PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions
Abstract
1. Introduction
2. PI3K Signaling in Neuronal Metabolism
3. The Role of PI3K in Neuroinflammation
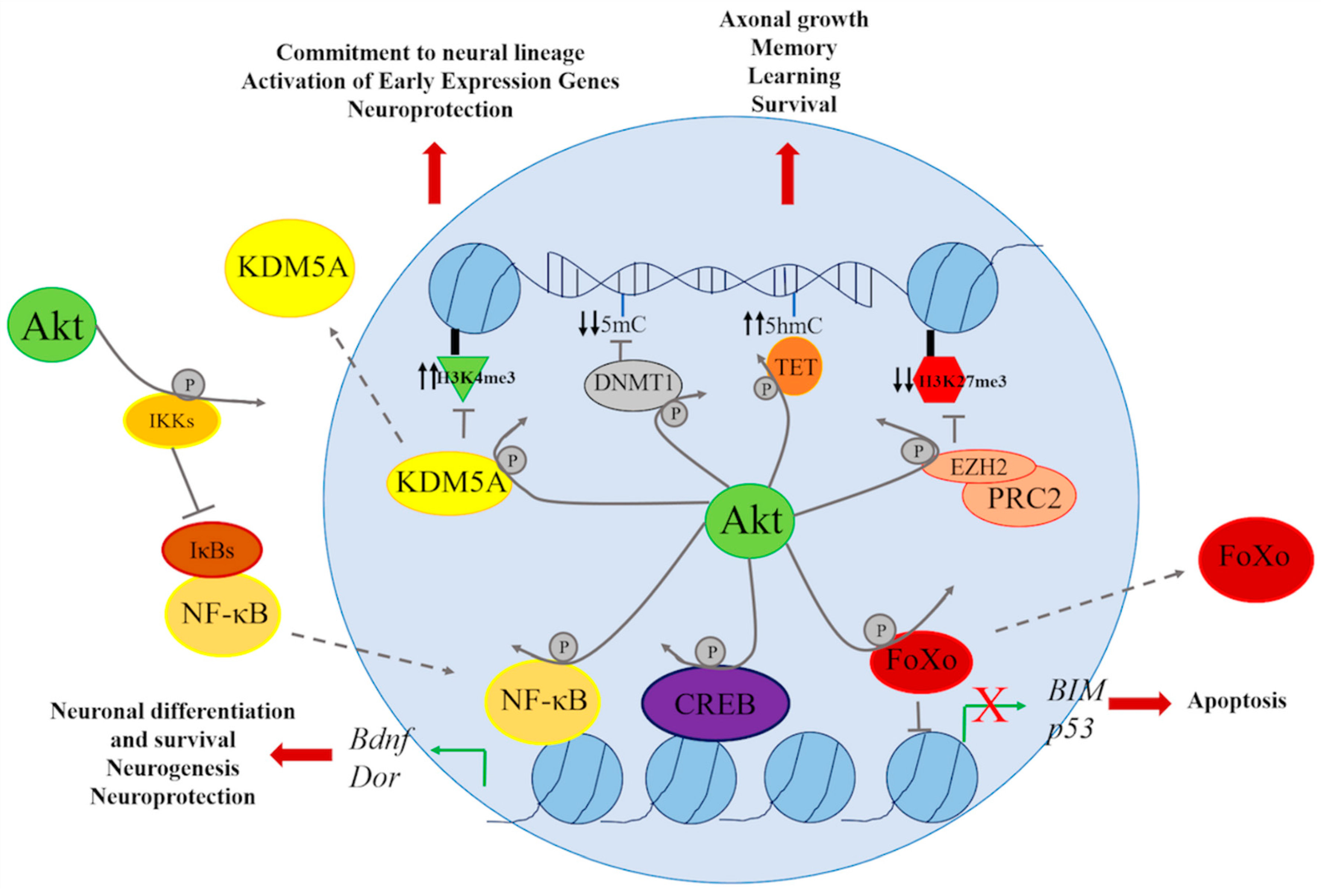
4. Genetic and Epigenetic Regulation through the PI3K/AKT Signaling Pathway
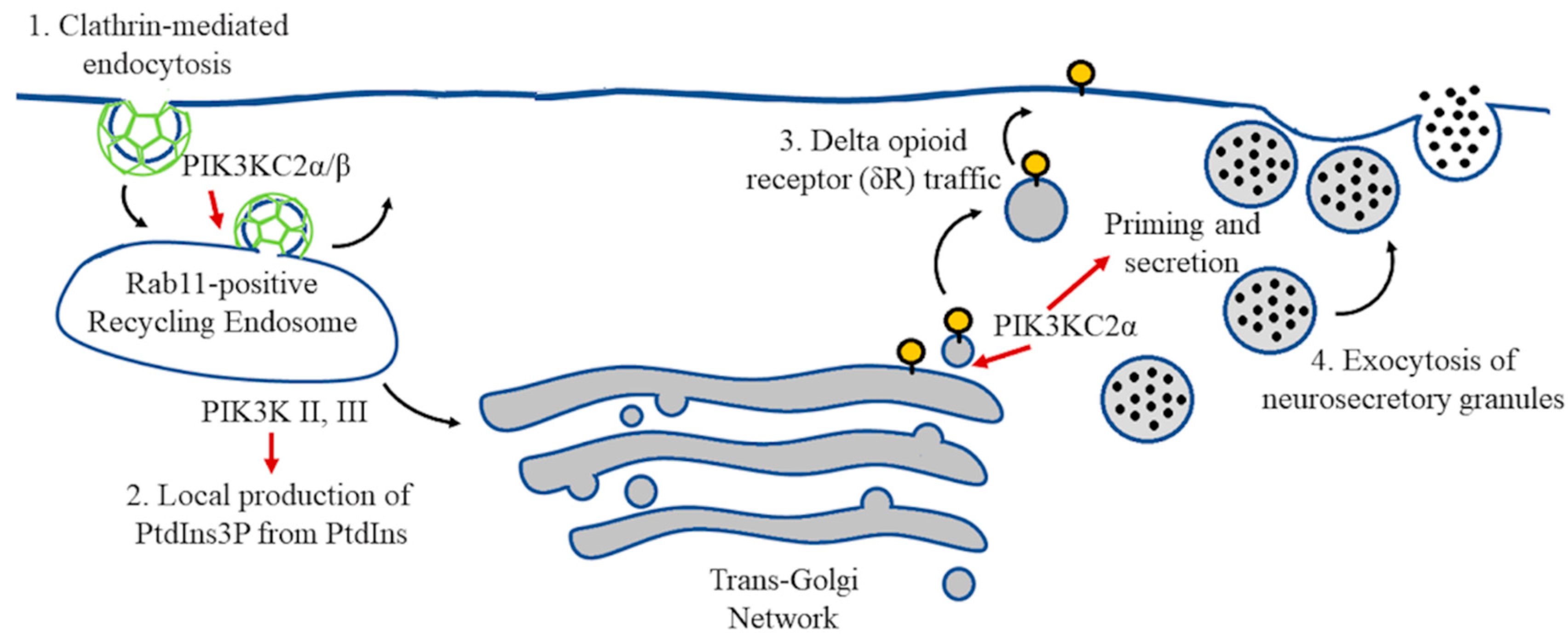
5. Vesicle Recycling and Neurotransmission
6. Class III PI3Ks in Neuronal Autophagy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Anderson, K.E.; Davidson, K.; Stephens, L.R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006, 34, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Costa, C.; Ciraolo, E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 2007, 194, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Maffucci, T. Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem. J. 2012, 443, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, K.; Dall’Armi, C.; Alcazar-Roman, A.; Ogasawara, Y.; Zhou, X.; Wang, F.; Yamamoto, A.; De Camilli, P.; Di Paolo, G. Regulation of Mammalian Autophagy by Class II and III PI 3-Kinases through PI3P Synthesis. PLoS ONE 2013, 8, e76405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-W.; Wang, Y.; Wang, T.; Zhang, K.-B.; Jiang, C.-Y.; Hu, F.-F.; Yuan, Y.; Bian, J.C.; Liu, X.Z.; Gu, J.H.; et al. Cadmium-induced autophagy promotes survival of rat cerebral cortical neurons by activating class III phosphoinositide 3-kinase/beclin-1/B-cell lymphoma 2 signaling pathways. Mol. Med. Rep. 2015, 12, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Van der Heide, L.P.; Ramakers, G.M.J.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol. 2006, 79, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; McIlroy, J.; Rordorf-Nikolic, T.; Orr, G.A.; Backer, J.M. Regulation of the p85/p110 phosphatidylinositol 3’-kinase: Stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol. Cell Biol. 1998, 18, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Pons, S. Phosphatidylinositol-3-OH kinase regulatory subunits are differentially expressed during development of the rat cerebellum. J. Neurobiol. 2001, 47, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.; Spangler, S.; Seeburg, D.P.; Hoogenraad, C.C.; Sheng, M. Control of Dendritic Arborization by the Phosphoinositide-3’-Kinase-Akt-Mammalian Target of Rapamycin Pathway. J. Neurosci. 2005, 25, 11300–11312. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.B.; Ye, K. Multiple Functions of Phosphoinositide-3 Kinase Enhancer (PIKE). Sci. World J. 2010, 10, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Cuesto, G.; Enriquez-Barreto, L.; Carames, C.; Cantarero, M.; Gasull, X.; Sandi, C.; Ferrús, A.; Acebes, Á.; Morales, M. Phosphoinositide-3-Kinase Activation Controls Synaptogenesis and Spinogenesis in Hippocampal Neurons. J. Neurosci. 2011, 31, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Kamiguchi, H. Phosphatidylinositol 3-kinase facilitates microtubule-dependent membrane transport for neuronal growth cone guidance. J. Biol. Chem. 2010, 285, 41740–41748. [Google Scholar] [CrossRef] [PubMed]
- Horwood, J.M.; Dufour, F.; Laroche, S.; Davis, S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur. J. Neurosci. 2006, 23, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Wang, J.; Li, B.-M. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn. Mem. 2008, 15, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Veyrac, A.; Dufour, F.; Horwood, J.; Laroche, S.; Davis, S. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS ONE 2009, 4, e7901. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, K.; Frank, R.; Szabó, J.; Knapp, L.; Kis, Z.; Farkas, T.; Vécsei, L.; Toldi, J. Acetyl-l-carnitine restores synaptic transmission and enhances the inducibility of stable LTP after oxygen-glucose deprivation. Neuroscience 2016, 332, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.; Eickholt, B.J. The neurodevelopmental implications of PI3K signaling. Curr. Top. Microbiol. Immunol. 2010, 346, 245–265. [Google Scholar] [PubMed]
- Rivière, J.-B.; Mirzaa, G.M.; O’Roak, B.J.; Beddaoui, M.; Alcantara, D.; Conway, R.L.; St-Onge, J.; Schwartzentruber, J.A.; Gripp, K.W.; Nikkel, S.M.; et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 2012, 44, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.A.; Mirzaa, G.M.; Ishak, G.E.; O’Roak, B.J.; Hiatt, J.B.; Roden, W.H.; Gunter, S.A.; Christian, S.L.; Collins, S.; Adams, C.; et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain 2015, 138, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Peng, J.; Yang, L.; Kong, H.; Yin, F. Interleukin-1β plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J. Neuroimmunol. 2015, 282, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Hillmann, P.; Noack, A.; Römermann, K.; Öhler, L.A.; Rageot, D.; Beaufils, F.; Melone, A.; Sele, A.M.; Wymann, M.P.; et al. The novel, catalytic mTORC1/2 inhibitor PQR620 and the PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain barrier and increase seizure threshold in a mouse model of chronic epilepsy. Neuropharmacology 2018, 140, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Hernández-Damián, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3β Signaling in Parkinson’s Disease Dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Mao, L.; Zahid, K.R.; Wen, J.; Zhang, L.; Zhang, M.; Duan, J.; Duan, J.; Yin, X.; et al. Histone deacetylase 1 promotes glioblastoma cell proliferation and invasion via activation of PI3K/AKT and MEK/ERK signaling pathways. Brain Res. 2018, 1692, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.M.; Filiz, G.; Brown, D.V.; Christie, M.; Waring, P.M.; Zhang, Y.; Haynes, J.M.; Pouton, C.; Flanagan, D.; Vincan, E.; et al. PI3K activation in neural stem cells drives tumorigenesis which can be ameliorated by targeting the cAMP response element binding protein. Neuro Oncol. 2018, 20, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Eickholt, B.J.; Ahmed, A.I.; Davies, M.; Papakonstanti, E.A.; Pearce, W.; Starkey, M.L.; Bilancio, A.; Need, A.C.; Smith, A.J.H.; Hall, S.M.; et al. Control of Axonal Growth and Regeneration of Sensory Neurons by the p110δ PI 3-Kinase. PLoS ONE 2007, 2, e869. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, J.M.; Fyffe-Maricich, S.L. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol. 2016, 283, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Chang, C.-W.; Kelly, S.M.; Bhattacharya, A.; McBride, S.M.J.; Danielson, S.W.; Jiang, M.Q.; Chan, C.B.; Ye, K.; Gibson, J.R.; et al. Increased Expression of the PI3K Enhancer PIKE Mediates Deficits in Synaptic Plasticity and Behavior in Fragile X Syndrome. Cell Rep. 2015, 11, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, K.; Liu, M.; Xu, M.; Hu, X.; Yan, R.; Förster, E.; Zhao, S. The effect of P85 on neuronal proliferation and differentiation during development of mouse cerebral cortex. Dev. Biol. 2018, 441, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Höland, K.; Boller, D.; Hagel, C.; Dolski, S.; Treszl, A.; Pardo, O.E.; Ćwiek, P.; Salm, F.; Leni, Z.; Shepherd, P.R.; et al. Targeting Class IA PI3K Isoforms Selectively Impairs Cell Growth, Survival, and Migration in Glioblastoma. PLoS ONE 2014, 9, e94132. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.M.; Poduri, A. Megalencephaly and hemimegalencephaly: Breakthroughs in molecular etiology. Am. J. Med. Genet. Part C Semin. Med. Genet. 2014, 166, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morrison, C.D.; Clegg, D.J.; Olson, R.; Baskin, D.G.; Myers, M.G., Jr.; Seeley, R.J.; Schwartz, M.W.; et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: A key mediator of insulin-induced anorexia. Diabetes 2003, 52, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Obici, S.; Zhang, B.B.; Karkanias, G.; Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002, 8, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Boghossian, S.; York, D.A.; Park-York, M. The effect of high fat diet and saturated fatty acids on insulin signaling in the amygdala and hypothalamus of rats. Brain Res. 2013, 1537, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pardini, A.W.; Nguyen, H.T.; Figlewicz, D.P.; Baskin, D.G.; Williams, D.L.; Kim, F.; Schwartz, M.W. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006, 1112, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.-W.; Oh, Y.; Kim, K.W.; Lee, S.; Williams, K.W.; Elmquist, J.K. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol. Metab. 2016, 5, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Romeo, F.; Inoue, S.; Niklison-Chirou, M.V.; Elia, A.J.; Dinsdale, D.; Morone, N.; Knight, R.A.; Mak, T.W.; Melino, G. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016, 23, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Leary, J.; Jahagirdar, V.; Sage, J.; McNay, E.C. Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4. Behav. Brain Res. 2018, 338, 32–39. [Google Scholar] [CrossRef] [PubMed]
- McNay, E.C.; Ong, C.T.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Z.; Wang, Y.; Wang, L.; Zhang, B.; Chen, G.; Wan, Q.; Chen, L. Akt3 deletion in mice impairs spatial cognition and hippocampal CA1 long long-term potentiation through downregulation of mTOR. Acta Physiol. 2018, e13167. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Lee, H.-R.; Sim, S.; Baek, J.; Yu, N.-K.; Choi, J.-H.; Ko, H.-G.; Lee, Y.S.; Park, S.W.; Kwak, C.; et al. PI3Kγ is required for NMDA receptor–dependent long-term depression and behavioral flexibility. Nat. Neurosci. 2011, 14, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Park, P.; Baek, G.-C.; Sim, S.-E.; Kang, S.J.; Lee, Y.; Ahn, S.H.; Lim, C.S.; Lee, Y.S.; Collingridge, G.L.; et al. Effects of PI3Kβ overexpression in the hippocampus on synaptic plasticity and spatial learning. Mol. Brain 2014, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yeh, S.H.; Lin, C.H.; Lu, K.T.; Leu, T.H.; Chang, W.C.; Gean, P.W. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 2001, 31, 841–851. [Google Scholar] [CrossRef]
- Seitz, C.; Hugle, M.; Cristofanon, S.; Tchoghandjian, A.; Fulda, S. The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int. J. Cancer 2013, 132, 2682–2693. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiu, J.; Liang, M.; Golinski, J.; van Leyen, K.; Jung, J.E.; You, Z.; Lo, E.H.; Degterev, A.; Whalen, M.J. Akt and mTOR mediate programmed necrosis in neurons. Cell Death Dis. 2014, 5, e1084. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira, C.R.; Rego, A.C. Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington’s disease knock-in striatal cells. Free Radic. Biol. Med. 2014, 74, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Lee, K.H.; Gabr, A.A.; Choi, G.E.; Kim, J.S.; Ko, S.H.; Han, H.J. Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Rodriguez-Ortiz, C.J.; Kitazawa, M. Infection, systemic inflammation, and Alzheimer’s disease. Microbes Infect. 2015, 17, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Avila-Muñoz, E.; Arias, C. When astrocytes become harmful: Functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res. Rev. 2014, 18, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; LaFerla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K.; Terwel, D.; Kummer, M.P. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. 2010, 117, 919–947. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.; Humpel, C. Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer’s disease and mild cognitive impairment. Exp. Gerontol. 2010, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Inflammatory processes in Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 741–749. [Google Scholar] [CrossRef]
- Troutman, T.D.; Bazan, J.F.; Pasare, C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 2012, 11, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.; Taboubi, S.; Torres, D.; Delbauve, S.; Hachani, A.; Whitehead, M.A.; Pearce, W.P.; Berenjeno, I.M.; Nock, G.; Filloux, A.; et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Huttenlocher, A. Leukocyte migration from a fish eye’s view. J. Cell Sci. 2012, 125, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Barberis, L.; Pasquali, C.; Bertschy-Meier, D.; Cuccurullo, A.; Costa, C.; Ambrogio, C.; Vilbois, F.; Chiarle, R.; Wymann, M.; Altruda, F.; et al. Leukocyte transmigration is modulated by chemokine-mediated PI3Kγ-dependent phosphorylation of vimentin. Eur. J. Immunol. 2009, 39, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.; Milne, L.; Hawkins, P. Moving towards a better understanding of chemotaxis. Curr. Biol. 2008, 18, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.J.; Milne, L.; Kulkarni, S.; Sasaki, T.; Walker, S.; Andrews, S.; Crabbe, T.; Finan, P.; Jones, G.; Jackson, S.; et al. PI(3)Kγ has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 2007, 9, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Deem, T.; Bruce, A.; Reutershan, J.; Wu, D. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J. Leukoc. Biol. 2006, 80, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.E.; Prigmore, E.; Calvez, R.; Hogan, C.; Dunn, G.A.; Hirsch, E.; Wymann, M.P.; Ridley, A.J. Requirement for PI 3-kinase γ in macrophage migration to MCP-1 and CSF-1. Exp. Cell Res. 2003, 290, 120–131. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Irie-Sasaki, J.; Jones, R.G.; Oliveira-dos-Santos, A.J.; Stanford, W.L.; Bolon, B.; Wakeham, A.; Itie, A.; Bouchard, D.; Kozieradzki, I.; et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000, 287, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Cianciulli, A.; Calvello, R.; Dragone, T.; Iacobazzi, F.; Panaro, M.A. The PI3K/Akt pathway is required for LPS activation of microglial cells. Immunopharmacol. Immunotoxicol. 2012, 34, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, S.; Pan, R.; Li, G.; Tang, H.; Jiang, M.; Xing, Y.; Jin, F.; Lin, L.; Dong, J. Curcumin Attenuates gp120-Induced Microglial Inflammation by Inhibiting Autophagy via the PI3K Pathway. Cell Mol. Neurobiol. 2018, 38, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-S.; Choi, M.-J.; Lee, Y.Y.; Moon, B.-I.; Park, J.-S.; Kim, H.-S. Suppression of Lipopolysaccharide-Induced Neuroinflammation by Morin via MAPK, PI3K/Akt, and PKA/HO-1 Signaling Pathway Modulation. J. Agric. Food Chem. 2017, 65, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Hwang, S.-Y.; Oh, E.-S.; Piao, H.Z.; Kim, K.-W.; Han, I.-O. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J. Immunol. 2004, 172, 7015–7023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, A.; Xu, L.; Zhang, X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience 2014, 269, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, Y.; Li, D.; Han, R.; Liu, G.; Kong, D.; Hao, J. Class I PI3K inhibitor ZSTK474 mediates a shift in microglial/macrophage phenotype and inhibits inflammatory response in mice with cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2016, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sherchan, P.; Wang, Y.; Reis, C.; Applegate, R.L.; Tang, J.; Zhang, J.H. Phosphoinositide 3-Kinase Gamma Contributes to Neuroinflammation in a Rat Model of Surgical Brain Injury. J. Neurosci. 2015, 35, 10390–10401. [Google Scholar] [CrossRef] [PubMed]
- Mozolewski, P.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Węgrzyn, G.; Bocheńska, K.; Banecki, B.; Gabig-Cimińska, M. Nonsteroidal anti-inflammatory drugs modulate cellular glycosaminoglycan synthesis by affecting EGFR and PI3K signaling pathways. Sci. Rep. 2017, 7, 43154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, W.; Su, Y.; Wei, Z.; Liu, J.; Kolluri, S.K.; Wu, H.; Cao, Y.; Chen, J.; Wu, Y.; et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell 2010, 17, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.T.; Bazan, N.G. Neuroprotectin D1 Induces Neuronal Survival and Downregulation of Amyloidogenic Processing in Alzheimer’s Disease Cellular Models. Mol. Neurobiol. 2011, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, A.B.; Taylor, J.; DeRuiter, S.L.; Yu, J.-Y.; Figueroa, C.; Kwok, R.P.; Turner, D.L. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol. Cell Biol. 2003, 23, 4417–4427. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, Y.; Camarillo, C.; Yao, Y.; Zhang, Y.; Xu, C.; Jiang, L. The anti-tumor histone deacetylase inhibitor SAHA and the natural flavonoid curcumin exhibit synergistic neuroprotection against amyloid-beta toxicity. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mantamadiotis, T. Towards targeting PI3K-dependent regulation of gene expression in brain cancer. Cancers 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Monteith, N.; Law, P.Y.; Loh, H.H. Dynamic association of p300 with the promoter of the G protein-coupled rat delta opioid receptor gene during NGF-induced neuronal differentiation. Biochem. Biophys. Res. Commun. 2010, 396, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Law, P.-Y.; Loh, H.H. NGF/PI3K signaling-mediated epigenetic regulation of delta opioid receptor gene expression. Biochem. Biophys. Res. Commun. 2008, 368, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Tamatani, M.; Matsuzaki, H.; Namikawa, K.; Kiyama, H.; Vitek, M.P.; Mitsuda, N.; Tohyama, M. Akt Activation Protects Hippocampal Neurons from Apoptosis by Inhibiting Transcriptional Activity of p53. J Biol. Chem. 2001, 276, 5256–5264. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Biswas, S.C. FoxO3a is activated and executes neuron death via Bim in response to β-amyloid. Cell Death Dis. 2013, 4, e625-12. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, Z.; Tsai, L.K.; Leeds, P.; Fessler, E.B.; Wang, J.; Chuang, D.M. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol. Psychiatry 2015, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; LaFerla, F.M.; Oddo, S.S.; Brewer, G.J. Reversible epigenetic histone modifications and Bdnf expression in neurons with aging and from a mouse model of Alzheimer’s disease. Age (Omaha) 2013, 35, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.J.; Lill, C.; Faull, R.; Curtis, M.A.; Dragunow, M. Increased acetyl and total histone levels in post-mortem Alzheimer’s disease brain. Neurobiol. Dis. 2015, 74, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.C.F.; Lemos, R.; Ferreiro, E.; Martins, M.; de Mendonça, A.; Santana, I.; Quteiro, T.F.; Pereira, C.M.F. Epigenetic regulation of BACE1 in Alzheimer’s disease patients and in transgenic mice. Neuroscience 2012, 220, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Deng, Y.; Yu, D.; Cao, H.; Wang, L.; Liu, L.; Yu, C.; Zhang, Y.; Guo, X.; Yu, G. Histone acetyltransferase p300 mediates histone acetylation of PS1 and BACE1 in a cellular model of Alzheimer’s disease. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spangle, J.M.; Roberts, T.M.; Zhao, J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jarome, T.J.; Perez, G.A.; Hauser, R.M.; Hatch, K.M.; Lubin, F.D. EZH2 Methyltransferase Activity Controls Pten Expression and mTOR Signaling During Fear Memory Reconsolidation. J. Neurosci. 2018, 38, 7635–7648. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Delvaux, E.; Nolz, J.; Tan, Y.; Grover, A.; Oddo, S.; Coleman, P.D. Aberrant intracellular localization of H3k4me3 demonstrates an early epigenetic phenomenon in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Li, Q.; Kong, X.; Ou, Z.; Zhang, L.; Gong, Z.; Long, D.; Li, J.; Zhang, M.; et al. PI3K/AKT/mTOR Signaling Mediates Valproic Acid-Induced Neuronal Differentiation of Neural Stem Cells through Epigenetic Modifications. Stem Cell Rep. 2017, 8, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Sarroca, S.; Ivanova, A.; Puigoriol-Illamola, D.; Aguado, F.; Camins, A.; Coral, S.; Pallas, M. Epigenetic mechanisms underlying cognitive impairment and Alzheimer disease hallmarks in 5XFAD mice. Aging (Albany NY) 2016, 8, 664–684. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.M.; Coleman, P.D.; Rutten, B.P.F.; Van den Hove, D.L.A. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide12 DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Kobayashi, N.; Ishii, J.; Shinagawa, S.; Nakayama, R.; Shibata, N.; Kueban, B.; Ohnuma, T.; Kondo, K.; Arai, H.; et al. Association between DNA methylation of the BDNF promoter region and clinical presentation in Alzheimer’s disease. Dement Geriatr. Cogn. Dis. Extra 2015, 5, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Muller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 2013, 499, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, A.; Zvelebil, M.J.; Wallasch, C.; Ullrich, A.; Waterfield, M.D.; Domin, J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol. Cell Biol. 2000, 20, 3817–3830. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, L.; Chen, J.; Zhao, H.; Wang, J.; Xu, S.; Huang, Z. miR-362-5p inhibits proliferation and migration of neuroblastoma cells by targeting phosphatidylinositol 3-kinase-C2β. FEBS Lett. 2015, 589, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.D.; Formoso, K.; Aparicio, G.I.; Frasch, A.C.C.; Scorticati, C. The Membrane Glycoprotein M6a Endocytic/Recycling Pathway Involves Clathrin-Mediated Endocytosis and Affects Neuronal Synapses. Front. Mol. Neurosci. 2017, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, A.N.; Praticò, D. Endosomal sorting and traf fi cking, the retromer complex and neurodegeneration. Mol. Psychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Petralia, R.S.; Kurushima, H.; Patel, H.; Jung, M.; Volk, L.; Chowdhury, S.; Shepherd, J.D.; Dehoff, M.; Li, Y.; et al. Arc/Arg3. 1 Regulates an Endosomal Pathway Essential for Activity-Dependent b -Amyloid Generation. Cell 2011, 147, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Domin, J.; Gaidarov, I.; Smith, M.E.; Keen, J.H.; Waterfield, M.D. The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles. J. Biol. Chem. 2000, 275, 11943–11950. [Google Scholar] [CrossRef] [PubMed]
- Gaidarov, I.; Smith, M.E.; Domin, J.; Keen, J.H. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 2001, 7, 443–449. [Google Scholar] [CrossRef]
- Meunier, F.A.; Osborne, S.L.; Hammond, G.R.V.; Cooke, F.T.; Parker, P.J.; Domin, J.; Schiavo, G. Phosphatidylinositol 3-Kinase C2α Is Essential for ATP-dependent Priming of Neurosecretory Granule Exocytosis. Mol. Biol. Cell 2005, 16, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Shiwarski, D.J.; Darr, M.; Telmer, C.A.; Bruchez, M.P.; Puthenveedu, M.A. PI3K class II α regulates δ-opioid receptor export from the trans-Golgi network. Mol. Biol. Cell 2017, 28, 2202–2219. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, A.G.; Ford, K.J.; Wang, T.; Fetter, R.D.; Tong, A.; Davis, G.W. A postsynaptic PI3K-cII dependent signaling controller for presynaptic homeostatic plasticity. Elife 2018, 7, e31535. [Google Scholar] [CrossRef] [PubMed]
- Byfield, M.P.; Murray, J.T.; Backer, J.M. hVps34 Is a Nutrient-regulated Lipid Kinase Required for Activation of p70 S6 Kinase. J. Biol. Chem. 2005, 280, 33076–33082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011, 278, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Ichimura, Y. MBSJ MCC Young Scientist Award 2009 REVIEW: Selective autophagy regulates various cellular functions. Genes Cells 2010, 15, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Gerónimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Castro-Obregón, S.; Massieu, L. Autophagy fails to prevent glucose deprivation/glucose reintroduction-induced neuronal death due to calpain-mediated lysosomal dysfunction in cortical neurons. Cell Death Dis. 2017, 8, e2911. [Google Scholar] [CrossRef] [PubMed]
- Issman-Zecharya, N.; Schuldiner, O. The PI3K Class III Complex Promotes Axon Pruning by Downregulating a Ptc-Derived Signal via Endosome-Lysosomal Degradation. Dev. Cell 2014, 31, 461–473. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alegría, K.; Flores-León, M.; Avila-Muñoz, E.; Rodríguez-Corona, N.; Arias, C. PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. Int. J. Mol. Sci. 2018, 19, 3725. https://doi.org/10.3390/ijms19123725
Sánchez-Alegría K, Flores-León M, Avila-Muñoz E, Rodríguez-Corona N, Arias C. PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. International Journal of Molecular Sciences. 2018; 19(12):3725. https://doi.org/10.3390/ijms19123725
Chicago/Turabian StyleSánchez-Alegría, Karina, Manuel Flores-León, Evangelina Avila-Muñoz, Nelly Rodríguez-Corona, and Clorinda Arias. 2018. "PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions" International Journal of Molecular Sciences 19, no. 12: 3725. https://doi.org/10.3390/ijms19123725
APA StyleSánchez-Alegría, K., Flores-León, M., Avila-Muñoz, E., Rodríguez-Corona, N., & Arias, C. (2018). PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. International Journal of Molecular Sciences, 19(12), 3725. https://doi.org/10.3390/ijms19123725




