Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder
Abstract
1. Introduction
2. Results
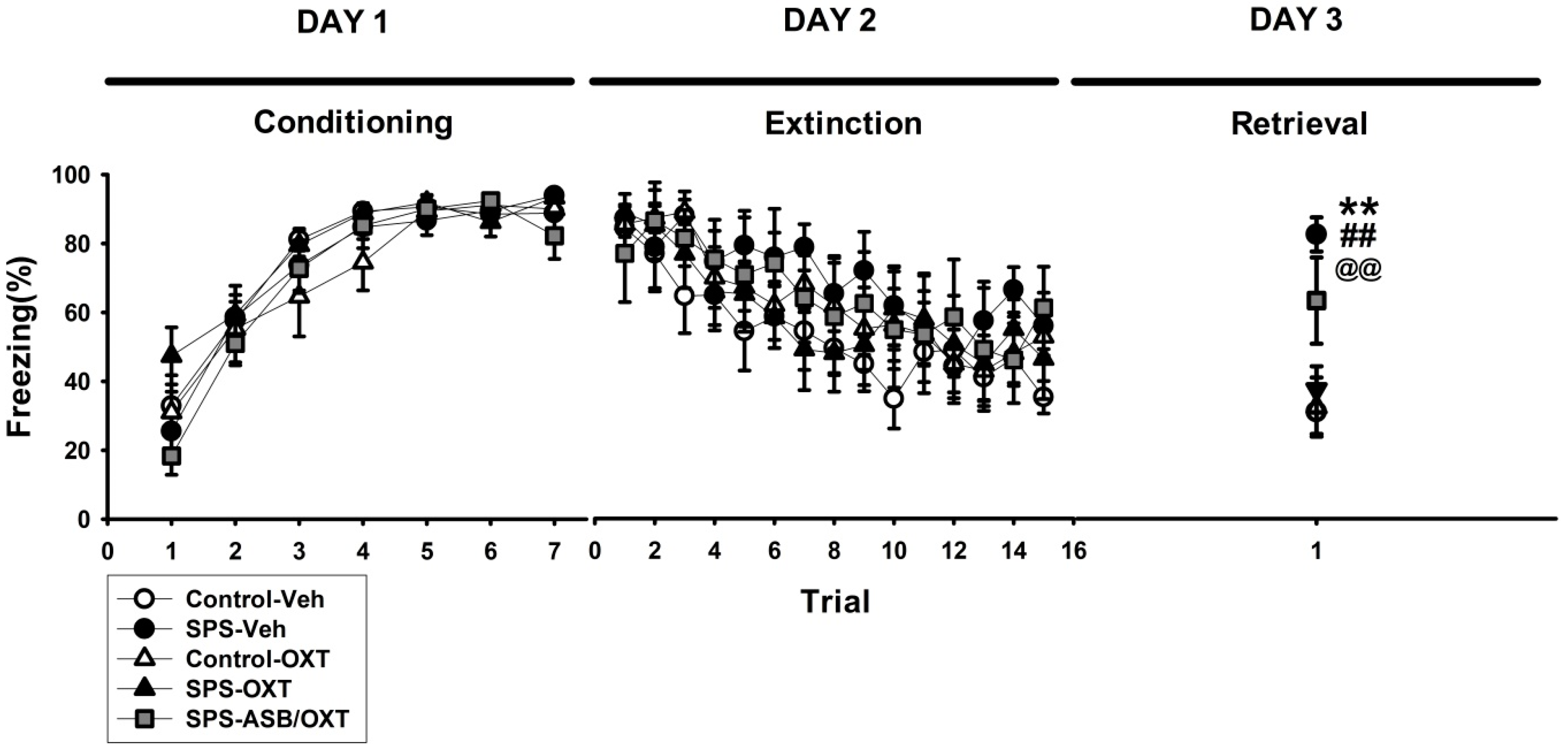
2.1. OXT (Oxytocin) Mitigated SPS-Induced Impairment of Fear Extinction
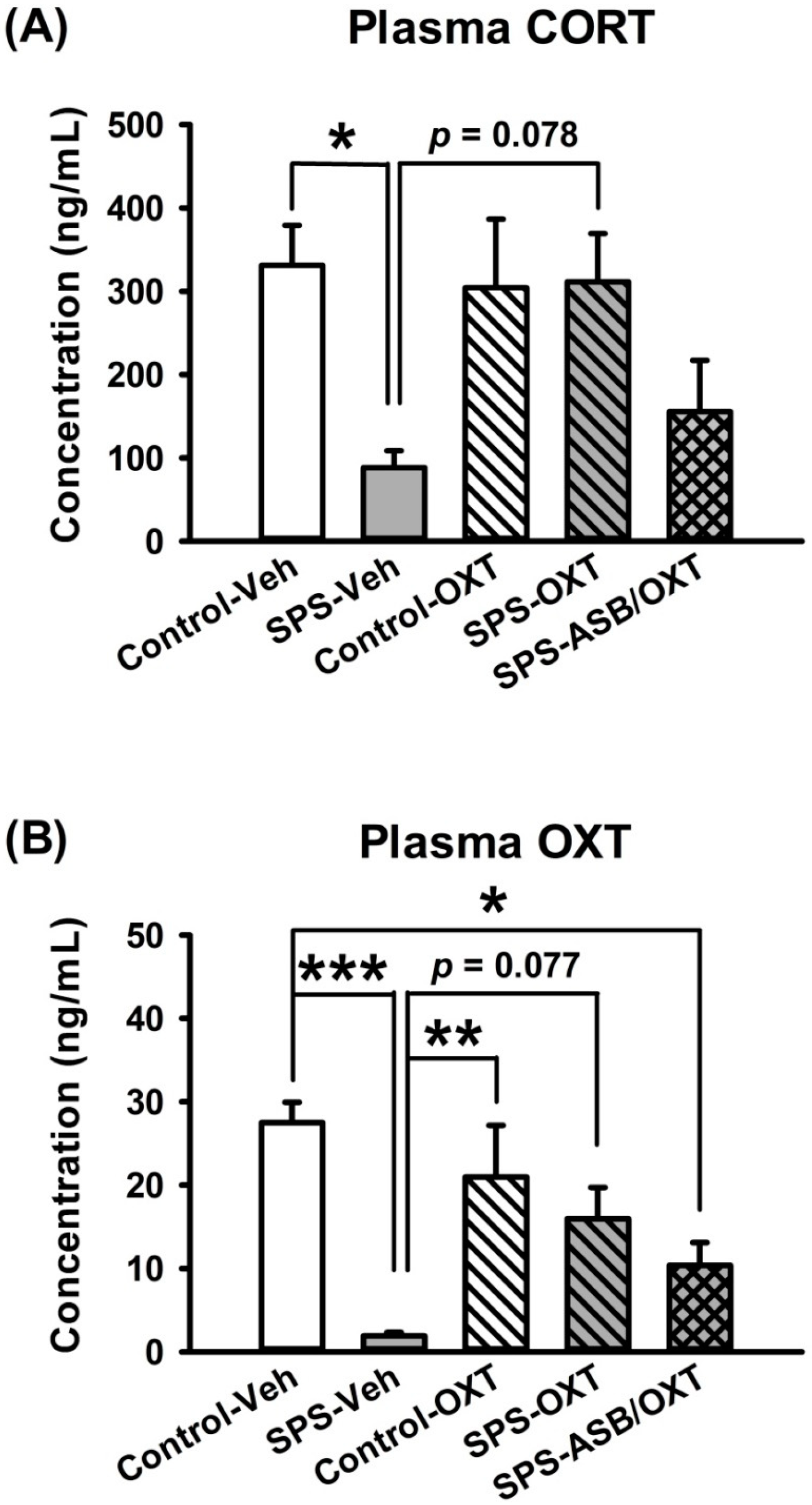
2.2. Intranasal OXT Eliminated SPS-Induced Reduction of Peripheral CORT and OXT Levels.
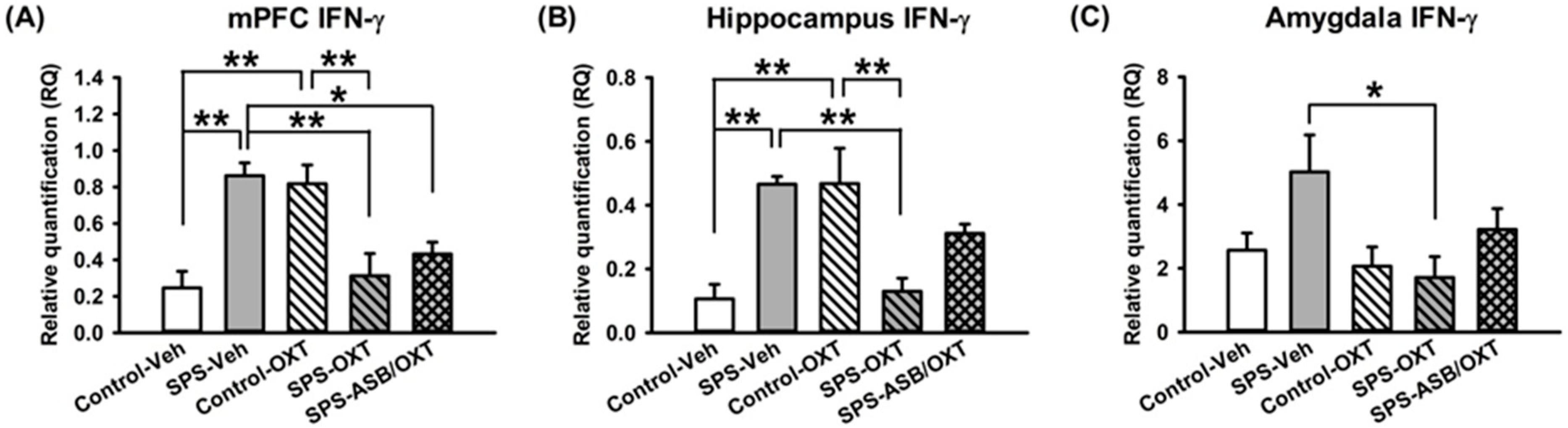
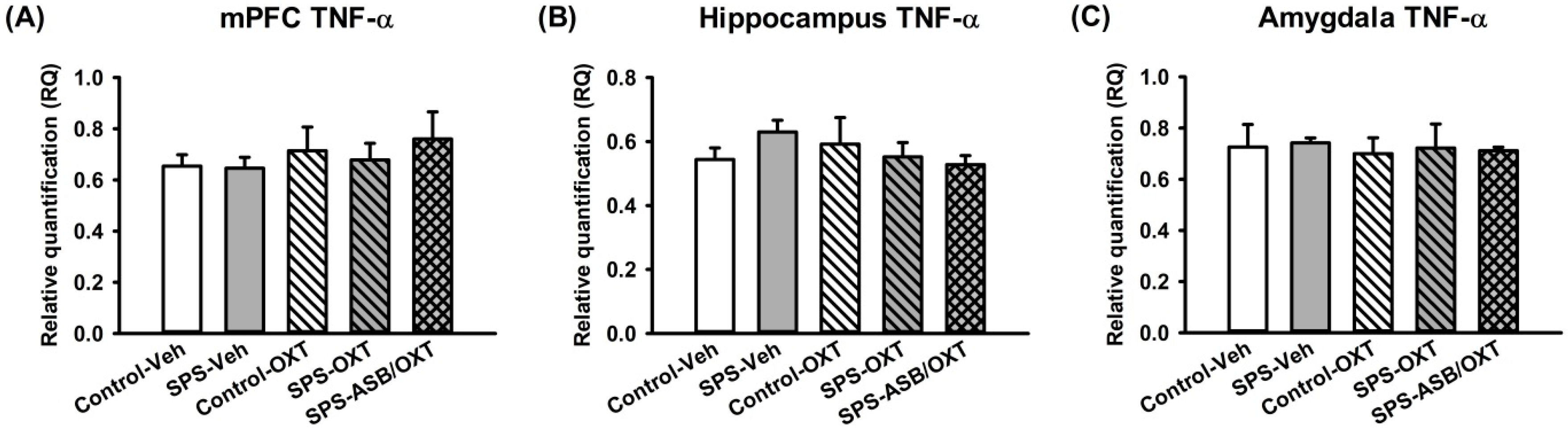
2.3. Dynamic Region-Dependent Changes of Central Pro-Inflammatory Cytokines
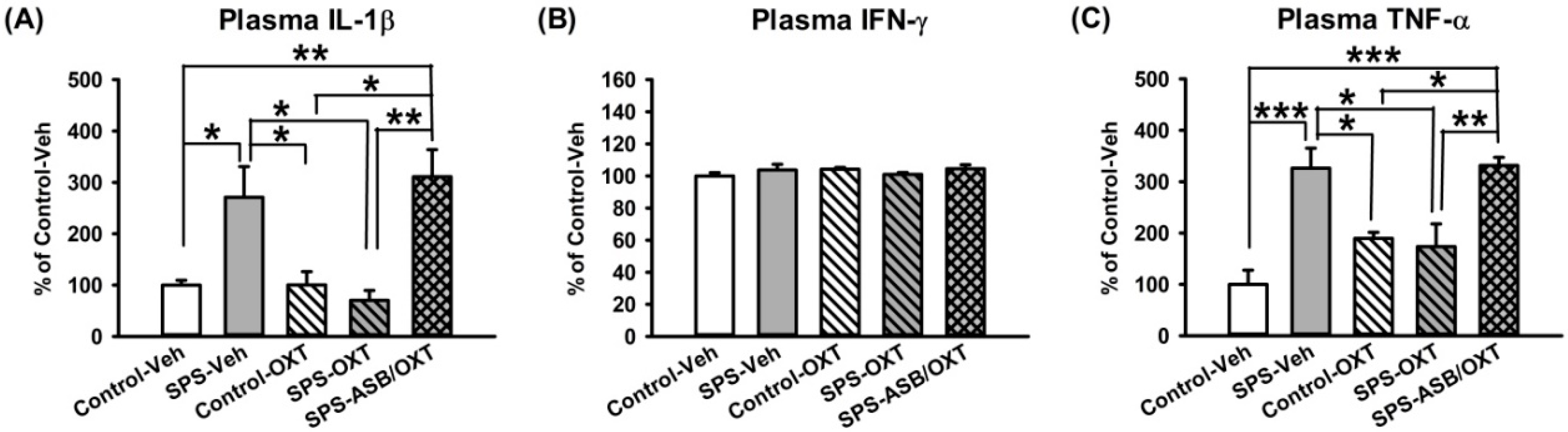
2.4. OXT Attenuated SPS-Induced Increment of Peripheral TNF-α and IL-1β Levels
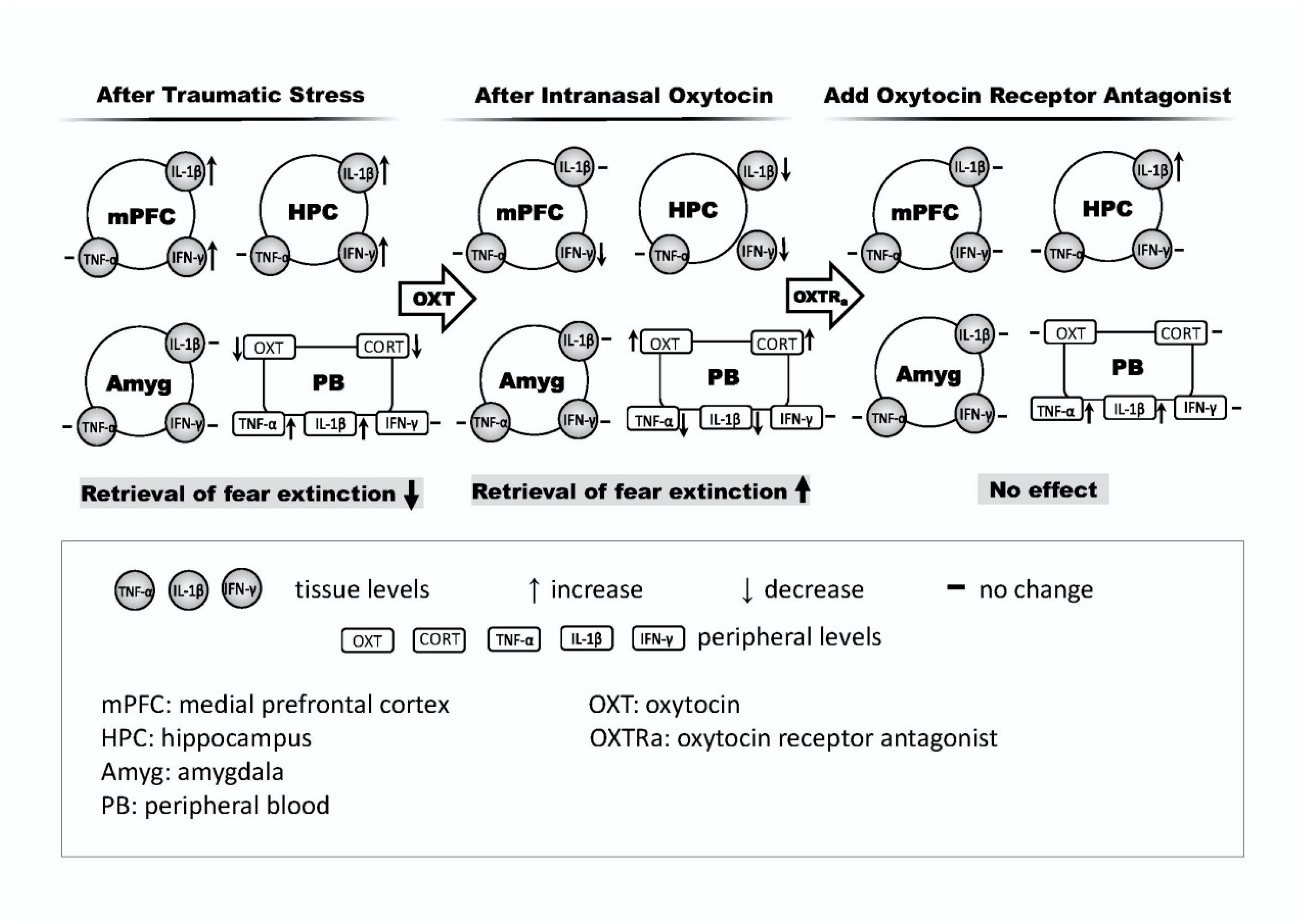
3. Discussion
4. Materials and Methods
4.1. Animals and PTSD Model
4.2. Experimental Design
4.3. Cue-Dependent Fear Conditioning-Extinction Test
4.4. Plasma OXT and CORT Analyses
4.5. Analysis of mRNA Expression
4.6. Plasma Cytokines Analyses
4.7. Data and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 2002, 346, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Cusack, K.; Forneris, C.A.; Wilkins, T.M.; Sonis, J.; Middleton, J.C.; Feltner, C.; Meredith, D.; Cavanaugh, J.; Brownley, K.A.; et al. Psychological and Pharmacological Treatments for Adults with Posttraumatic Stress Disorder (PTSD); Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013.
- Zoellner, L.A.; Pruitt, L.D.; Farach, F.J.; Jun, J.J. Understanding heterogeneity in PTSD: Fear, dysphoria, and distress. Depress. Anxiety 2014, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Abelson, J.L.; King, A.P.; Sripada, R.K.; Wang, X.; Gaines, L.M.; Liberzon, I. Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci. 2014, 34, 13435–13443. [Google Scholar] [CrossRef] [PubMed]
- Blechert, J.; Michael, T.; Vriends, N.; Margraf, J.; Wilhelm, F.H. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res. Ther. 2007, 45, 2019–2033. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Pitman, R.K.; Ellis, C.B.; Gold, A.L.; Shin, L.M.; Lasko, N.B.; Zeidan, M.A.; Handwerger, K.; Orr, S.P.; Rauch, S.L. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 2009, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Lin, A.H.; Verkerk, R.; Delmeire, L.; van Gastel, A.; van der Planken, M.; Scharpé, S. Serotonergic and noradrenergic markers of post-traumatic stress disorder with and without major depression. Neuropsychopharmacology 1999, 20, 188–197. [Google Scholar] [CrossRef]
- Van Elzakker, M.B.; Kathryn Dahlgren, M.; Caroline Davis, F.; Dubois, S.; Shin, L.M. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014, 113, 3–18. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Averill, L.A.; Akiki, T.J.; Raza, M.; Averill, C.L.; Gomaa, H.; Adikey, A.; Krystal, J.H. The Neurobiology and Pharmacotherapy of Posttraumatic Stress Disorder. Annu. Rev. Pharmacol. Toxicol. 2018, 59. [Google Scholar] [CrossRef]
- Lopatina, O.L.; Komleva, Y.K.; Gorina, Y.V.; Higashida, H.; Salmina, A.B. Neurobiological Aspects of Face Recognition: The Role of Oxytocin. Front. Behav. Neurosci. 2018, 12, 195. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, W.; Geng, Y.; Chen, Y.; Zhao, Z.; Ma, X.; Xu, L.; Becker, B.; Kendrick, K.M. Oxytocin Facilitates Approach Behavior to Positive Social Stimuli via Decreasing Anterior Insula Activity. Int. J. Neuropsychopharmacol. 2018, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Hand, A.; Jarnecke, A.M.; Moran-Santa Maria, M.M.; Brady, K.T.; Joseph, J.E. Effects of oxytocin on working memory and executive control system connectivity in posttraumatic stress disorder. Exp. Clin. Psychopharmacol. 2018, 26, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Frijling, J.L. Preventing PTSD with oxytocin: Effects of oxytocin administration on fear neurocircuitry and PTSD symptom development in recently trauma-exposed individuals. Eur. J. Psychotraumatol. 2017, 8, 1302652. [Google Scholar] [CrossRef] [PubMed]
- Renicker, M.D.; Cysewski, N.; Palmer, S.; Nakonechnyy, D.; Keef, A.; Thomas, M.; Magori, K.; Daberkow, D.P. Ameliorating Impact of Prophylactic Intranasal Oxytocin on Signs of Fear in a Rat Model of Traumatic Stress. Front. Behav. Neurosci. 2018, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- McGorry, P.D.; Hartmann, J.A.; Spooner, R.; Nelson, B. Beyond the “at risk mental state” concept: Transitioning to transdiagnostic psychiatry. World Psychiatry 2018, 17, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Amini-Khoei, H.; Mohammadi-Asl, A.; Amiri, S.; Hosseini, M.J.; Momeny, M.; Hassanipour, M.; Rastegar, M.; Haj-Mirzaian, A.; Mirzaian, A.H.; Sanjarimoghaddam, H.; et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 169–178. [Google Scholar] [CrossRef]
- Hurlemann, R.; Marsh, N. Deciphering the modulatory role of oxytocin in human altruism. Rev. Neurosci. 2017, 28, 335–342. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Priftis, K.N. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef]
- Pohl, T.T.; Young, L.J.; Bosch, O.J. Lost connections: Oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. Int. J. Psychophysiol. 2018. [Google Scholar] [CrossRef]
- Deslauriers, J.; Powell, S.; Risbrough, V.B. Immune signaling mechanisms of PTSD risk and symptom development: Insights from animal models. Curr. Opin. Behav. Sci. 2017, 14, 123–132. [Google Scholar] [CrossRef]
- Gill, J.M.; Saligan, L.; Woods, S.; Page, G. PTSD is associated with an excess of inflammatory immune activities. Perspect. Psychiatr. Care 2009, 45, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Rothbaum, A.O.; Jovanovic, T.; Almli, L.M.; Bradley, B.; Rothbaum, B.O.; Gillespie, C.F.; Ressler, K.J. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 2015, 172, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Felger, J.C. The Immunology of Behavior-Exploring the Role of the Immune System in Brain Health and Illness. Neuropsychopharmacology 2017, 42, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Murugan, S.; Zhang, C.; Boyadjieva, N. Regulation of cancer progression by β-endorphin neuron. Cancer Res. 2012, 72, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; Ebenezer, P.J.; McLaughlin, L.D.; Francis, J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE 2014, 9, e89104. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Krstov, M.; Young, E.A. Stress-restress: Effects on ACTH and fast feedback. Psychoneuroendocrinology 1997, 22, 443–453. [Google Scholar] [CrossRef]
- Liberzon, I.; Lopez, J.F.; Flagel, S.B.; Vazquez, D.M.; Young, E.A. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: Relevance to post-traumatic stress disorder. J. Neuroendocrinol. 1999, 11, 11–17. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, K.L.; Tung, C.S.; Liu, Y.P. Hyperbaric oxygen therapy restored traumatic stress-induced dysregulation of fear memory and related neurochemical abnormalities. Behav. Brain Res. 2018. [Google Scholar] [CrossRef]
- Lin, C.C.; Tung, C.S.; Liu, Y.P. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology 2016, 233, 1135–1146. [Google Scholar] [CrossRef]
- Lin, C.C.; Tung, C.S.; Lin, P.H.; Huang, C.L.; Liu, Y.P. Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur. Neuropsychopharmacol. 2016, 26, 1484–1495. [Google Scholar] [CrossRef]
- Knox, D.; George, S.A.; Fitzpatrick, C.J.; Rabinak, C.A.; Maren, S.; Liberzon, I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn. Mem. 2012, 19, 43–49. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morinobu, S.; Takei, S.; Fuchikami, M.; Matsuki, A.; Yamawaki, S.; Liberzon, I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress. Anxiety 2009, 26, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, M.; Gill, J.M.; Luckenbaugh, D.A.; Gold, P.W.; Collin, C.; Bonne, O.; Plumb, K.; Polignano, E.; West, K.; Charney, D. Low early morning plasma cortisol in posttraumatic stress disorder is associated with co-morbid depression but not with enhanced glucocorticoid feedback inhibition. Psychoneuroendocrinology 2010, 35, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Strohle, A.; Scheel, M.; Modell, S.; Holsboer, F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J. Psychiatry. Res. 2008, 42, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, C.; Li, X.; Zhang, P.; Liu, B.; Wang, H.; Wang, Y.; Yang, Y.; Wu, Y.; Li, H.; et al. Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav. Brain Res. 2018, 336, 236–243. [Google Scholar] [CrossRef]
- Acheson, D.; Feifel, D.; de Wilde, S.; McKinney, R.; Lohr, J.; Risbrough, V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology 2013, 229, 199–208. [Google Scholar] [CrossRef]
- Eckstein, M.; Becker, B.; Scheele, D.; Scholz, C.; Preckel, K.; Schlaepfer, T.E.; Grinevich, V.; Kendrick, K.M.; Maier, W.; Hurlemann, R. Oxytocin facilitates the extinction of conditioned fear in humans. Biol. Psychiatry 2015, 78, 194–202. [Google Scholar] [CrossRef]
- Lefevre, A.; Mottolese, R.; Dirheimer, M.; Mottolese, C.; Duhamel, J.R.; Sirigu, A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep. 2017, 7, 17222. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Jones, M.E.; Lebonville, C.L.; Barrus, D.; Lysle, D.T. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology 2015, 40, 1289–1296. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Walder, K.; Maes, M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol. Neurobiol. 2016, 53, 1195–1219. [Google Scholar] [CrossRef]
- Vankelecom, H.; Andries, M.; Billiau, A.; Denef, C. Evidence that folliculo-stellate cells mediate the inhibitory effect of interferon-γ on hormone secretion in rat anterior pituitary cell cultures. Endocrinology 1992, 130, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Czerniawski, J.; Guzowski, J.F. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J. Neurosci. 2014, 34, 12470–12480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dong, Y.; Xu, Z.; Gompf, H.S.; Ward, S.A.; Xue, Z.; Miao, C.; Zhang, Y.; Chamberlin, N.L.; Xie, Z. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol. Dis. 2012, 48, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Young, E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 1997, 22, 411–422. [Google Scholar] [CrossRef]
- Jimenez, A.; Young, L.J.; Triana-Del Rio, R.; LaPrairie, J.L.; Gonzalez-Mariscal, G. Neuroanatomical distribution of oxytocin receptor binding in the female rabbit forebrain: Variations across the reproductive cycle. Brain Res. 2015, 1629, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pare, D.; Royer, S.; Smith, Y.; Lang, E.J. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann. N. Y. Acad. Sci. 2003, 985, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Quirk, G.J.; Likhtik, E.; Pelletier, J.G.; Pare, D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003, 23, 8800–8807. [Google Scholar] [CrossRef]
- Spengler, F.B.; Schultz, J.; Scheele, D.; Essel, M.; Maier, W.; Heinrichs, M.; Hurlemann, R. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol. Psychiatry 2017, 82, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhaes, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Koenig, S.; Bredehoft, J.; Perniss, A.; Fuchs, F.; Roth, J.; Rummel, C. Age Dependent Hypothalamic and Pituitary Responses to Novel Environment Stress or Lipopolysaccharide in Rats. Front. Behave. Neurosci. 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Rasie Abdullahi, P.; Eskandarian, S.; Ghanbari, A.; Rashidy-Pour, A. Oxytocin receptor antagonist atosiban impairs consolidation, but not reconsolidation of contextual fear memory in rats. Brain Res. 2018, 1695, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.; Stanfield, B.R.; Staib, J.M.; David, N.P.; Keller, S.M.; DePietro, T. Neural circuits via which single prolonged stress exposure leads to fear extinction retention deficits. Learn. Mem. 2016, 23, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.H.; Chung, C.; Kim, J.J.; Choi, S.Y.; Han, J.S. Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci. Rep. 2015, 5, 18540. [Google Scholar] [CrossRef]
- Labonte, B.; Azoulay, N.; Yerko, V.; Turecki, G.; Brunet, A. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Transl. Psychiatry 2014, 4, e368. [Google Scholar] [CrossRef]
- Zak, P.J.; Kurzban, R.; Matzner, W.T. The neurobiology of trust. Ann. N. Y. Acad. Sci. 2004, 1032, 224–227. [Google Scholar] [CrossRef]
- Amsen, D.; de Visser, K.E.; Town, T. Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 2009, 511, 107–142. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Lin, C.-C.; Chen, C.-C.; Tzeng, N.-S.; Liu, Y.-P. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. Int. J. Mol. Sci. 2018, 19, 3848. https://doi.org/10.3390/ijms19123848
Wang S-C, Lin C-C, Chen C-C, Tzeng N-S, Liu Y-P. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. International Journal of Molecular Sciences. 2018; 19(12):3848. https://doi.org/10.3390/ijms19123848
Chicago/Turabian StyleWang, Sheng-Chiang, Chen-Cheng Lin, Chun-Chuan Chen, Nian-Sheng Tzeng, and Yia-Ping Liu. 2018. "Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder" International Journal of Molecular Sciences 19, no. 12: 3848. https://doi.org/10.3390/ijms19123848
APA StyleWang, S.-C., Lin, C.-C., Chen, C.-C., Tzeng, N.-S., & Liu, Y.-P. (2018). Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. International Journal of Molecular Sciences, 19(12), 3848. https://doi.org/10.3390/ijms19123848




