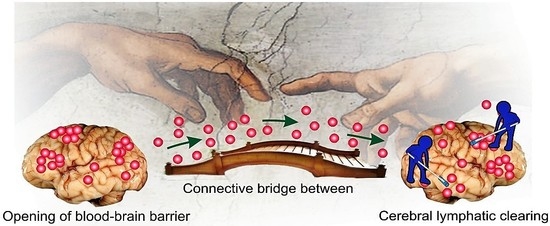
Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses
Abstract
1. Lymphatics and Central Nervous System Health
2. Where and How Lymph is Generated in the Brain?
3. The Gaps in the Lymphatic Anatomy in the Brain
4. Problems with Current Concepts about Lymphatic Functions in the Brain
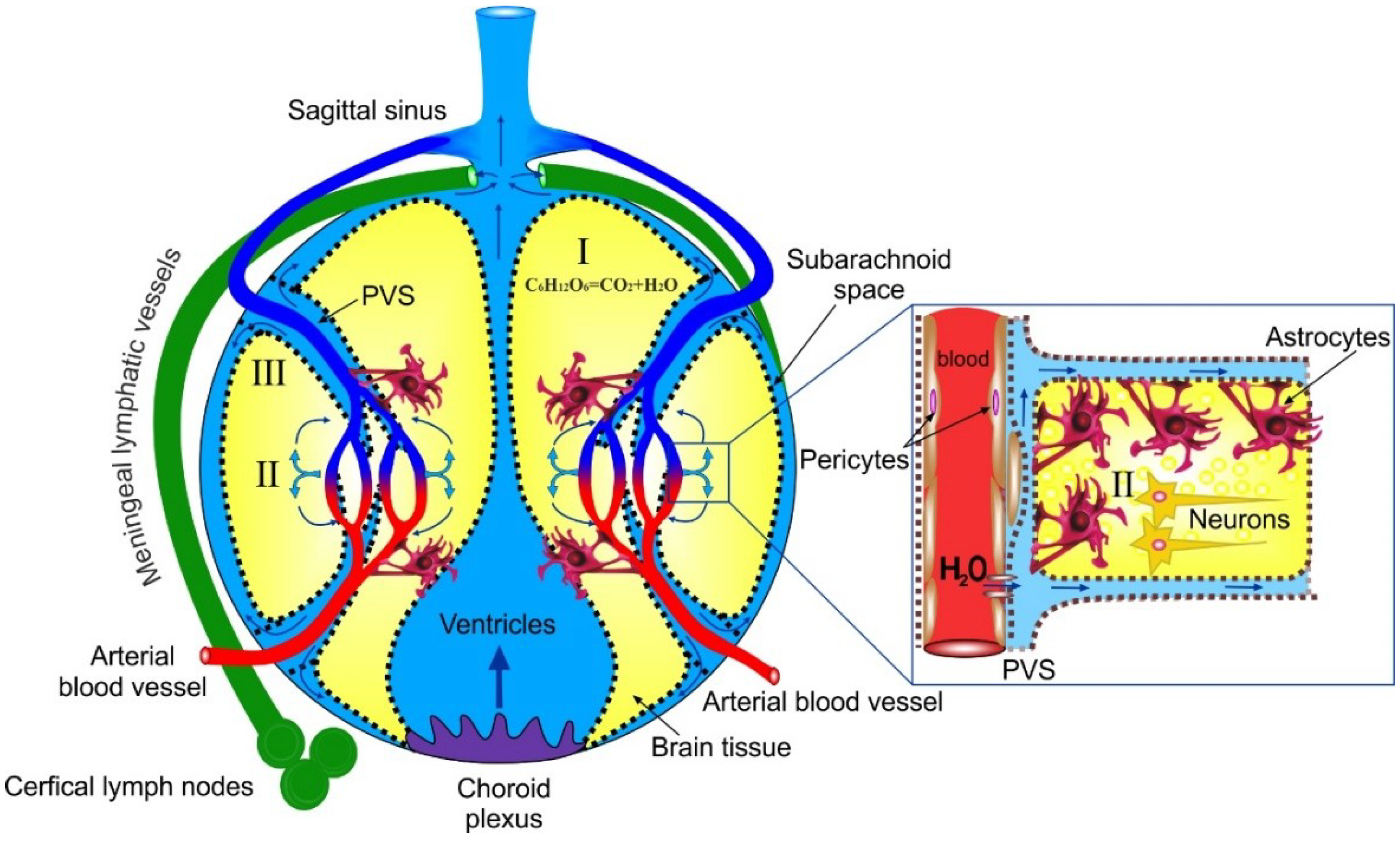
5. Mechanisms of ISF Fluid Transport in the Brain
6. Conceptual Problems in Glymphatic Mechanisms of ISF Transport in the Brain
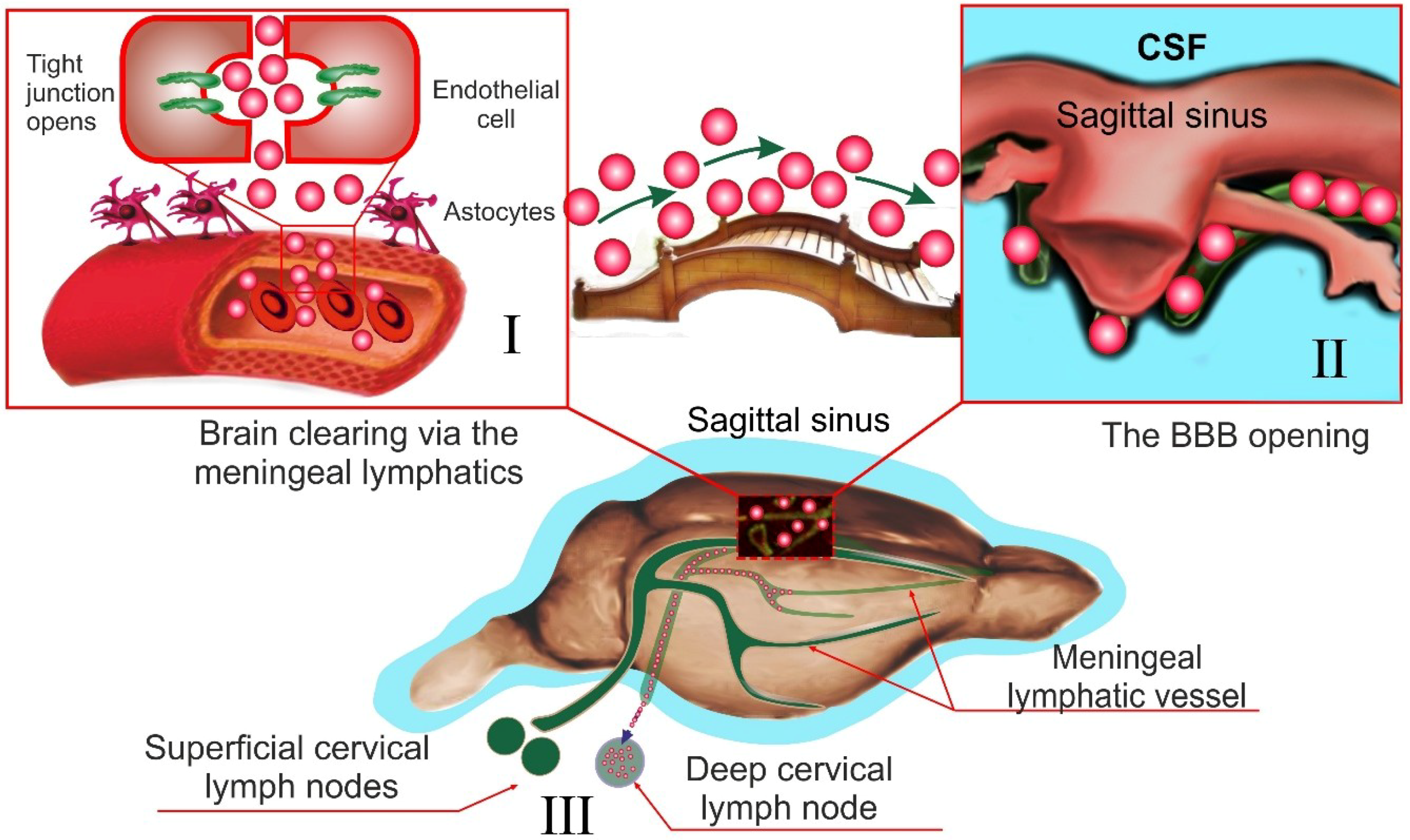
7. Alternative Notions for Lymphatic Functions in the Brain
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APQ(s)-4 | Aquaporin(s)-4 |
| ATP-K(+) channels | Adenosine Tri-Phosphate Potassium Channels |
| BBB | Blood–Brain Barrier |
| CCL21 | Chemokine (C-C motif ligand 21) |
| CSF | Cerebral Spinal Fluid |
| ECF | Extracellular Fluid |
| GNRs | Gold Nano Roads |
| HA | Hyaluronan |
| ISF | Interstitial Fluid |
| ITGA9 | Intergrin-α9 |
| LYVE-1 | Lymphatic Vessel Endothelial Hyaluronan Receptor 1 |
| NCD | Nasolacrimal Duct |
| PD | Photodynamic |
| PDPN | Podoplanin |
| PVS | Perivascular Space |
References
- Lohrberg, M.; Wilting, J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 2016, 366, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Patlak, C.S. Secretion and bulk flow of interstitial fluid. In Physiology and Pharmacology of the Blood-Brain Barrier; Bradbury, M.W.B., Cuatrecasas, P., Herken, H., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 1992; Volume 103, pp. 245–261. [Google Scholar]
- Davson, H.; Segal, M.B. Physiology of the CSF and Blood-Brain Barriers; CRC Press: Boca Raton, FL, USA, 1996; p. 832. [Google Scholar]
- Brierley, J.B.; Field, E.J. The connexions of the spinal sub-arachnoid space with the lymphatic system. J. Anat. 1948, 82, 153–166. [Google Scholar] [PubMed]
- Bradbury, M.W.; Cole, D.F. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J. Physiol. 1980, 299, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W. Lymphatics and the central nervous system. Trends Neurosci. 1981, 4, 100–101. [Google Scholar] [CrossRef]
- Mezey, E.; Palkovits, M. Neuroanatomy: Forgotten finding of brain lymphatics. Nature 2015, 524, 415. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W.; Cserr, H.F.; Westrop, R.J. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 1981, 240, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W.; Westrop, R.J. Factors influencing exit of substances from cerebrospinal-fluid into deep cervical lymph of the rabbit. J. Physiol. 1983, 339, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat—Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Papaiconomou, C. Cerebrospinal fluid transport: A lymphatic perspective. News Physiol. Sci. 2002, 17, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Zakharov, A.; Koh, L.; Armstrong, D. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol. Appl. Neurobiol. 2005, 31, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Nagra, G.; Koh, L.; Zakharov, A.; Armstrong, D.; Johnston, M. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.; Zakharov, A.; Johnston, M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Knopf, P.M. Cervical lymphatics, the blood-brain barrier and the immureactivity of the brain: A new view. Immunol. Today 1992, 13, 507–512. [Google Scholar] [CrossRef]
- Cserr, H.F.; Harling-Berg, C.J.; Knopf, P.M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Dissing-Olesen, L.; Hong, S.; Stevens, B. New Brain Lymphatic Vessels drain old concepts. EBioMedicine 2015, 2, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- His, W. Ueber ein perivaskulaeres Kanalsystem in den nervoesen Central-Organen und ueber dessen Beziehungen zum Lymphsystem. Zentralbl. Med. Wiss. 1865, 5, 127–141. [Google Scholar]
- Schwalbe, G. Die Arachnoidalraum, ein Lymphraum und sein Zusammenhang mit den Perichorioidalraum. Zentralbl. Med. Wiss. 1869, 7, 465–467. [Google Scholar]
- Weed, L.H. The pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J. Med. Res. 1914, 31, 51–91. [Google Scholar] [PubMed]
- Iliff, J.F.; Wang, M.; Liao, Y.; Nedergaard, M.A. Paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Goldman, S.; Nedergaard, M. Clearing the mind: Implications of dural lymphatic vessels for brain function. Lancet Neurol. 2015, 14, 977–979. [Google Scholar] [CrossRef]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Caversaccio, M.; Peschel, O.; Arnold, W. Connections between the cerebrospinal fluid space and the lymphatic system of the head and neck in humans. In Intracranial and Intralabyrinthine Fluids; Ernst, A., Marchbanks, R., Samii, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Engelhardt, B.; Carare, R.; Bechmann, I.; Flügel, A.; Laman, J.; Weller, R. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016, 132, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Bucchieri, F.; Farina, F.; Zummo, G.; Cappello, F. Lymphatic vessels of the dura mater: A new discovery? J. Anat. 2015, 27, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Chehonin, V.; Borisova, E.; Fedosov, I.; Namykin, A.; Abdurashitov, A.; Shirokov, A.; Khlebtsov, B.; Lyubun, Y.; Navolokin, N.; et al. Photodynamic opening of the blood-brain barrier and pathways of brain clearing pathways. J. Biophotonics 2018, 11, e201700287. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Bragin, D.; Bragina, O.; Shushunova, N.; Maslyakova, G.; Navolokin, N.; Bucharskaya, A.; Tuchin, V.; et al. Application of optical coherent tomography for in vivo monitoring of the meningeal lymphatic vessels during opening of blood-brain barrier: Mechanisms of brain clearing. JBO 2017, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.T.; Tigges, J.; Arnold, W. Subarchnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol. 1979, 105, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Mollanji, R.; Bozanovic-Sosic, R.; Zakharov, A.; Makarian, L.; Johnston, M.G. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Foldi, M. Lymphogenous encephalopathy. In Lymph and the Lymphatic System; Mayerson, H.S., Ed.; Charlie C. Thomas Publisher: Springfield, IL, USA, 1968; pp. 169–198. [Google Scholar]
- Foldi, M.; Csillik, B.; Zoltan, O.T. Lymphatic drainage of the brain. Experientia 1968, 24, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Chen, L.; Xia, Z. Effects of cervical-lymphatic blockade on brain edema and infarction volume in cerebral ischemic rats. Chin. J. Physiol. 2006, 49, 258–265. [Google Scholar] [PubMed]
- Radjavi, A.; Smirnov, I.; Derecki, N.; Kipnis, J. Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 2014, 19, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Lu, X.; Wei, S.; Wang, J.; Xiang, D. The effect of blocking the cervical lymphatic drainage of rabbit on its cerebral structure and function in the acute lymphostasis stage. In Progress in Lymphology XIV; Witte, M.H., Witte, C.L., Eds.; International Society of Lymphology: Zurich, Switzerland, 1994; pp. 742–746. [Google Scholar]
- Lymph—Definition and More from the Free Merriam-Webster Dictionary. Available online: www.merriam-webster.com (accessed on 29 May 2010).
- Lauralee, S. Human Physiology from Cells to Systems, 8th ed.; Nelson Education: Scarborough, ON, Canada, 2015. [Google Scholar]
- Rapoport, S.I. Blood–Brain Barrier in Physiology and Medicine; Raven Press: New York, NY, USA, 1976. [Google Scholar]
- Abbott, N.J.; Bundgaard, M.; Cserr, H.F. Tightness of the blood–brain barrier and evidence for brain interstitial fluid flow in the cuttlefish. J. Physiol. 1985, 368, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Mascagni, P.; Bellini, G.B. Istoria Completa Dei Vasi Linfatici; Presso Eusebio Pacini e Figlio: Florence, Italy, 1816; Volume II, p. 195. [Google Scholar]
- Lukić, I.K.; Glunčić, V.; Ivkić, G.; Hubenstorf, M.; Marušić, A. Virtual dissection: A lesson from the 18th century. Lancet 2003, 362, 2110–2113. [Google Scholar] [CrossRef]
- Zheng, M.; Kimura, S.; Nio-Kobayashi, J.; Iwanaga, T. The selective distribution of LYVE-1-expressing endothelial cells and reticular cells in the reticulo-endothelial system. Biomed. Res 2016, 37, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Ni, J.; Wang, S.-X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D. LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-specific Receptor for Hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G. The lymphatics revisited: New perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc. Med. 2003, 13, 1–7. [Google Scholar] [CrossRef]
- Jackson, D.G.; Prevo, R.; Clasper, S.; Banerji, S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001, 22, 317–321. [Google Scholar] [CrossRef]
- Grzegorek, I.; Drozdz, K.; Chmielewska, M.; Gomulkiewicz, A.; Jablonska, K.; Piotrowska, A.; Karczewski, M.; Janczak, D.; Podhorska-Okolow, M.; Dziegiel, P.; et al. Arterial wall lymphangiogenesis is increased in the human iliac atherosclerotic arteries: Involvement of CCR7 receptor. Lymphat. Res. Biol. 2014, 12, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.J.; Gale, N.W.; Harvey, N.L. Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dyn. 2008, 237, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Lim, S.Y.; Tan, S.K.; Jackson, D.C.; Ginhoux, F.; Angeli, F. Hyaluronan Receptor LYVE-1-Expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 2018, 49, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002, 225, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; von der Weid, P.Y. Lymphatic system: An active pathway for immune protection. Semin. Cell Dev. Biol. 2015, 38, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Harvey, N.L. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 244, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Schacht, V.; Ramirez, M.I.; Hong, Y.K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1 alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Astarita, J.L. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283. [Google Scholar] [CrossRef] [PubMed]
- Bazigou, E. Integrin-a9 is required for fbronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 2009, 17, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bradstreet, J.J.; Ruggiero, M.; Pacini, S. Commentary: Structural and functional features of central nervous system lymphatic vessels. Front. Neurosci. 2015, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W. Multiple sclerosis: Presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science 1979, 203, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Bergsneider, M. Evolving concepts of cerebrospinal fluid. Neurosurg. Clin. N. Am. 2001, 36, 631–638. [Google Scholar] [CrossRef]
- Davson, H.; Welch, K.; Segal, M.B. The Physiology and Pathophysiology of the Cerebrospinal Fluid; Churchill Livingstone: London, UK, 1987. [Google Scholar]
- Poplack, D.G.; Bleyer, W.A.; Wood, J.H.; Kostolich, M.; Savitch, J.L.; Ommaya, A.K. A primate model for study of methotrexate pharmacokinetics in the central nervous system. Cancer Res. 1977, 37, 1982–1985. [Google Scholar] [PubMed]
- Gomez, D.G.; Ehrmann, J.E.; Potts, D.G.; Pavese, A.M.; Gilanian, A. The arachnoid granulation of the newborn human: An ultrastructural study. Int. J. Dev. Neurosci. 1983, 1, 139–147. [Google Scholar] [CrossRef]
- Johanson, C.E. Ventricles and cerebrospinal fluid. In Neuroscience in Medicine; Conn, P.M., Ed.; Lippincott: Philadelphia, PA, USA, 1995; p. 171196. [Google Scholar]
- Johnston, M.; Boulton, M.; Flessner, M. Cerebrospinal fluid absorption revisited: Do extracranial lymphatics play a role? Neuroscience 2000, 6, 77–87. [Google Scholar] [CrossRef]
- Zwillinger, H. Die Lymphbahnen des oberen Nasalschnittes und deren Beziehungen zu den perimeningealen Lymphraumen. Eur. Arch. Otorhinolaryngol. 1912, 26, 66–78. [Google Scholar]
- Yamada, S.; De Pasquale, M.; Patlak, C.S.; Cserr, H.F. Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am. J. Physiol. 1991, 261, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Persaud, T.; Torchia, M. The Developing Human, 10th ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Heindl, L.M.; Hofmann-Rummelt, C.; Adler, W.; Bosch, J.J.; Holbach, L.M.; Naumann, G.O.; Kruse, F.E.; Cursiefen, C. Tumor-associated lymphangiogenesis in the development of conjunctival melanoma. Investig. Ophthalomol. Vis. Sci. 2011, 52, 7074–7083. [Google Scholar] [CrossRef] [PubMed]
- Dithmar, S.; Diaz, C.E.; Grossniklaus, H.E. Intraocular melanoma spread to regional lymph nodes: Report of two cases. Retina 2000, 20, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, K.F.; Kersten, R.; Daouk, N.; Blodi, F.C. Metastatic squamous cell carcinoma of the conjunctiva. Ophthalmology 1988, 95, 318–321. [Google Scholar] [CrossRef]
- Thorne, R.G.; Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA 2006, 103, 5567–5572. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol. 1981, 240, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Szentistvanyi, I.; Patlak, C.S.; Ellis, R.A.; Cserr, H.F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 1984, 246, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Fraser, P.A.; Cserr, H.F. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991, 545, 103–113. [Google Scholar] [CrossRef]
- Nicholson, C.; Kamali-Zare, P.; Tao, L. Brain extracellular space as a diffusion barrier. Comput. Vis. Sci. 2011, 14, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, E.; Meves, H. Die Feinstrucktur des moleculären Rindengraues und ihre physiologisches Bedeutung. Cell Tissue Res. 1959, 49, 569–604. [Google Scholar] [CrossRef]
- Johnston, P.V.; Roots, B.I. Nerve Membranes. A Study of the Biological and Chemical Aspects of Neuron-Glia Relationships; Pergamon: Oxford, UK, 1972. [Google Scholar]
- Villegas, G.M.; Fernández, J. Permeability to thorium dioxide of the intercellular spaces of the frog cerebral hemisphere. Exp. Neurol. 1966, 15, 18–36. [Google Scholar] [CrossRef]
- Van Harreveld, A. The extracellular space in the vertebrate central nervous system. In The Structure and Function of Nervous Tissue; Bourne, G.H., Ed.; Academic: New York, NY, USA, 1972; Volume IV, pp. 447–511. [Google Scholar]
- Van Harreveld, A.; Crowell, J.; Malhotra, S.K. A study of extracellular space in central nervous tissue by freeze-substitution. J. Cell Biol. 1965, 25, 117–137. [Google Scholar] [CrossRef]
- Syková, E. Extracellular K+ accumulation in the central nervous system. Prog. Biophys. Mol. Biol. 1983, 42, 135–189. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Tuz, K. Volume changes in neurons: Hyperexcitability and neuronal death. In Mechanisms and Significance of Cell Volume Regulation; Karger Publishers: Basel, Switzerland, 2006; Volume 152, pp. 221–240. [Google Scholar]
- Zhou, N.; Gordon, G.R.; Feighan, D.; Mac Vicar, B.A. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb. Cortex 2010, 20, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Andrew, R.D.; Kirov, S.A. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 2009, 57, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.M.; Baillie, L.D.; Mulligan, S.J. Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. PLoS ONE 2012, 7, e51124. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Vargová, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.R.; Binder, D.K.; Fiacco, T.A. Turning down the volume: Astrocyte volume change in the generation and termination of epileptic seizures. Neurobiol. Dis. 2017, 104, 24–32. [Google Scholar] [CrossRef] [PubMed]
- McBain, C.J.; Traynelis, S.F.; Dingledine, R. Regional variation of extracellular space in the hippocampus. Science 1990, 249, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’Donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; Takano, T. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Diem, A.K.; Tan, M.; Bressloff, N.W.; Hawkes, C.; Morris, A.W.; Weller, R.O.; Carare, R.O. A Simulation Model of Periarterial Clearance of Amyloid-β from the Brain. Front. Aging Neurosci. 2016, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kwee, I.L.; Igarashi, H.; Suzuki, Y. Aquaporin-4 functionality and Virchow-Robin space water dynamics: Physiological model for neurovascular coupling and glymphatic flow. Int. J. Mol. Sci. 2017, 18, 1798. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.L.D.S.; Ferreira, A.C.A.D.F.; de Oliveira-Souza, R. Pseudotumor Cerebri and Glymphatic dysfunction. Front. Neurol. 2018, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, V.; Wang, X.; Korhonen, V.; Keinänen, T.; Tuovinen, T.; Autio, J.; Nedergaard, M. Ultra-fast magnetic resonance encephalography of physiological brain activity–Glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. 2016, 36, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; De Zélicourt, D.; Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Sci. Rep. 2016, 6, 38635. [Google Scholar] [CrossRef] [PubMed]
- Diem, A.K.; MacGregor Sharp, M.; Gatherer, M.; Bressloff, N.W.; Carare, R.O.; Richardson, G. Arterial pulsations cannot drive intramural periarterial drainage: Significance for Aβ drainage. Front. Neurosci. 2017, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Yao, X.; Dix, J.A.; Jin, B.J.; Verkman, A.S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 2017, 6, e27679. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Verkman, A.S. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: Game changer or unproven speculation? FASEB J. 2017, 32, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Minamitani, H.; Fukuoka, Y.; Ehara, H.; Sekizuka, E.; Oshio, C.; Tsuchiya, M. Application of microscopic laser Doppler velocimeter for analysis of arterial pulse wave in microcirculation. In Proceedings of the 12th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Philadelphia, PA, USA, 1–4 November 1990. [Google Scholar]
- Yeh, C.; Hu, S.; Maslov, K.I.; Wang, L.V. Photoacoustic microscopy of blood pulse wave. J. Biomed. Opt. 2012, 17, 070504. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; De Zélicourt, D.; Kurtcuoglu, V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci. Rep. 2015, 5, 15024. [Google Scholar] [CrossRef] [PubMed]
- Bacyinski, A.; Xu, M.; Wang, W.; Hu, J. The paravascular pathway for brain waste clearance: Current understanding, significance and controversy. Front. Neuroanat. 2017, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The glymphatic system and waste clearance with brain aging: A review. Gerontology 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hannocks, M.J.; Pizzo, M.E.; Huppert, J.; Deshpande, T.; Abbott, N.J.; Thorne, R.G.; Sorokin, L. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 2018, 38, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Holter, K.E.; Kehlet, B.; Devor, A.; Sejnowski, T.J.; Dale, A.M.; Omholt, S.W.; Ottersen, O.P.; Nagelhus, E.A.; Mardal, K.A.; Pettersen, K.H. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl. Acad. Sci. USA 2017, 114, 9894–9899. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, L.; Ding, G.; Davoodi-Bojd, E.; Li, Q.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z. Impairment of the glymphatic system after diabetes. Cereb. Blood Flow Metab. 2016, 7, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Lu, M.L.; Yang, E.; Peng, W.; Mestre, H.; Hitomi, E.; Deane, R.; Nedergaard, M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2016, 37, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J. Neurosci. 2017, 37, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Nedergaard, M. The glymphatic system in central nervous system health and disease: Past, present, and future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Navolokin, N.; Borisova, E.; Shirokov, S.; Abdurashitov, A.; Dubrovskiy, A.; Klimova., M.; Bodrova, A.; Nowave, E.; Khorovodov, A.; et al. Recruitment of the meningeal lymphatics by the blood-brain barrier. Front. Physiol. 2018. under review. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Kurths, J.; Borisova, E.; Sokolovsky, S.; Mantareva, N.; Angelov, I.; Shirokov, A.; Navolokin, N.; Shushunova, N.; Khorovodov, A.; et al. Photodynamic opening of blood-brain barrier. Biomed. Opt. Express 2017, 1, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Pavlov, A.; Shirokov, A.; Navolokin, N.; Pavlova, O.; Gekalyuk, A.; Ulanova, M.; Shushunova, N.; Bodrova, A.; et al. Laser speckle imaging and wavelet analysis of cerebral blood flow associated with the opening of the blood-brain barrier by sound. Chin. Opt. Lett. 2017, 15, 090002. [Google Scholar] [CrossRef]
| The Markers of the Lymphatic Endothelium | The Functional Characteristics of Proteins Expressed in the Lymphatic Endothelium |
|---|---|
| LYVE-1—Lymphatic vessel endothelial hyaluronan receptor 1 | Hyaluronan (HA) is an element of skin and mesenchymal tissues that regulates cell migration in the course of wound healing, inflammation, and embryonic morphogenesis [50]. LYVE-1 is a specific transmembrane receptor of HA, which was first described by Banerji in 1999 [51]. LYVE-1 was found primarily on both the luminal and abluminal surface of lymphatic endothelial cells [51,52]. The functional role of LYVE-1 is still the subject of debate, but there are evidences that LYVE-1 plays an important role in hyaluronan transport and turnover, or in hyaluronan localization to the surface of lymphatic endothelium providing for migration CD44+ leukocytes or tumor cells [53]. Notice, there are some findings where expression of Lyve-1 was also observed in the human iliac atherosclerotic arteries [54], in the embryonic blood vessels [55], in macrophages [56], the reticulo-endothelial system [48]. Therefore, the specificity of Lyve-1 as a marker for the lymphatic vessels is not strong enough. |
| Prox1—Prospero homeobox protein 1 | Transcription factor regulating the process of growth and differentiation of endothelial cells of lymphatic vessels [57] |
| CCL21-Chemokine (C-C motif ligand 21) | It is secreted by endothelial cells of lymphatic vessels and is involved in activation of T-lymphocyte movement, migration of lymphocytes to other organs, and dendritic cells into lymph nodes [58]. |
| VEGFR3—Vascular endothelial growth factor receptor 3 | VEGFR3 is a receptor that triggers the lymphangiogenesis, i.e., the formation of new lymphatic vessels [59]. In transgenic mice with absence of VEGFR3 gene, the meningeal lymphatic vessels do not develop, and lymph node hypoplasia is noted [21]. |
| PDPN—Podoplanin | PDPN is integral membrane protein, which is responsible for the normal development of the network of lymphatic vessels, providing drainage of the intercellular fluid. If the synthesis is broken, lymphedema is formed [60]. The PDPN activation is accompanied by lymphangiogenesis, which is regarded as an important indicator of tumor growth [61]. |
| ITGA9—Integrin-α9 | ITGA9 is a protein, which is a part of the valves in the lymphatic vessels [62]. |
| Objects | The Side of Injection of Tracer (Radio-Iodinated Albumin) | Time of Lymph Collection (h) | Lymph Recovery (%) * |
|---|---|---|---|
| Rabbit | Caudate nucleus | 25 | 47 |
| Internal capsule | 25 | 22 | |
| Brain | 25 | 18 | |
| CSF | 6 | 30 | |
| Cat | CSF | 8 | 14 |
| Sheep | CSF | 26 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semyachkina-Glushkovskaya, O.; Postnov, D.; Kurths, J. Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses. Int. J. Mol. Sci. 2018, 19, 3818. https://doi.org/10.3390/ijms19123818
Semyachkina-Glushkovskaya O, Postnov D, Kurths J. Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses. International Journal of Molecular Sciences. 2018; 19(12):3818. https://doi.org/10.3390/ijms19123818
Chicago/Turabian StyleSemyachkina-Glushkovskaya, Oxana, Dmitry Postnov, and Jürgen Kurths. 2018. "Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses" International Journal of Molecular Sciences 19, no. 12: 3818. https://doi.org/10.3390/ijms19123818
APA StyleSemyachkina-Glushkovskaya, O., Postnov, D., & Kurths, J. (2018). Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses. International Journal of Molecular Sciences, 19(12), 3818. https://doi.org/10.3390/ijms19123818