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol. Appl. Pharmacol. 2008, 229, 44–55. [Google Scholar] [CrossRef]
- Zerbi, G.; Barbon, A.; Bengalli, R.; Lucotti, A.; Catelani, T.; Tampieri, F.; Gualtieri, M.; D’Arienzo, M.; Morazzoni, F.; Camatini, M. Graphite particles induce ros formation in cell free systems and human cells. Nanoscale 2017, 9, 13640–13650. [Google Scholar] [CrossRef]
- Anderson, A.; Grose, J.; Pahl, S.; Thompson, R.; Wyles, K. Microplastics in personal care products: Exploring perceptions of environmentalists, beauticians and students. Mar. Pollut. Bull. 2016, 113, 454–460. [Google Scholar] [CrossRef]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet. Dermatol. 2018. [Google Scholar] [CrossRef]
- Hosny, A.E.-D.M.; Kashef, M.T.; Taher, H.A.; El-Bazza, Z.E. The use of unirradiated and γ-irradiated zinc oxide nanoparticles as a preservative in cosmetic preparations. Int. J. Nanomed. 2017, 12, 6799. [Google Scholar] [CrossRef]
- Haque, E.; Ward, A. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G. Evaluation of chronic nanosilver toxicity to adult zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, L.; Li, Y.; Duan, Z.; Chen, W.; Alvarez, P.J. Developmental toxicity in zebrafish (danio rerio) embryos after exposure to manufactured nanomaterials: Buckminsterfullerene aggregates (NC60) and fullerol. Environ. Toxicol. Chem. Int. J. 2007, 26, 976–979. [Google Scholar] [CrossRef]
- Zhu, S.; Oberdörster, E.; Haasch, M.L. Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, daphnia and fathead minnow. Mar. Environ. Res. 2006, 62, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. Idtracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, B.; Tuichiev, S.; Tabarov, S.K.; Shepelevskii, A.; Shibaev, L. X-ray diffraction analysis of C60 fullerene powder and fullerene soot. Tech. Phys. 2005, 50, 1458–1461. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D.J.I. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Basel, Switzerland, 2012; pp. 231–246. [Google Scholar]
- Ahmed, O.; Seguin, D.; Gerlai, R.J.B. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R.J. Quantification of shoaling behaviour in zebrafish (danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Sun, M.; Zhang, Q.; Li, T.; Li, X.; Xu, J.; Zhao, X.; Chen, D.; Feng, X. Reversal of reserpine-induced depression and cognitive disorder in zebrafish by sertraline and traditional chinese medicine (TCM). Behav. Brain Funct. 2018, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Floyd-Rump, T.; Horstmann-Dehn, L.; Atkinson, S.; Skaugstad, C. Effect of ichthyophonus on blood plasma chemistry of spawning chinook salmon and their resulting offspring in a yukon river tributary. Dis. Aquat. Org. 2017, 122, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Heerlein, K.; Schulze, A.; Hotz, L.; Bartsch, P.; Mairbaurl, H. Hypoxia decreases cellular ATP demand and inhibits mitochondrial respiration of A549 cells. Am. J. Respir. Cell Mol. Biol. 2005, 32, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bogo, M.R.; Dal Forno, G.O.; Kist, L.W.; Britto, R.S.; Guterres, S.S.; Bonan, C.D.; Külkamp-Guerreiro, I.C.; Fritsch, R.S.; de Azevedo, M.B.; Monserrat, J. Intraperitoneal exposure to nano/microparticles of fullerene () increases acetylcholinesterase activity and lipid peroxidation in adult zebrafish (danio rerio) brain. BioMed Res. Int. 2013, 2013, 623789. [Google Scholar]
- Zarrindast, M.-R.; Khakpai, F. The modulatory role of dopamine in anxiety-like behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar]
- Liu, Q.; Cui, Q.; Li, X.J.; Jin, L. The applications of buckminsterfullerene C60 and derivatives in orthopaedic research. Connect. Tissue Res. 2014, 55, 71–79. [Google Scholar] [CrossRef]
- Lens, M. Recent progresses in application of fullerenes in cosmetics. Recent Patents Biotechnol. 2011, 5, 67–73. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Xin, Y.; Zhao, M.J.A.T. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (danio rerio) after a long-term exposure. Aquat. Toxicol. 2011, 101, 493–499. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Zhang, X.; Zhao, Z.; Liu, H.; George, R.; Wilson-Rawls, J.; Chang, Y.; Chen, Y.J.C. Disruption of zebrafish (danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 2011, 83, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chan, C.M.; Veca, L.M.; Poon, W.L.; Chan, P.K.; Qu, L.; Sun, Y.-P.; Cheng, S.H.J.T. Acute and long-term effects after single loading of functionalized multi-walled carbon nanotubes into zebrafish (danio rerio). Toxicol. Appl. Pharmacol. 2009, 235, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.; Henry, T.; Handy, R.J.A. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquatic Toxicol. 2013, 126, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Roursgaard, M.; Jensen, K.A.; Poulsen, S.S.; Larsen, S.T. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin. Pharmacol. Toxicol. 2008, 103, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.W.; Wilson, S.R.; Schuster, D.I. Biological applications of fullerenes. Bioorgan. Med. Chem. 1996, 4, 767–779. [Google Scholar] [CrossRef]
- Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; John Wiley & Sons: New York, USA, 2006. [Google Scholar]
- Pickering, K.; Wiesner, M. Fullerol-sensitized production of reactive oxygen species in aqueous solution. Environ. Sci. Technol. 2005, 39, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Taurozzi, J.S.; Pandey, A.K.; Shan, W.; Miller, S.M.; Hashsham, S.A.; Tarabara, V.V. Stable colloidal dispersions of C60 fullerenes in water: Evidence for genotoxicity. Environ. Sci. Technol. 2006, 40, 7394–7401. [Google Scholar] [CrossRef]
- Kamat, J.; Devasagayam, T.; Priyadarsini, K.; Mohan, H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology 2000, 155, 55–61. [Google Scholar] [CrossRef]
- Prat, F.; Stackow, R.; Bernstein, R.; Qian, W.; Rubin, Y.; Foote, C.S. Triplet-state properties and singlet oxygen generation in a homologous series of functionalized fullerene derivatives. J. Phys. Chem. A 1999, 103, 7230–7235. [Google Scholar] [CrossRef]
- Isakovic, A.; Markovic, Z.; Todorovic-Markovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Li, Y.; Wang, Y.; Yang, L. A large-scale association study for nanoparticle C60 uncovers mechanisms of nanotoxicity disrupting the native conformations of DNA/rna. Nucleic Acids Res. 2012, 40, 7622–7632. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Chiu, I.-J.; Cheng, F.-Y.; Lee, Y.-H.; Wang, Y.-J.; Hsu, Y.-H.; Chiu, H.-W. The role of hypoxia-inducible factor-1α in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Particle Fibre Toxicol. 2015, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, X.J. Acetylcholinesterase and apoptosis. FEBS J. 2008, 275, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Toiber, D.; Berson, A.; Greenberg, D.; Melamed-Book, N.; Diamant, S.; Soreq, H. N-acetylcholinesterase-induced apoptosis in alzheimer’s disease. PLoS ONE 2008, 3, e3108. [Google Scholar] [CrossRef] [PubMed]
- Senger, M.R.; Seibt, K.J.; Ghisleni, G.C.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Aluminum exposure alters behavioral parameters and increases acetylcholinesterase activity in zebrafish (danio rerio) brain. Cell Biol. Toxicol. 2011, 27, 199–205. [Google Scholar] [CrossRef]
- Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Ethanol alters acetylcholinesterase activity and gene expression in zebrafish brain. Toxicol. Lett. 2007, 174, 25–30. [Google Scholar] [CrossRef]
- Oberdörster, E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ. Health Perspect. 2004, 112, 1058. [Google Scholar] [CrossRef]
- Shinohara, N.; Matsumoto, T.; Gamo, M.; Miyauchi, A.; Endo, S.; Yonezawa, Y.; Nakanishi, J. Is lipid peroxidation induced by the aqueous suspension of fullerene C60 nanoparticles in the brains of cyprinus carpio? Environ. Sci. Technol. 2008, 43, 948–953. [Google Scholar] [CrossRef]
- Shipelin, V.; Arianova, E.; Trushina, E.; Avren’eva, L.; Batishcheva, S.; Cherkashin, A.; Soto, S.; Lashneva, N.; Gmoshinskiĭ, I.; Khotimchenko, S. Toxicological and sanitary characteristics of fullerene C60 administered to the rat gastrointestinal tract. Gigiena i Sanitariia 2012, 90–94. [Google Scholar]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.N.; Hoffmann, R.G.; Hoke, E.S.; Tanguay, R.L. Bisphenol a exposure during early development induces sex-specific changes in adult zebrafish social interactions. J. Toxicol. Environ. Health Part A 2015, 78, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Zebra fish: An uncharted behavior genetic model. Behav. Genet. 2003, 33, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef]

| Biomarker | Control | C60 (1ppm) | C60 (2ppm) | Unit | Significance | ANOVA F value | p value |
|---|---|---|---|---|---|---|---|
| Muscle | |||||||
| ROS | 4.643 ± 0.356 a | 5.353 ± 0.586 a | 15.710 ± 1.881 b | U/μg total protein | YES | F (2, 6) = 28.740 | p = 0.0008 |
| 4-HNE | 1.028 ± 0.101 | 1.192 ± 0.223 | 1.005 ± 0.182 | U/μg total protein | NO | F (2, 6) = 0.341 | p = 0.7272 |
| MDA | 0.163 ± 0.011 | 0.096 ± 0.016 | 0.143 ± 0.021 | ng/μg total protein | NO | F (2, 6) = 4.416 | p = 0.0662 |
| TBARS | 3.317 ± 0.045 a | 3.922 ± 0.541 a | 20.240 ± 2.066 b | ng/μg total protein | YES | F (2, 6) = 60.600 | p = 0.0001 |
| catalase | 1.157 ± 0.003 a | 1.662 ± 0.200 a | 0.499 ± 0.045 b | U/μg total protein | YES | F (2, 6) = 24.300 | p = 0.0013 |
| SOD | 3.443 ± 0.199 a | 4.825 ± 0.787 a | 1.004 ± 0.204 b | U/μg total protein | YES | F (2, 6) = 16.050 | p = 0.0039 |
| ssDNA | 0.717 ± 0.007 a | 0.791 ± 0.162 a | 3.702 ± 0.229 b | U/μg total protein | YES | F (2, 6) = 110.600 | p < 0.0001 |
| catecholamine | 4.031 ± 0.063 a | 3.793 ± 0.732 a | 15.240 ± 1.847 b | ng/μg total protein | YES | F (2, 6) = 32.480 | p = 0.0006 |
| cortisol | 11.700 ± 0.413 a | 35.090 ± 5.455 b | 43.020 ± 5.051 b | pg/μg total protein | YES | F (2, 6) = 14.340 | p = 0.0052 |
| TNF-α | 5.877 ± 0.076 a | 5.603 ± 1.080 a | 19.880 ± 2.414 b | pg/μg total protein | YES | F (2, 6) = 28.600 | p = 0.0009 |
| IL1β | 0.406 ± 0.010 a | 0.365 ± 0.071 a | 2.651 ± 0.242 b | ng/μg total protein | YES | F (2, 6) = 80.890 | p < 0.0001 |
| Hif-1α | 13.880 ± 0.738 | 21.880 ± 4.270 | 19.170 ± 2.221 | pg/μg total protein | NO | F (2, 6) = 2.095 | p = 0.2042 |
| creatine kinase | 2.092 ± 0.019 a | 2.037 ± 0.412 a | 5.300 ± 0.473 b | pg/μg total protein | YES | F (2, 6) = 26.600 | p = 0.0010 |
| ATP | 293.400 ± 8.753 a | 257.900 ± 57.280 a | 89.440 ± 15.460 b | ng/μg total protein | YES | F (2, 6) = 9.909 | p = 0.0126 |
| creatinine | 5.715 ± 0.133 | 3.916 ± 1.222 | 5.543 ± 0.435 | U/μg total protein | NO | F (2, 6) = 1.738 | p = 0.2538 |
| metallothionine | 13.840 ± 0.537 | 13.470 ± 0.727 | 12.610 ± 0.564 | pg/μg total protein | NO | F (2, 6) = 1.055 | p = 0.4049 |
| Gill | |||||||
| ROS | 2.923 ± 0.193 a | 3.386 ± 0.644 a | 7.235 ± 1.219 b | U/μg total protein | YES | F (2, 6) = 8.675 | P = 0.0170 |
| 4-HNE | 0.552 ± 0.115 a | 1.048 ± 0.030 b | 0.792 ± 0.070 a,b | U/μg total protein | YES | F (2, 6) = 9.693 | p = 0.0132 |
| MDA | 0.123 ± 0.007 a | 0.114 ± 0.012 a | 0.024 ± 0.006 b | ng/μg total protein | YES | F (2, 6) = 39.050 | p = 0.0004 |
| TBARS | 2.504 ± 0.074 a | 3.135 ± 0.534 a | 6.630 ± 1.230 b | ng/μg total protein | YES | F (2, 6) = 8.213 | p = 0.0191 |
| Catalase | 0.875 ± 0.044 a | 0.815 ± 0.052 a | 0.481 ± 0.022 b | U/μg total protein | YES | F (2, 6) = 26.470 | p = 0.0011 |
| SOD | 2.501 ± 0.071 a | 2.946 ± 0.473 a | 1.088 ± 0.082 b | U/μg total protein | YES | F (2, 6) = 11.990 | p = 0.0080 |
| ssDNA | 0.522 ± 0.018 a | 0.941 ± 0.056 a | 1.555 ± 0.204 b | U/μg total protein | YES | F (2, 6) = 17.930 | p = 0.0029 |
| catecholamine | 3.088 ± 0.087 a | 5.676 ± 0.349 a, c | 10.430 ± 2.156 b, c | ng/μg total protein | YES | F (2, 6) = 8.717 | p = 0.0168 |
| cortisol | 6.024 ± 0.451 a | 11.830 ± 1.165 b | 13.010 ± 1.320 b | pg/μg total protein | YES | F (2, 6) = 12.710 | p = 0.0070 |
| TNF-α | 4.404 ± 0.138 a | 8.020 ± 0.482 a | 12.220 ± 1.465 b | pg/μg total protein | YES | F (2, 6) = 19.150 | p = 0.0025 |
| IL1β | 0.334 ± 0.007 a | 0.631 ± 0.045 a | 1.302 ± 0.117 b | ng/μg total protein | YES | F (2, 6) = 46.850 | p = 0.0002 |
| Hif-1α | 9.727 ± 1.484 a | 22.580 ± 3.800 b | 13.490 ± 2.089 a,b | pg/μg total protein | YES | F (2, 6) = 6.240 | p = 0.0342 |
| creatine kinase | 1.496 ± 0.091 a | 2.820 ± 0.168 b | 2.085 ± 0.243 a,b | pg/μg total protein | YES | F (2, 6) = 13.810 | p = 0.0057 |
| ATP | 142.700 ± 15.940 a | 208.400 ± 20.080 a,b | 112.300 ± 12.880 a,c | ng/μg total protein | YES | F (2, 6) = 8.790 | p = 0.0165 |
| creatinine | 5.035 ± 1.256 | 4.843 ± 0.768 | 5.036 ± 0.850 | U/μg total protein | NO | F (2, 6) = 0.013 | p = 0.9873 |
| metallothionine | 9.871 ± 0.340 | 12.770 ± 1.227 | 11.950 ± 0.820 | pg/μg total protein | NO | F (2, 6) = 2.927 | p = 0.1297 |
| Biomarker | Control | C60 (1ppm) | C60 (2ppm) | Unit | Significance | ANOVA F value | p value |
|---|---|---|---|---|---|---|---|
| ACh | 22.750 ± 0.373 a | 15.520 ± 1.686 b | 9.309 ± 0.641 c | U/μg totalprotein | YES | F (2, 6) = 39.99 | p = 0.0003 |
| AChE | 3.102 ± 0.079 a | 3.272 ± 0.504 a | 7.527 ± 0.284 b | U/μg totalprotein | YES | F (2, 6) = 55.34 | p = 0.0001 |
| Dopamine | 11.860 ± 2.769 a | 11.940 ± 1.420 a | 34.530 ± 5.335 b | pg/μg totalprotein | YES | F (2, 6) = 13.42 | p = 0.0061 |
| GABA | 0.057 ± 0.003 a | 0.057 ± 0.001 a | 0.159 ± 0.013 b | ug/μg totalprotein | YES | F (2, 6) = 59.43 | p = 0.0001 |
| melatonin | 3.459 ± 0.143 a | 1.759 ± 0.035 b | 2.493 ± 0.301 b | pg/μg totalprotein | YES | F (2, 6) = 19.43 | p = 0.0024 |
| glutamate | 0.633 ± 0.069 a | 0.392 ± 0.024 a,b | 0.379 ± 0.055 b | ug/μg totalprotein | YES | F (2, 6) = 7.312 | p = 0.0246 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Lai, Y.-H.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity. Int. J. Mol. Sci. 2018, 19, 3853. https://doi.org/10.3390/ijms19123853
Sarasamma S, Audira G, Juniardi S, Sampurna BP, Lai Y-H, Hao E, Chen J-R, Hsiao C-D. Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity. International Journal of Molecular Sciences. 2018; 19(12):3853. https://doi.org/10.3390/ijms19123853
Chicago/Turabian StyleSarasamma, Sreeja, Gilbert Audira, Stevhen Juniardi, Bonifasius Putera Sampurna, Yu-Heng Lai, Erwei Hao, Jung-Ren Chen, and Chung-Der Hsiao. 2018. "Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity" International Journal of Molecular Sciences 19, no. 12: 3853. https://doi.org/10.3390/ijms19123853
APA StyleSarasamma, S., Audira, G., Juniardi, S., Sampurna, B. P., Lai, Y.-H., Hao, E., Chen, J.-R., & Hsiao, C.-D. (2018). Evaluation of the Effects of Carbon 60 Nanoparticle Exposure to Adult Zebrafish: A Behavioral and Biochemical Approach to Elucidate the Mechanism of Toxicity. International Journal of Molecular Sciences, 19(12), 3853. https://doi.org/10.3390/ijms19123853








