Genetic Overlap between General Cognitive Function and Schizophrenia: A Review of Cognitive GWASs
Abstract
1. Introduction
2. General Cognitive Function (g)
3. Fluid Intelligence
4. Educational Attainment
5. Genes and Functions Related to General Cognitive Function
6. Polygenic Risk Score Analysis and Genetic Correlation between General Cognitive Function and Schizophrenia
7. Genetic Correlations between General Cognitive Function and Socioeconomic and Health-Related Outcomes
8. Intelligence Decline in Schizophrenia
- (a)
- Deteriorated group: patients with a difference of 10 points or more between premorbid IQ and present IQ;
- (b)
- Preserved group: patients with a difference of less than 10 points between premorbid IQ and present IQ (premorbid IQ above 90);
- (c)
- Compromised group: patients with a difference of less than 10 points between premorbid IQ and present IQ (premorbid IQ below 90).
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GWASs | Genome-Wide Association Studies |
| PGC | Psychiatric Genomics Consortium |
| COGENT | Cognitive Genomics Consortium |
| CHARGE | Heart and Aging Research in Genomic Epidemiology consortium |
| UKB | UK Biobank |
| IQ | Intelligence Quotient |
| PCA | Principal Component Analysis |
| WAIS | Wechsler Adult Intelligence Scale |
| AVLT | Auditory verbal learning Test |
| VNR | Verbal–Numerical Reasoning |
| LDSC | Linkage Disequilibrium Score Regression |
References
- Gottfredson, S.L. Why g matters: The complexity of everyday life. Intelligence 1997, 24, 79–132. [Google Scholar]
- Batty, G.D.; Deary, I.J.; Gottfredson, L.S. Premorbid (early life) IQ and later mortality risk: Systematic review. Ann. Epidemiol. 2007, 17, 278–288. [Google Scholar]
- Kahn, R.S.; Keefe, R.S. Schizophrenia is a cognitive illness: Time for a change in focus. JAMA Psychiatry 2013, 70, 1107–1112. [Google Scholar]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar]
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar]
- Sawada, K.; Kanehara, A.; Sakakibara, E.; Eguchi, S.; Tada, M.; Satomura, Y.; Suga, M.; Koike, S.; Kasai, K. Identifying neurocognitive markers for outcome prediction of global functioning in individuals with first-episode and ultra-high-risk for psychosis. Psychiatry Clin. Neurosci. 2017, 71, 318–327. [Google Scholar]
- Fujino, H.; Sumiyoshi, C.; Sumiyoshi, T.; Yasuda, Y.; Yamamori, H.; Ohi, K.; Fujimoto, M.; Hashimoto, R.; Takeda, M.; Imura, O. Predicting employment status and subjective quality of life in patients with schizophrenia. Schizophr. Res. Cogn. 2016, 3, 20–25. [Google Scholar]
- Sumiyoshi, C.; Harvey, P.D.; Takaki, M.; Okahisa, Y.; Sato, T.; Sora, I.; Nuechterlein, K.H.; Subotnik, K.L.; Sumiyoshi, T. Factors predicting work outcome in Japanese patients with schizophrenia: Role of multiple functioning levels. Schizophr. Res. Cogn. 2015, 2, 105–112. [Google Scholar]
- Ohi, K.; Shimada, T.; Nemoto, K.; Kataoka, Y.; Yasuyama, T.; Kimura, K.; Okubo, H.; Uehara, T.; Kawasaki, Y. Cognitive clustering in schizophrenia patients, their first-degree relatives and healthy subjects is associated with anterior cingulate cortex volume. Neuroimage Clin. 2017, 16, 248–256. [Google Scholar]
- Simeone, J.C.; Ward, A.J.; Rotella, P.; Collins, J.; Windisch, R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990 horizontal line 2013: A systematic literature review. BMC Psychiatry 2015, 15, 193. [Google Scholar]
- Fujino, H.; Sumiyoshi, C.; Sumiyoshi, T.; Yasuda, Y.; Yamamori, H.; Ohi, K.; Fujimoto, M.; Umeda-Yano, S.; Higuchi, A.; Hibi, Y.; et al. Performance on the Wechsler Adult Intelligence Scale-III in Japanese patients with schizophrenia. Psychiatry Clin. Neurosci. 2014, 68, 534–541. [Google Scholar]
- Ohi, K.; Hashimoto, R.; Yasuda, Y.; Fukumoto, M.; Nemoto, K.; Ohnishi, T.; Yamamori, H.; Takahashi, H.; Iike, N.; Kamino, K.; et al. The AKT1 gene is associated with attention and brain morphology in schizophrenia. World J. Biol. Psychiatry 2013, 14, 100–113. [Google Scholar]
- Fukumoto, M.; Hashimoto, R.; Ohi, K.; Yasuda, Y.; Yamamori, H.; Umeda-Yano, S.; Iwase, M.; Kazui, H.; Takeda, M. Relation between remission status and attention in patients with schizophrenia. Psychiatry Clin. Neurosci. 2014, 68, 234–241. [Google Scholar]
- Ohi, K.; Hashimoto, R.; Yasuda, Y.; Fukumoto, M.; Yamamori, H.; Umeda-Yano, S.; Fujimoto, M.; Iwase, M.; Kazui, H.; Takeda, M. Influence of the NRGN gene on intellectual ability in schizophrenia. J. Hum. Genet. 2013, 58, 700–705. [Google Scholar]
- Ohi, K.; Hashimoto, R.; Ikeda, M.; Yamamori, H.; Yasuda, Y.; Fujimoto, M.; Umeda-Yano, S.; Fukunaga, M.; Fujino, H.; Watanabe, Y.; et al. Glutamate Networks Implicate Cognitive Impairments in Schizophrenia: Genome-Wide Association Studies of 52 Cognitive Phenotypes. Schizophr. Bull. 2015, 41, 909–918. [Google Scholar]
- Horiguchi, M.; Ohi, K.; Hashimoto, R.; Hao, Q.; Yasuda, Y.; Yamamori, H.; Fujimoto, M.; Umeda-Yano, S.; Takeda, M.; Ichinose, H. Functional polymorphism (C-824T) of the tyrosine hydroxylase gene affects IQ in schizophrenia. Psychiatry Clin. Neurosci. 2014, 68, 456–462. [Google Scholar]
- Hashimoto, R.; Noguchi, H.; Hori, H.; Ohi, K.; Yasuda, Y.; Takeda, M.; Kunugi, H. Association between the dysbindin gene (DTNBP1) and cognitive functions in Japanese subjects. Psychiatry Clin. Neurosci. 2009, 63, 550–556. [Google Scholar]
- Badcock, J.C.; Dragovic, M.; Waters, F.A.; Jablensky, A. Dimensions of intelligence in schizophrenia: Evidence from patients with preserved, deteriorated and compromised intellect. J. Psychiatr. Res. 2005, 39, 11–19. [Google Scholar]
- Ikebuchi, E.; Sato, S.; Yamaguchi, S.; Shimodaira, M.; Taneda, A.; Hatsuse, N.; Watanabe, Y.; Sakata, M.; Satake, N.; Nishio, M.; et al. Does improvement of cognitive functioning by cognitive remediation therapy effect work outcomes in severe mental illness? A secondary analysis of a randomized controlled trial. Psychiatry Clin. Neurosci. 2017, 71, 301–308. [Google Scholar]
- Kremen, W.S.; Vinogradov, S.; Poole, J.H.; Schaefer, C.A.; Deicken, R.F.; Factor-Litvak, P.; Brown, A.S. Cognitive decline in schizophrenia from childhood to midlife: A 33-year longitudinal birth cohort study. Schizophr. Res. 2010, 118, 1–5. [Google Scholar]
- Meier, M.H.; Caspi, A.; Reichenberg, A.; Keefe, R.S.; Fisher, H.L.; Harrington, H.; Houts, R.; Poulton, R.; Moffitt, T.E. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: Evidence from a population-representative longitudinal study. Am. J. Psychiatry 2014, 171, 91–101. [Google Scholar]
- Sheitman, B.B.; Murray, M.G.; Snyder, J.A.; Silva, S.; Goldman, R.; Chakos, M.; Volavka, J.; Lieberman, J.A. IQ scores of treatment-resistant schizophrenia patients before and after the onset of the illness. Schizophr. Res. 2000, 46, 203–207. [Google Scholar]
- Leeson, V.C.; Sharma, P.; Harrison, M.; Ron, M.A.; Barnes, T.R.; Joyce, E.M. IQ trajectory, cognitive reserve, and clinical outcome following a first episode of psychosis: A 3-year longitudinal study. Schizophr. Bull. 2011, 37, 768–777. [Google Scholar]
- Bilder, R.M.; Goldman, R.S.; Robinson, D.; Reiter, G.; Bell, L.; Bates, J.A.; Pappadopulos, E.; Willson, D.F.; Alvir, J.M.; Woerner, M.G.; et al. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. Am. J. Psychiatry 2000, 157, 549–559. [Google Scholar]
- Hill, S.K.; Schuepbach, D.; Herbener, E.S.; Keshavan, M.S.; Sweeney, J.A. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr. Res. 2004, 68, 49–63. [Google Scholar]
- Hoff, A.L.; Sakuma, M.; Wieneke, M.; Horon, R.; Kushner, M.; DeLisi, L.E. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am. J. Psychiatry 1999, 156, 1336–1341. [Google Scholar]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar]
- Swagerman, S.C.; de Geus, E.J.; Kan, K.J.; van Bergen, E.; Nieuwboer, H.A.; Koenis, M.M.; Hulshoff Pol, H.E.; Gur, R.E.; Gur, R.C.; Boomsma, D.I. The Computerized Neurocognitive Battery: Validation, aging effects, and heritability across cognitive domains. Neuropsychology 2016, 30, 53–64. [Google Scholar]
- Husted, J.A.; Lim, S.; Chow, E.W.; Greenwood, C.; Bassett, A.S. Heritability of neurocognitive traits in familial schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 845–853. [Google Scholar]
- Berrettini, W.H. Genetic bases for endophenotypes in psychiatric disorders. Dialogues Clin. Neurosci. 2005, 7, 95–101. [Google Scholar]
- Chen, W.J.; Liu, S.K.; Chang, C.J.; Lien, Y.J.; Chang, Y.H.; Hwu, H.G. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am. J. Psychiatry 1998, 155, 1214–1220. [Google Scholar]
- Posthuma, D.; de Geus, E.J.; Boomsma, D.I. Perceptual speed and IQ are associated through common genetic factors. Behav. Genet. 2001, 31, 593–602. [Google Scholar]
- Polderman, T.J.; Benyamin, B.; de Leeuw, C.A.; Sullivan, P.F.; van Bochoven, A.; Visscher, P.M.; Posthuma, D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015, 47, 702–709. [Google Scholar]
- Toulopoulou, T.; Goldberg, T.E.; Mesa, I.R.; Picchioni, M.; Rijsdijk, F.; Stahl, D.; Cherny, S.S.; Sham, P.; Faraone, S.V.; Tsuang, M.; et al. Impaired intellect and memory: A missing link between genetic risk and schizophrenia? Arch. Gen. Psychiatry 2010, 67, 905–913. [Google Scholar]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.; Farh, K.H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar]
- Lencz, T.; Knowles, E.; Davies, G.; Guha, S.; Liewald, D.C.; Starr, J.M.; Djurovic, S.; Melle, I.; Sundet, K.; Christoforou, A.; et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: A report from the Cognitive Genomics consorTium (COGENT). Mol. Psychiatry 2014, 19, 168–174. [Google Scholar]
- Davies, G.; Armstrong, N.; Bis, J.C.; Bressler, J.; Chouraki, V.; Giddaluru, S.; Hofer, E.; Ibrahim-Verbaas, C.A.; Kirin, M.; Lahti, J.; et al. Genetic contributions to variation in general cognitive function: A meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53949). Mol. Psychiatry 2015, 20, 183–192. [Google Scholar]
- Davies, G.; Marioni, R.E.; Liewald, D.C.; Hill, W.D.; Hagenaars, S.P.; Harris, S.E.; Ritchie, S.J.; Luciano, M.; Fawns-Ritchie, C.; Lyall, D.; et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol. Psychiatry 2016, 21, 758–767. [Google Scholar]
- Trampush, J.W.; Yang, M.L.; Yu, J.; Knowles, E.; Davies, G.; Liewald, D.C.; Starr, J.M.; Djurovic, S.; Melle, I.; Sundet, K.; et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: A report from the COGENT consortium. Mol. Psychiatry 2017, 22, 336–345. [Google Scholar]
- Sniekers, S.; Stringer, S.; Watanabe, K.; Jansen, P.R.; Coleman, J.R.I.; Krapohl, E.; Taskesen, E.; Hammerschlag, A.R.; Okbay, A.; Zabaneh, D.; et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 2017, 49, 1107–1112. [Google Scholar]
- Lam, M.; Trampush, J.W.; Yu, J.; Knowles, E.; Davies, G.; Liewald, D.C.; Starr, J.M.; Djurovic, S.; Melle, I.; Sundet, K.; et al. Large-Scale Cognitive GWAS Meta-Analysis Reveals Tissue-Specific Neural Expression and Potential Nootropic Drug Targets. Cell Rep. 2017, 21, 2597–2613. [Google Scholar]
- Davies, G.; Lam, M.; Harris, S.E.; Trampush, J.W.; Luciano, M.; Hill, W.D.; Hagenaars, S.P.; Ritchie, S.J.; Marioni, R.E.; Fawns-Ritchie, C.; et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 2018, 9, 2098. [Google Scholar]
- Savage, J.E.; Jansen, P.R.; Stringer, S.; Watanabe, K.; Bryois, J.; de Leeuw, C.A.; Nagel, M.; Awasthi, S.; Barr, P.B.; Coleman, J.R.I.; et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 2018, 50, 912–919. [Google Scholar]
- Davies, G.; Tenesa, A.; Payton, A.; Yang, J.; Harris, S.E.; Liewald, D.; Ke, X.; Le Hellard, S.; Christoforou, A.; Luciano, M.; et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol. Psychiatry 2011, 16, 996–1005. [Google Scholar]
- Benyamin, B.; Pourcain, B.; Davis, O.S.; Davies, G.; Hansell, N.K.; Brion, M.J.; Kirkpatrick, R.M.; Cents, R.A.; Franic, S.; Miller, M.B.; et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol. Psychiatry 2014, 19, 253–258. [Google Scholar]
- Kirkpatrick, R.M.; McGue, M.; Iacono, W.G.; Miller, M.B.; Basu, S. Results of a “GWAS plus:” general cognitive ability is substantially heritable and massively polygenic. PLoS ONE 2014, 9, e112390. [Google Scholar]
- Ohi, K.; Sumiyoshi, C.; Fujino, H.; Yasuda, Y.; Yamamori, H.; Fujimoto, M.; Sumiyoshi, T.; Hashimoto, R. A Brief Assessment of Intelligence Decline in Schizophrenia As Represented by the Difference between Current and Premorbid Intellectual Quotient. Front. Psychiatry 2017, 8, 293. [Google Scholar]
- Hashimoto, R.; Ikeda, M.; Ohi, K.; Yasuda, Y.; Yamamori, H.; Fukumoto, M.; Umeda-Yano, S.; Dickinson, D.; Aleksic, B.; Iwase, M.; et al. Genome-wide association study of cognitive decline in schizophrenia. Am. J. Psychiatry 2013, 170, 683–684. [Google Scholar]
- Plomin, R.; Kovas, Y. Generalist genes and learning disabilities. Psychol. Bull. 2005, 131, 592–617. [Google Scholar]
- Johnson, W.; Nijenhuis, J.T.; Bouchard, T.J., Jr. Still just 1 g: Consistent results from five test batteries. Intelligence 2008, 36, 81–95. [Google Scholar]
- Salthouse, T.A. Localizing age-related individual differences in a hierarchical structure. Intelligence 2004, 32. [Google Scholar]
- Craik, F.I.; Bialystok, E. Cognition through the lifespan: Mechanisms of change. Trends Cogn. Sci. 2006, 10, 131–138. [Google Scholar]
- Deary, I.J.; Penke, L.; Johnson, W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010, 11, 201–211. [Google Scholar]
- Rietveld, C.A.; Medland, S.E.; Derringer, J.; Yang, J.; Esko, T.; Martin, N.W.; Westra, H.J.; Shakhbazov, K.; Abdellaoui, A.; Agrawal, A.; et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science 2013, 340, 1467–1471. [Google Scholar]
- Okbay, A.; Beauchamp, J.P.; Fontana, M.A.; Lee, J.J.; Pers, T.H.; Rietveld, C.A.; Turley, P.; Chen, G.B.; Emilsson, V.; Meddens, S.F.; et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 2016, 533, 539–542. [Google Scholar]
- Smeland, O.B.; Frei, O.; Kauppi, K.; Hill, W.D.; Li, W.; Wang, Y.; Krull, F.; Bettella, F.; Eriksen, J.A.; Witoelar, A.; et al. Identification of Genetic Loci Jointly Influencing Schizophrenia Risk and the Cognitive Traits of Verbal-Numerical Reasoning, Reaction Time, and General Cognitive Function. JAMA Psychiatry 2017, 74, 1065–1075. [Google Scholar]
- Hubbard, L.; Tansey, K.E.; Rai, D.; Jones, P.; Ripke, S.; Chambert, K.D.; Moran, J.L.; McCarroll, S.A.; Linden, D.E.; Owen, M.J.; et al. Evidence of Common Genetic Overlap Between Schizophrenia and Cognition. Schizophr. Bull. 2016, 42, 832–842. [Google Scholar]
- McIntosh, A.M.; Gow, A.; Luciano, M.; Davies, G.; Liewald, D.C.; Harris, S.E.; Corley, J.; Hall, J.; Starr, J.M.; Porteous, D.J.; et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol. Psychiatry 2013, 73, 938–943. [Google Scholar]
- Nakahara, S.; Medland, S.; Turner, J.A.; Calhoun, V.D.; Lim, K.O.; Mueller, B.A.; Bustillo, J.R.; O’Leary, D.S.; Vaidya, J.G.; McEwen, S.; et al. Polygenic risk score, genome-wide association, and gene set analyses of cognitive domain deficits in schizophrenia. Schizophr. Res. 2018, 201, 393–399. [Google Scholar] [CrossRef]
- Liebers, D.T.; Pirooznia, M.; Seiffudin, F.; Musliner, K.L.; Zandi, P.P.; Goes, F.S. Polygenic Risk of Schizophrenia and Cognition in a Population-Based Survey of Older Adults. Schizophr. Bull. 2016, 42, 984–991. [Google Scholar]
- Hatzimanolis, A.; Bhatnagar, P.; Moes, A.; Wang, R.; Roussos, P.; Bitsios, P.; Stefanis, C.N.; Pulver, A.E.; Arking, D.E.; Smyrnis, N.; et al. Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168B, 392–401. [Google Scholar]
- Germine, L.; Robinson, E.B.; Smoller, J.W.; Calkins, M.E.; Moore, T.M.; Hakonarson, H.; Daly, M.J.; Lee, P.H.; Holmes, A.J.; Buckner, R.L.; et al. Association between polygenic risk for schizophrenia, neurocognition and social cognition across development. Transl. Psychiatry 2016, 6, e924. [Google Scholar]
- Hagenaars, S.P.; Harris, S.E.; Davies, G.; Hill, W.D.; Liewald, D.C.; Ritchie, S.J.; Marioni, R.E.; Fawns-Ritchie, C.; Cullen, B.; Malik, R.; et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112 151) and 24 GWAS consortia. Mol. Psychiatry 2016, 21, 1624–1632. [Google Scholar]
- Van Scheltinga, A.F.; Bakker, S.C.; van Haren, N.E.; Derks, E.M.; Buizer-Voskamp, J.E.; Cahn, W.; Ripke, S.; Ophoff, R.A.; Kahn, R.S. Schizophrenia genetic variants are not associated with intelligence. Psychol. Med. 2013, 43, 2563–2570. [Google Scholar]
- Bulik-Sullivan, B.K.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar]
- Swanson, C.L., Jr.; Gur, R.C.; Bilker, W.; Petty, R.G.; Gur, R.E. Premorbid educational attainment in schizophrenia: Association with symptoms, functioning, and neurobehavioral measures. Biol. Psychiatry 1998, 44, 739–747. [Google Scholar]
- Power, R.A.; Steinberg, S.; Bjornsdottir, G.; Rietveld, C.A.; Abdellaoui, A.; Nivard, M.M.; Johannesson, M.; Galesloot, T.E.; Hottenga, J.J.; Willemsen, G.; et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat. Neurosci. 2015, 18, 953–955. [Google Scholar]
- Bansal, V.; Mitjans, M.; Burik, C.A.P.; Karlsson Linner, R.; Okbay, A.; Rietveld, C.A.; Begemann, M.; Bonn, S.; Ripke, S.; de Vlaming, R.; et al. Genome-wide association study results for educational attainment aid in identifying genetic heterogeneity of schizophrenia. bioRxiv 2018. [Google Scholar] [CrossRef]
- Fujino, H.; Sumiyoshi, C.; Yasuda, Y.; Yamamori, H.; Fujimoto, M.; Fukunaga, M.; Miura, K.; Takebayashi, Y.; Okada, N.; Isomura, S.; et al. Estimated cognitive decline in patients with schizophrenia: A multicenter study. Psychiatry Clin. Neurosci. 2017, 71, 294–300. [Google Scholar]
- Sumiyoshi, C.; Fujino, H.; Sumiyoshi, T.; Yasuda, Y.; Yamamori, H.; Ohi, K.; Fujimoto, M.; Takeda, M.; Hashimoto, R. Usefulness of the Wechsler Intelligence Scale short form for assessing functional outcomes in patients with schizophrenia. Psychiatry Res. 2016, 245, 371–378. [Google Scholar]
- Weickert, T.W.; Goldberg, T.E.; Gold, J.M.; Bigelow, L.B.; Egan, M.F.; Weinberger, D.R. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch. Gen. Psychiatry 2000, 57, 907–913. [Google Scholar]
- Kremen, W.S.; Seidman, L.J.; Faraone, S.V.; Tsuang, M.T. IQ decline in cross-sectional studies of schizophrenia: Methodology and interpretation. Psychiatry Res. 2008, 158, 181–194. [Google Scholar]
- Potter, A.I.; Nestor, P.G. IQ subtypes in schizophrenia: Distinct symptom and neuropsychological profiles. J. Nerv. Ment. Dis. 2010, 198, 580–585. [Google Scholar]
- Mercado, C.L.; Johannesen, J.K.; Bell, M.D. Thought disorder severity in compromised, deteriorated, and preserved intellectual course of schizophrenia. J. Nerv. Ment. Dis. 2011, 199, 111–116. [Google Scholar]
- Ammari, N.; Heinrichs, R.W.; Pinnock, F.; Miles, A.A.; Muharib, E.; McDermid Vaz, S. Preserved, deteriorated, and premorbidly impaired patterns of intellectual ability in schizophrenia. Neuropsychology 2014, 28, 353–358. [Google Scholar]
- Wells, R.; Swaminathan, V.; Sundram, S.; Weinberg, D.; Bruggemann, J.; Jacomb, I.; Cropley, V.; Lenroot, R.; Pereira, A.M.; Zalesky, A.; et al. The impact of premorbid and current intellect in schizophrenia: Cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015, 1, 15043. [Google Scholar]
- Weinberg, D.; Lenroot, R.; Jacomb, I.; Allen, K.; Bruggemann, J.; Wells, R.; Balzan, R.; Liu, D.; Galletly, C.; Catts, S.V.; et al. Cognitive Subtypes of Schizophrenia Characterized by Differential Brain Volumetric Reductions and Cognitive Decline. JAMA Psychiatry 2016, 73, 1251–1259. [Google Scholar]
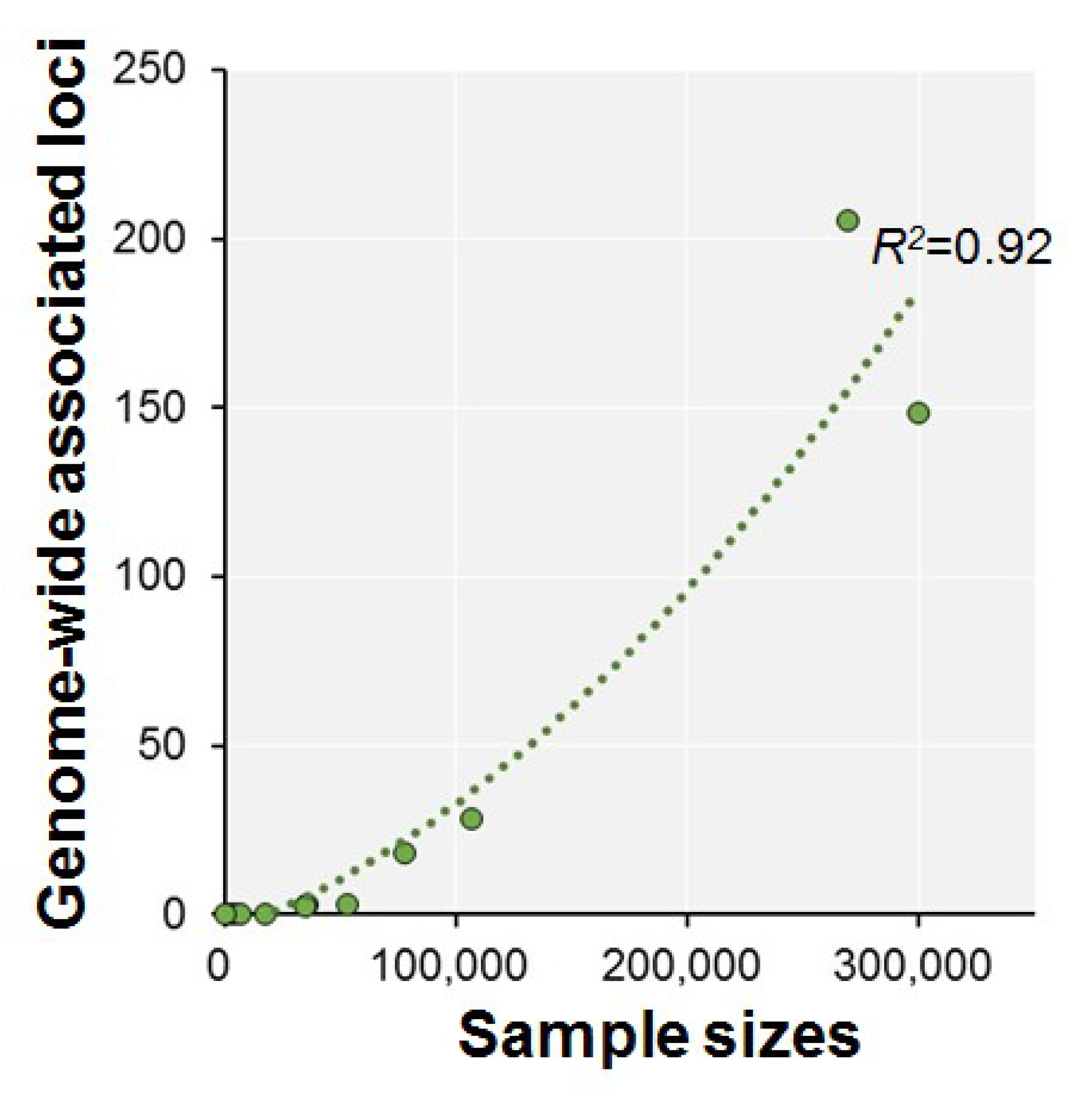
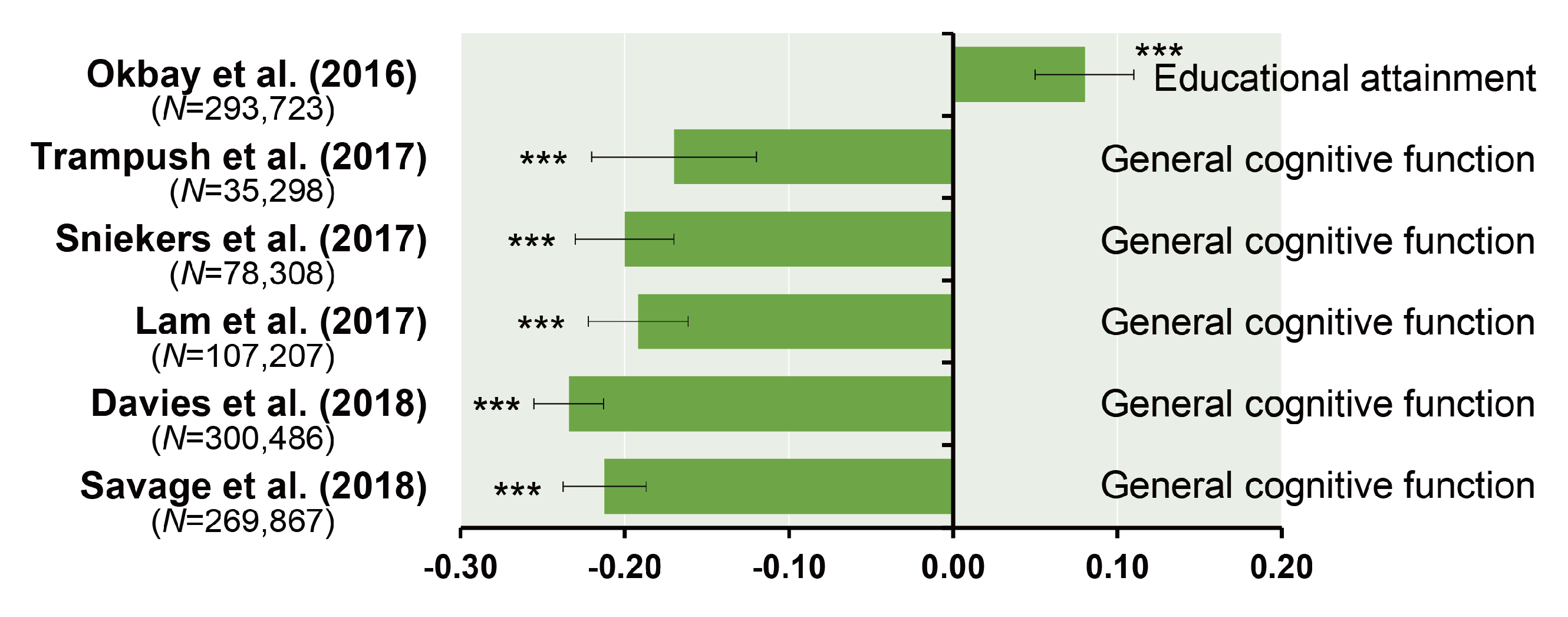
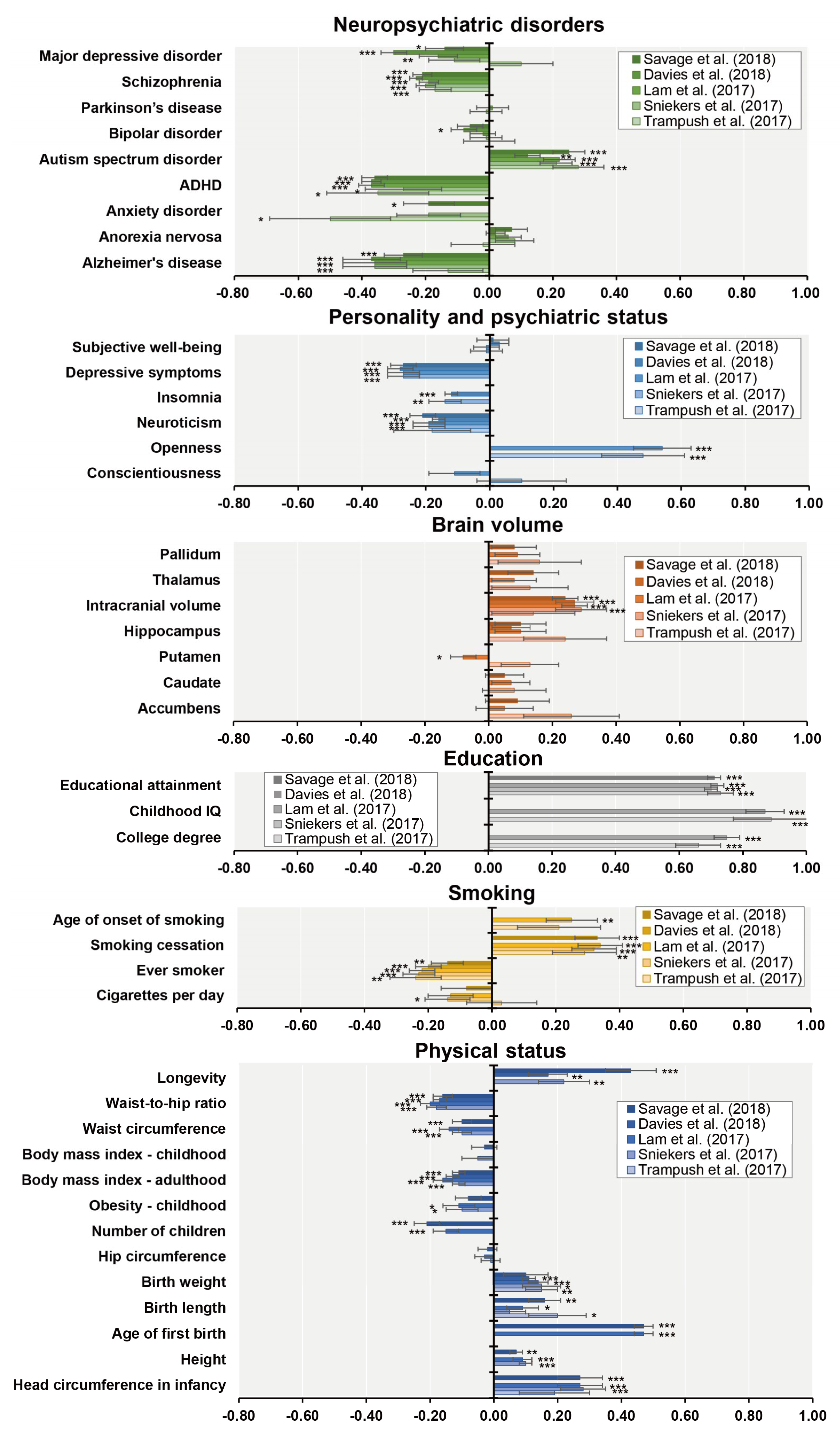
| Authors (year) | n | Phenotypes | Ethnics | Participants | Consortium | Age Range | GWS Loci | SNP Hits | GWS Gene |
|---|---|---|---|---|---|---|---|---|---|
| Ohi et al. (2015) [15] | 411 | g or IQ | Japanese | Psychiatric healthy subjects | Osaka University | 18–66 | 0 | 0 | NA |
| Davies et al. (2011) [44] | 3511 | g | Caucasian | Nonclinical healthy samples | CAGES, LBC1921, LBC1936, ABC1936, etc. | 64.6–79.1 * | 0 | 0 | 1 |
| Lencz et al. (2014) [36] | 5000 | g | Caucasian | General population (epidemiologically representative cohorts or mentally healthy cohorts) | COGENT | 15.9–69.5 * | 0 | 0 | NA |
| Benyamin et al. (2014) [45] | 17,989 | g or IQ | European | Children | CHIC | 6–18 | 0 | 0 | 0 |
| Kirkpatrick et al. (2014) [46] | 7100 | IQ | Caucasian | Community-based family study samples | MTFS, SIBS | 11.8–43.3 * | 0 | 0 | 0 |
| Davies et al. (2015) [37] | 53,949 | g | European | Population-based cohorts | CHARGE | >45 | 3 | 13 | 1 |
| Davies et al. (2016) [38] | 36,035 | Fluid intelligence (VNR) | White British | Touchscreen-based community-dwelling individuals | UKB | 40–73 | 3 | 149 | 17 |
| Trampush et al. (2017) [39] | 35,298 | g | European | General population | COGENT | 8–96 | 2 | 7 | 7 |
| Sniekers et al. (2017) [40] | 78,308 | g, IQ or Fluid (VNR) | European | Web-base and touchscreen-based community-dwelling individuals and population-based cohorts | UKB, CHIC, MTFS, etc. | 8–78 | 18 | 336 | 47 |
| Lam et al. (2017) [41] | 107,207 | g, IQ or Fluid (VNR) | European | Web-base and touchscreen-based community-dwelling individuals and population-based cohorts | COGENT, UKB, CHIC, etc. | 8–96 | 28 | 469 | 73 |
| Davies et al. (2018) [42] | 300,486 | g or Fluid (VNR) | European | Web-base and touchscreen-based community-dwelling individuals and population-based cohorts | CHARGE, COGENT, UKB | 16–102 | 148 | 11,600 | 709 |
| Savage et al. (2018) [43] | 269,867 | g, IQ or Fluid (VNR) | European | Epidemiological cohorts | COGENT, UKB, etc. | 5–98 | 205 | 12,110 | 507 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohi, K.; Sumiyoshi, C.; Fujino, H.; Yasuda, Y.; Yamamori, H.; Fujimoto, M.; Shiino, T.; Sumiyoshi, T.; Hashimoto, R. Genetic Overlap between General Cognitive Function and Schizophrenia: A Review of Cognitive GWASs. Int. J. Mol. Sci. 2018, 19, 3822. https://doi.org/10.3390/ijms19123822
Ohi K, Sumiyoshi C, Fujino H, Yasuda Y, Yamamori H, Fujimoto M, Shiino T, Sumiyoshi T, Hashimoto R. Genetic Overlap between General Cognitive Function and Schizophrenia: A Review of Cognitive GWASs. International Journal of Molecular Sciences. 2018; 19(12):3822. https://doi.org/10.3390/ijms19123822
Chicago/Turabian StyleOhi, Kazutaka, Chika Sumiyoshi, Haruo Fujino, Yuka Yasuda, Hidenaga Yamamori, Michiko Fujimoto, Tomoko Shiino, Tomiki Sumiyoshi, and Ryota Hashimoto. 2018. "Genetic Overlap between General Cognitive Function and Schizophrenia: A Review of Cognitive GWASs" International Journal of Molecular Sciences 19, no. 12: 3822. https://doi.org/10.3390/ijms19123822
APA StyleOhi, K., Sumiyoshi, C., Fujino, H., Yasuda, Y., Yamamori, H., Fujimoto, M., Shiino, T., Sumiyoshi, T., & Hashimoto, R. (2018). Genetic Overlap between General Cognitive Function and Schizophrenia: A Review of Cognitive GWASs. International Journal of Molecular Sciences, 19(12), 3822. https://doi.org/10.3390/ijms19123822





