Effects of Enhanced Hydrophilic Titanium Dioxide-Coated Hydroxyapatite on Bone Regeneration in Rabbit Calvarial Defects
Abstract
:1. Introduction
2. Results
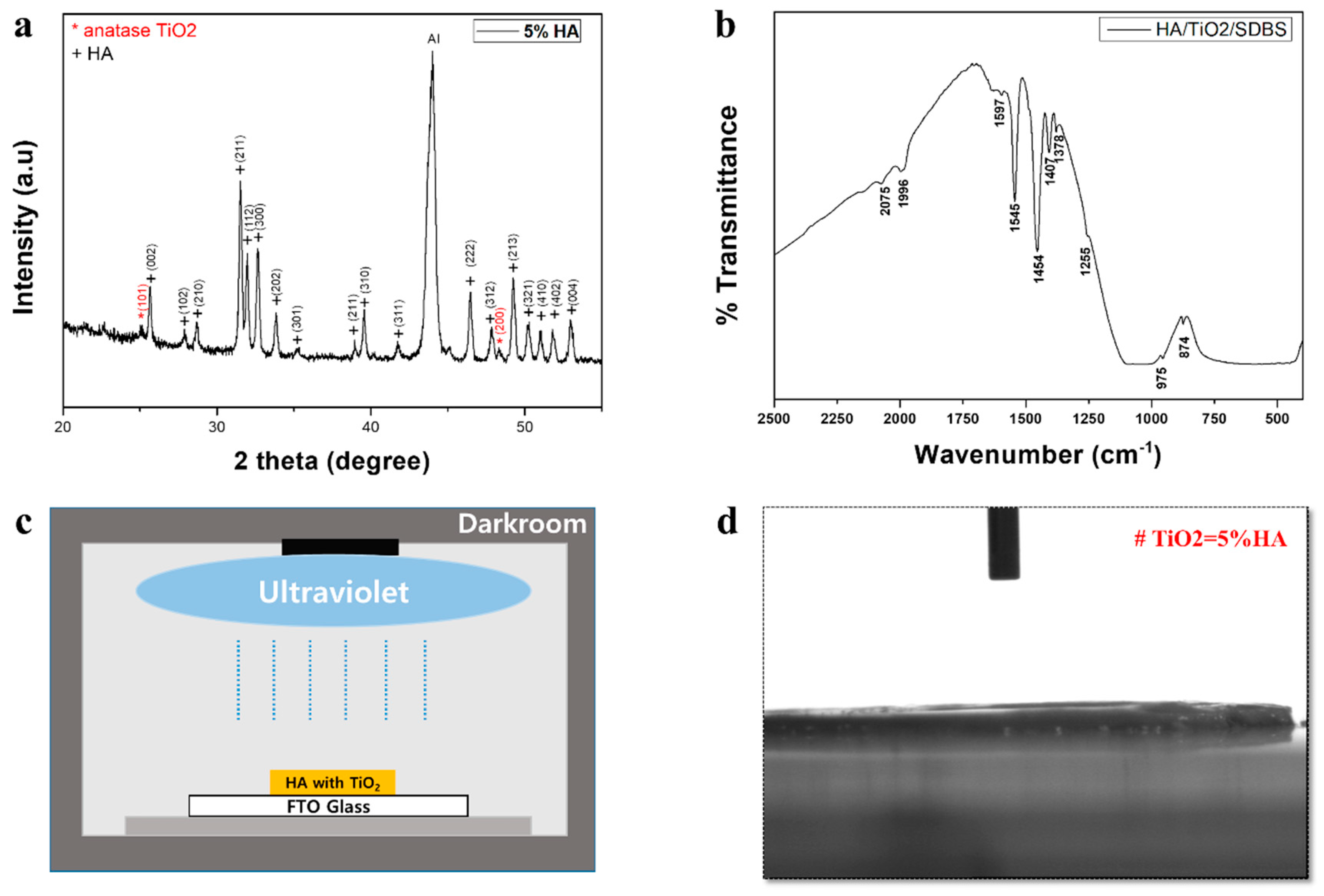
2.1. Surface Characteristics
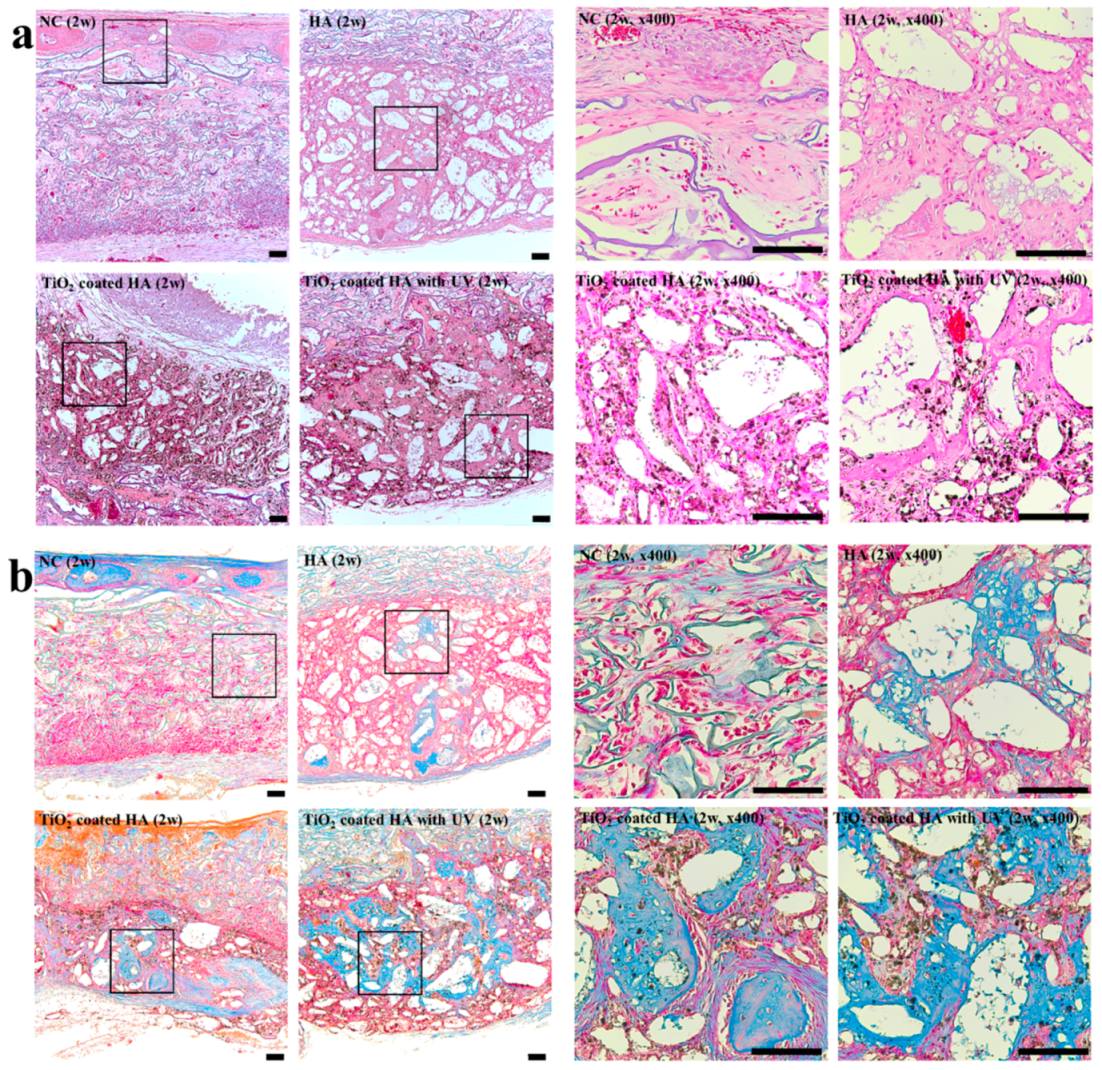
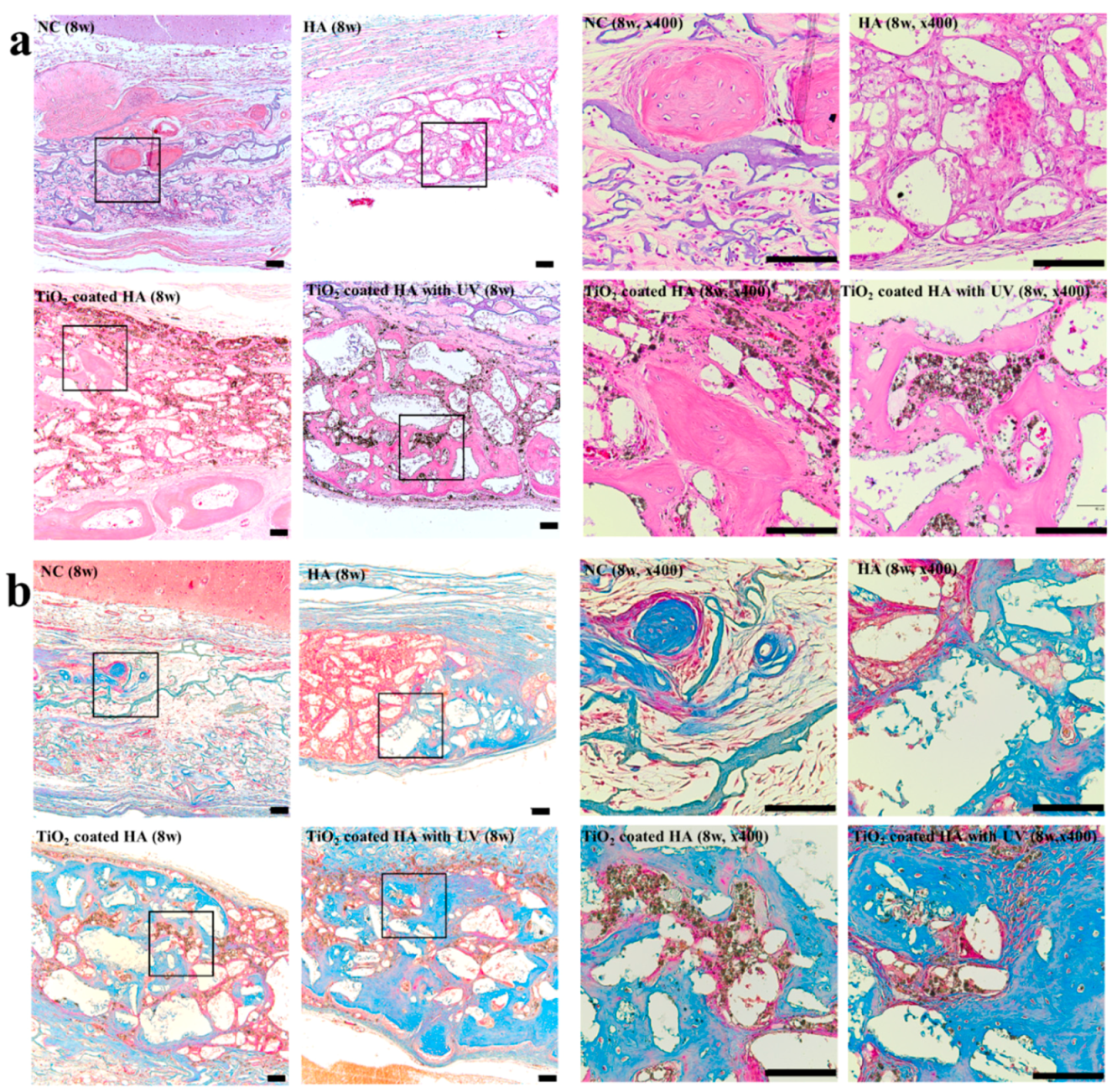
2.2. Histology
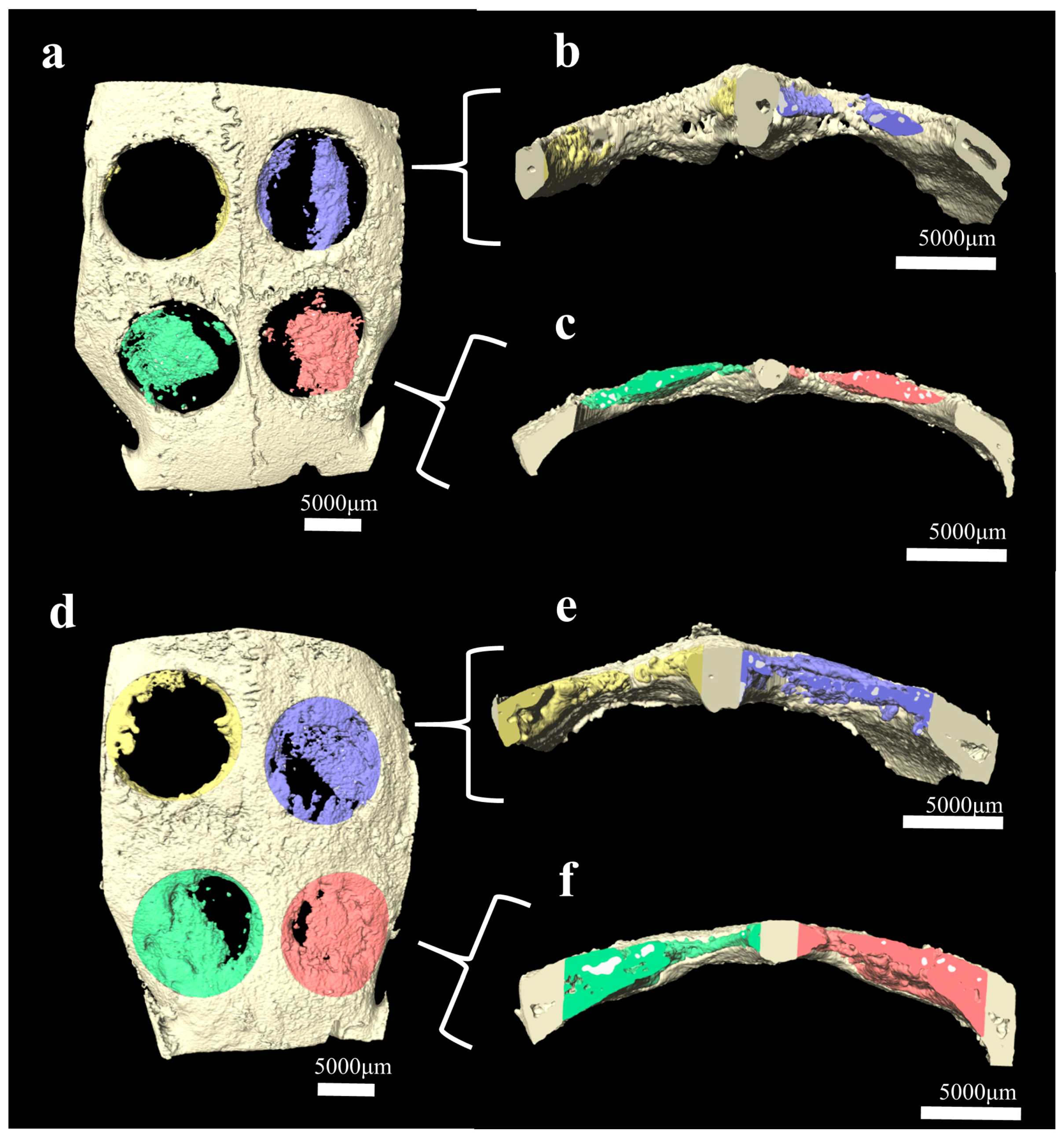
2.3. Histomorphometry
3. Discussion
4. Materials and Methods
4.1. Preparation of Bone Graft Materials
4.2. Wettability Test
4.3. UV Photofunctionalization
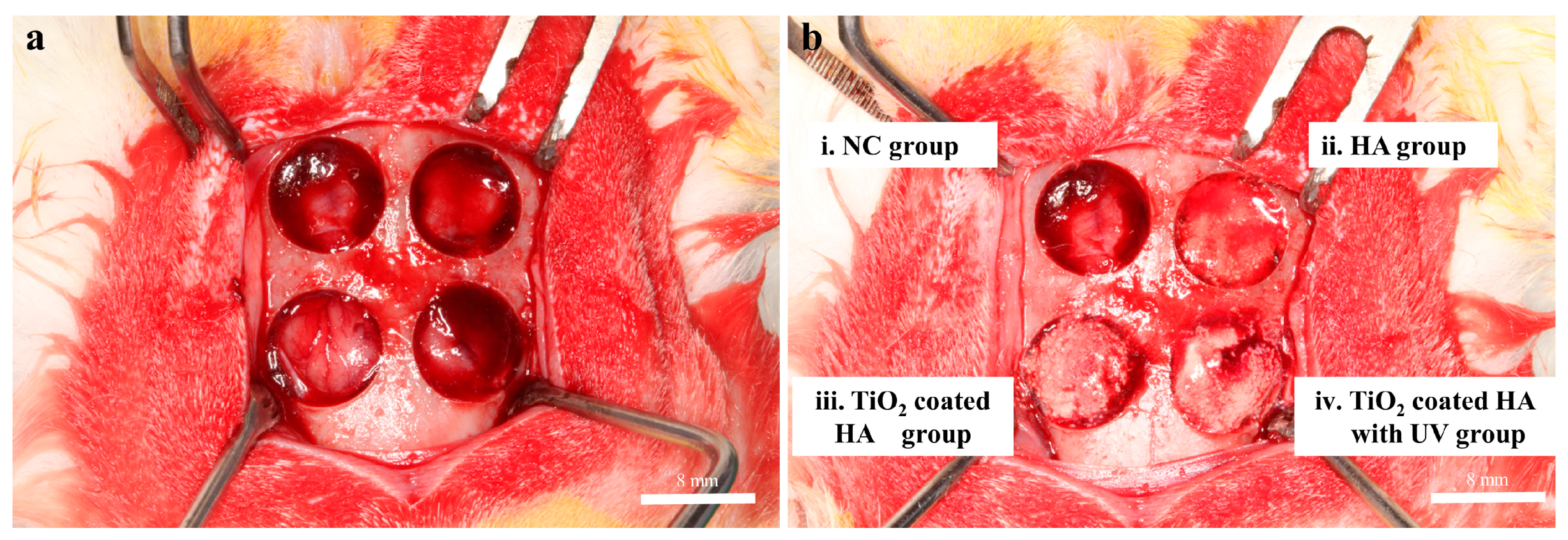
4.4. Animal Study
4.5. X-ray Microcomputed Tomography Analysis
4.6. Surface Characteristics and Histological and Histomorphometric Analyses
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.W.; Kim, J.H.; Moon, S.Y. Effect of hydroxyapatite on critical-sized defect. Maxillofac. Plast. Reconstr. Surg. 2016, 38. [Google Scholar] [CrossRef] [PubMed]
- Zane, A.; Zuo, R.; Villamena, F.A.; Rockenbauer, A.; Foushee, A.M.D.; Flores, K.; Dutta, P.K.; Nagy, A. Biocompatibility and antibacterial activity of nitrogen-doped titanium dioxide nanoparticles for use in dental resin formulations. Int. J. Nanomed. 2016, 11, 6459–6470. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Osteoinduction of porous bioactive titanium metal. Biomaterials 2004, 25, 443–450. [Google Scholar] [CrossRef]
- Gonzalez, J.; Mirza-Rosca, J. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Franchi, M.; Bacchelli, B.; Martini, D.; De Pasquale, V.; Orsini, E.; Ottani, V.; Fini, M.; Giavaresi, G.; Giardino, R.; Ruggeri, A. Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials 2004, 25, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Niska, K.; Pyszka, K.; Tukaj, C.; Wozniak, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. Titanium dioxide nanoparticles enhance production of superoxide anion and alter the antioxidant system in human osteoblast cells. Int. J. Nanomed. 2015, 10, 1095–1107. [Google Scholar] [CrossRef]
- Mani, P.; Sharma, H.K.; Gautam, A.; Singh, T.; Verma, S.K.; Hussain, R. hydroxyapatite (HA) attenuate TiO2 toxicuty in bio-system triggering E. coli and mouse bone marrow mono nuclear cells (BMMNC’S). Int. J. Life Sci. Pharma Res. 2017, 7, L46–L57. [Google Scholar]
- Okumura, A.; Goto, M.; Goto, T.; Yoshinari, M.; Masuko, S.; Katsuki, T.; Tanaka, T. Substrate affects the initial attachment and subsequent behavior of human osteoblastic cells (Saos-2). Biomaterials 2001, 22, 2263–2271. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, J.; Huang, B.; Wu, X.; QIAO, W.; Luo, X.; Chen, Z. Ultraviolet irradiation enhanced bioactivity and biological response of mesenchymal stem cells on micro-arc oxidized titanium surfaces. Dent. Mater. J. 2015, 34, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implant. 2014, 29. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, J.; Chen, X.; Wang, X.; He, F.; Wang, H. The In Vivo Bone Response of Ultraviolet-Irradiated Titanium Implants Modified with Spontaneously Formed Nanostructures: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 2016, 31, 776–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Areid, N.; Peltola, A.; Kangasniemi, I.; Ballo, A.; Närhi, T.O. Effect of ultraviolet light treatment on surface hydrophilicity and human gingival fibroblast response on nanostructured titanium surfaces. Clin. Exp. Dent. Res. 2018, 4, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimbo, R.; Ono, D.; Hirakawa, Y.; Odatsu, T.; Tanaka, T.; Sawase, T. Accelerated photo-induced hydrophilicity promotes osseointegration: An animal study. Clin. Implant Dent. Relat. Res. 2011, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lim, H.C.; Lee, E.U.; Park, J.Y.; Lee, J.S.; Lee, D.W.; Jung, U.W.; Choi, S.H. Paracrine effect of the bone morphogeneticprotein-2 at the experimental site on healing of the adjacent control site: A study in the rabbit calvarial defect model. J. Periodontal Implant Sci. 2014, 44, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.Y.; Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Cho, K.S.; Choi, S.H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavenus, S.; Trichet, V.; Le Chevalier, S.; Hoornaert, A.; Louarn, G.; Layrolle, P. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedicine 2012, 7, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.Q.; Yamaguchi, Y.; Yamatoya, K.; Horikoshi, S.; Nakata, K. Rewritable superhemophobic and superhemophilic wettability pattern based on titanium dioxide. Mater. Lett. 2018, 213, 51–53. [Google Scholar] [CrossRef]
- Areid, N.; Kangasniemi, I.; Söderling, E.; Närhi, T.O. Ultraviolet photofunctionalization of nanostructured titanium surfaces enhances thrombogenicity and platelet response. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, P.; Liao, Y.; Wang, J.; Chen, H.; Sun, H.; Huang, N. Effect of the duration of UV irradiation on the anticoagulant properties of titanium dioxide films. ACS Appl. Mater. Interfaces 2015, 7, 4423–4432. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, N.; Ueno, T.; Suzuki, T.; Iwasa, F.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implant. 2010, 25, 49–62. [Google Scholar]
- Ishijima, M.; Soltanzadeh, P.; Hirota, M.; Tsukimura, N.; SHIGAMI, T.; Ogawa, T. Enhancing osteoblast-affinity of titanium scaffolds for bone engineering by use of ultraviolet light treatment. Biomed. Res. 2015, 36, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Park, J.-H.; Kim, S.-J.; Kang, M.-Y.; Kang, M.-K. Rat Mastoid Bullae Obliteration with Hydroxyapatite: Histopathologic Results with Various Size of Hydroxyapatite. Korean J. Otorhinolaryngol. Head Neck Surg. 2010, 53. [Google Scholar] [CrossRef]
- Chang, B.-S.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
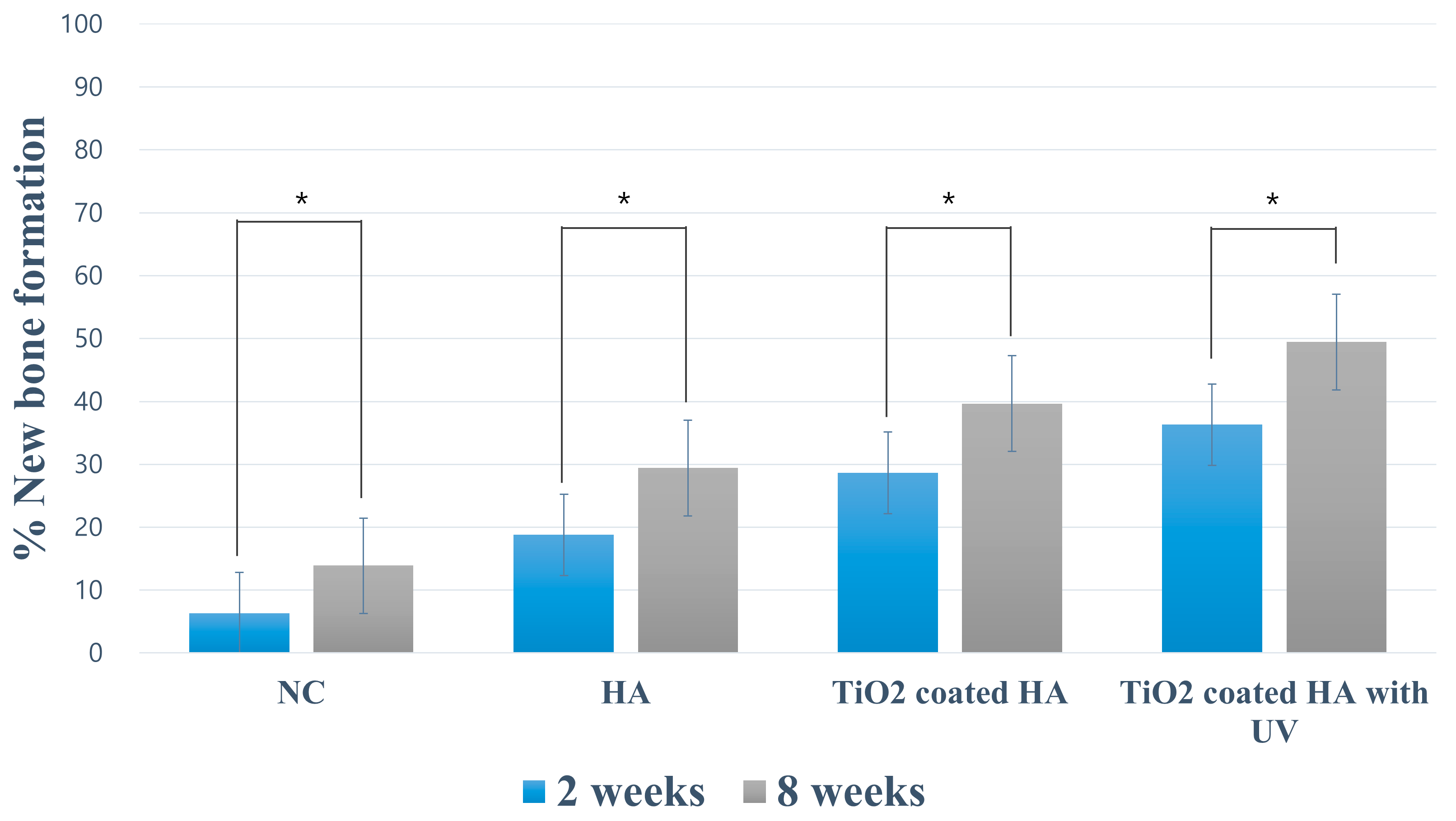
| Negative Control | Porous HA | TiO2-Coated HA | TiO2-Coated HA with UV | ||
|---|---|---|---|---|---|
| 2 weeks | % NB | 6.36 ± 1.72 | 18.78 ± 4.60 a | 28.63 ± 2.75 a, b | 36.32 ± 6.87 a, b, c |
| % RBM | 10.56 ± 1.62 | 10.90 ± 1.78 | 14.53 ± 2.15 b | ||
| 8 weeks | % NB | 13.90 ± 3.68 | 29.42 ± 5.81 a | 39.68 ± 6.70 a, b | 49.46 ± 6.54 a, b, c |
| % RBM | 12.14 ± 5.22 | 14.64 ± 2.82 | 13.38 ± 3.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Bark, C.W.; Quy, H.V.; Seo, S.-J.; Lim, J.-H.; Kang, S.-A.; Lee, Y.; Lee, J.-M.; Suh, J.-Y.; Kim, Y.-G. Effects of Enhanced Hydrophilic Titanium Dioxide-Coated Hydroxyapatite on Bone Regeneration in Rabbit Calvarial Defects. Int. J. Mol. Sci. 2018, 19, 3640. https://doi.org/10.3390/ijms19113640
Lee J-E, Bark CW, Quy HV, Seo S-J, Lim J-H, Kang S-A, Lee Y, Lee J-M, Suh J-Y, Kim Y-G. Effects of Enhanced Hydrophilic Titanium Dioxide-Coated Hydroxyapatite on Bone Regeneration in Rabbit Calvarial Defects. International Journal of Molecular Sciences. 2018; 19(11):3640. https://doi.org/10.3390/ijms19113640
Chicago/Turabian StyleLee, Ji-Eun, Chung Wung Bark, Hoang Van Quy, Seung-Jun Seo, Jae-Hong Lim, Sung-A Kang, Youngkyun Lee, Jae-Mok Lee, Jo-Young Suh, and Yong-Gun Kim. 2018. "Effects of Enhanced Hydrophilic Titanium Dioxide-Coated Hydroxyapatite on Bone Regeneration in Rabbit Calvarial Defects" International Journal of Molecular Sciences 19, no. 11: 3640. https://doi.org/10.3390/ijms19113640
APA StyleLee, J.-E., Bark, C. W., Quy, H. V., Seo, S.-J., Lim, J.-H., Kang, S.-A., Lee, Y., Lee, J.-M., Suh, J.-Y., & Kim, Y.-G. (2018). Effects of Enhanced Hydrophilic Titanium Dioxide-Coated Hydroxyapatite on Bone Regeneration in Rabbit Calvarial Defects. International Journal of Molecular Sciences, 19(11), 3640. https://doi.org/10.3390/ijms19113640





