Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis
Abstract
1. Introduction
2. Results
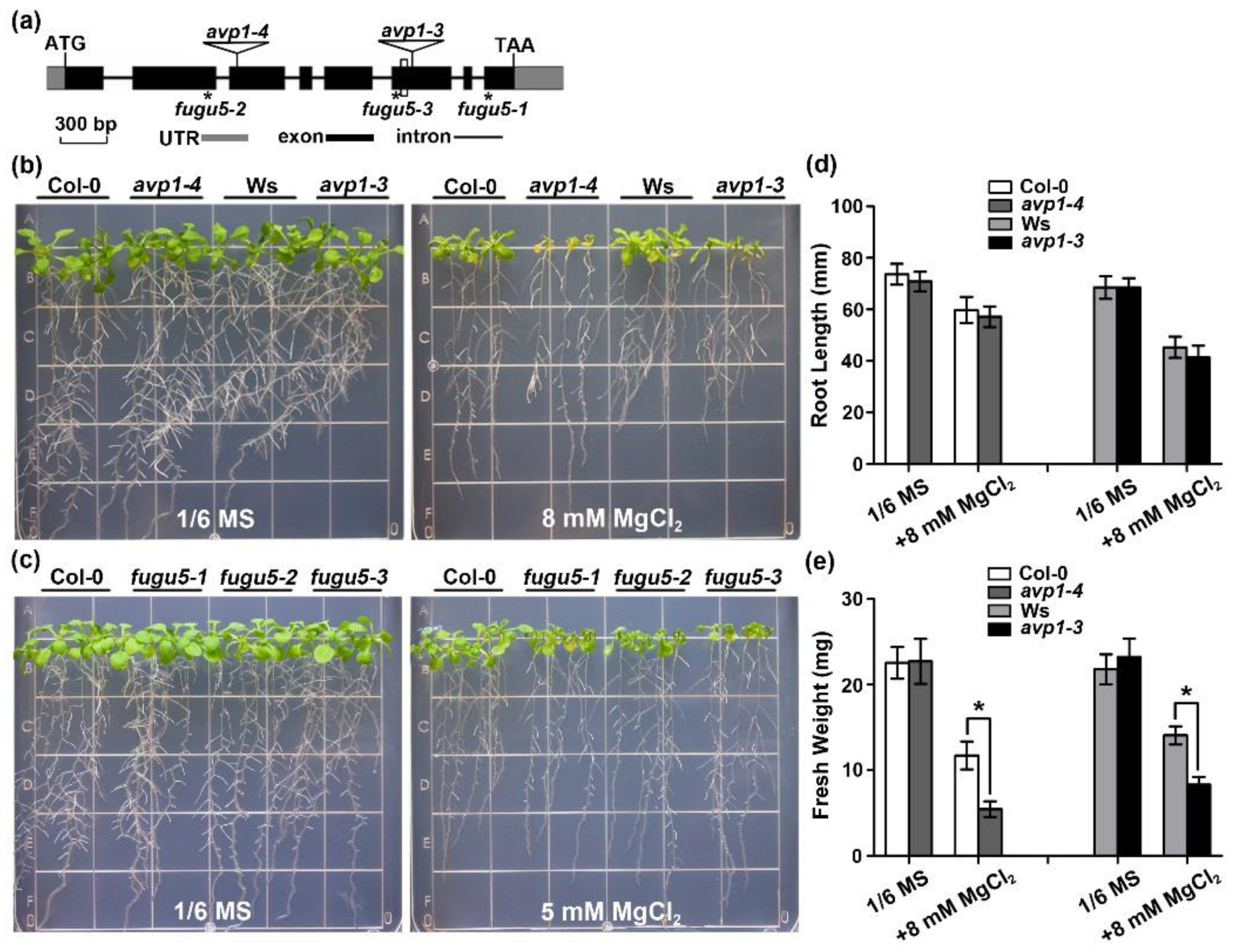
2.1. The avp1 Mutant Is Hypersensitive to High External Magnesium Conditions
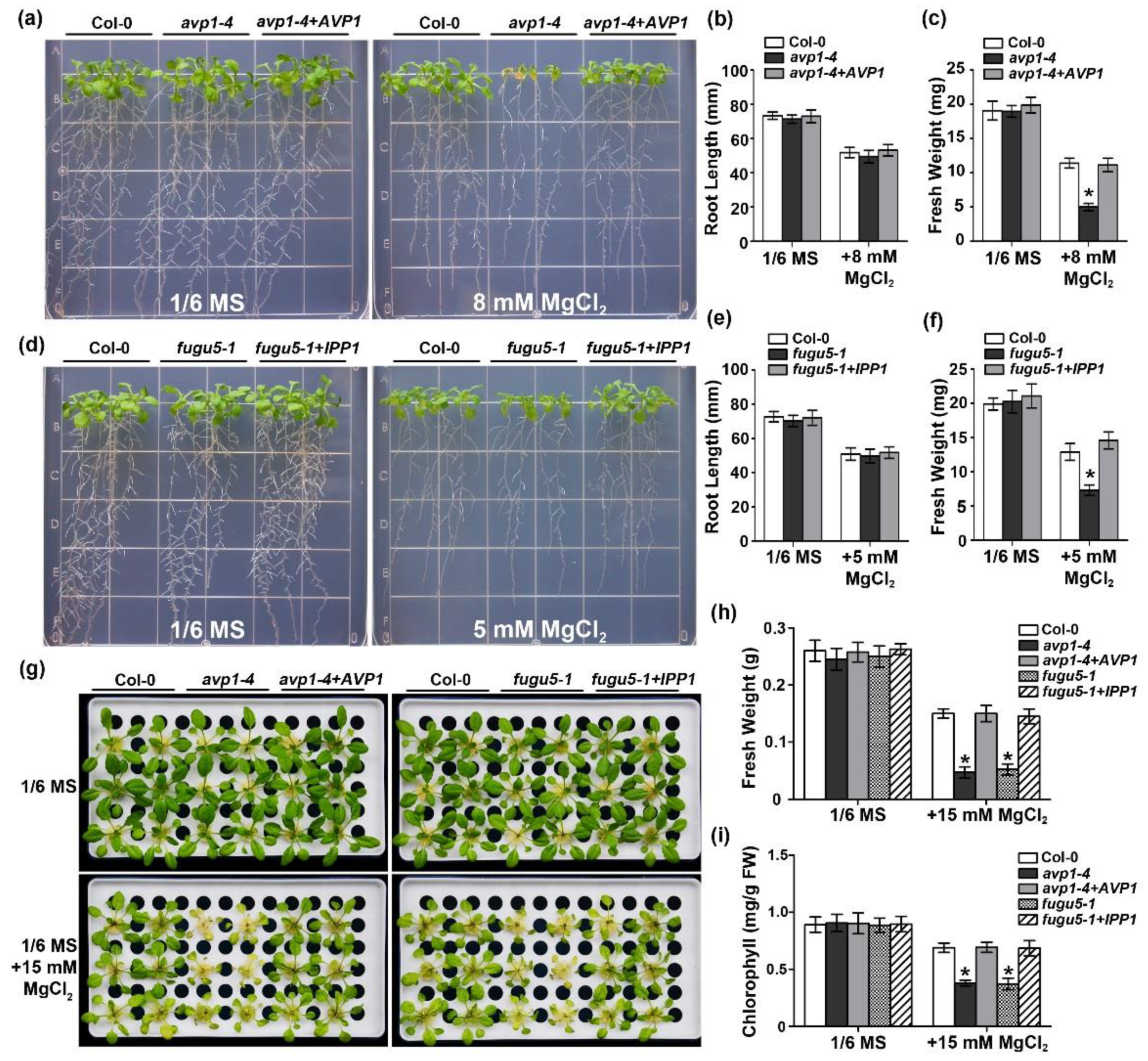
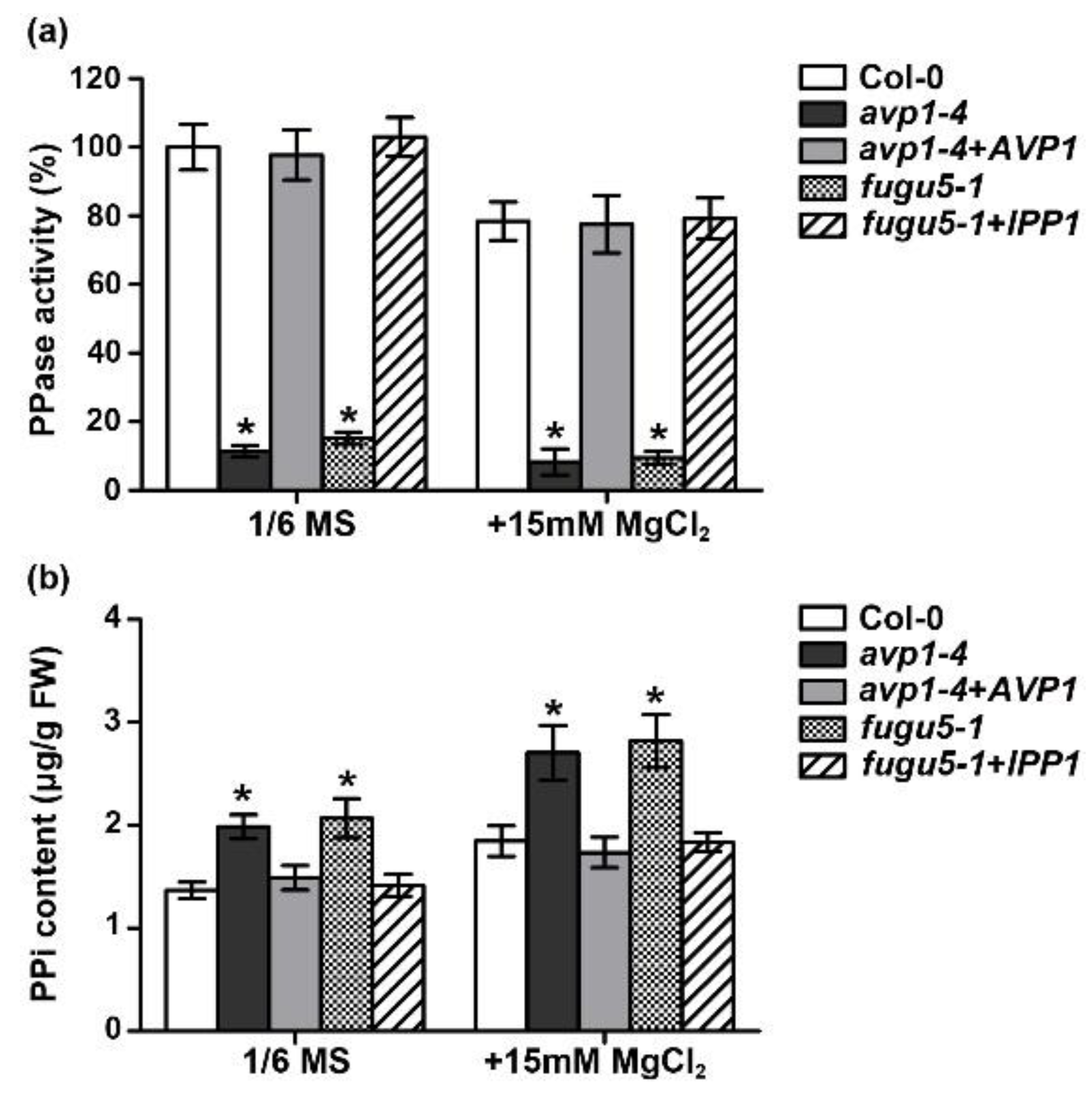
2.2. The Enzymatic Pyrophosphatase Activity Is Required for High-Mg Tolerance in Plants
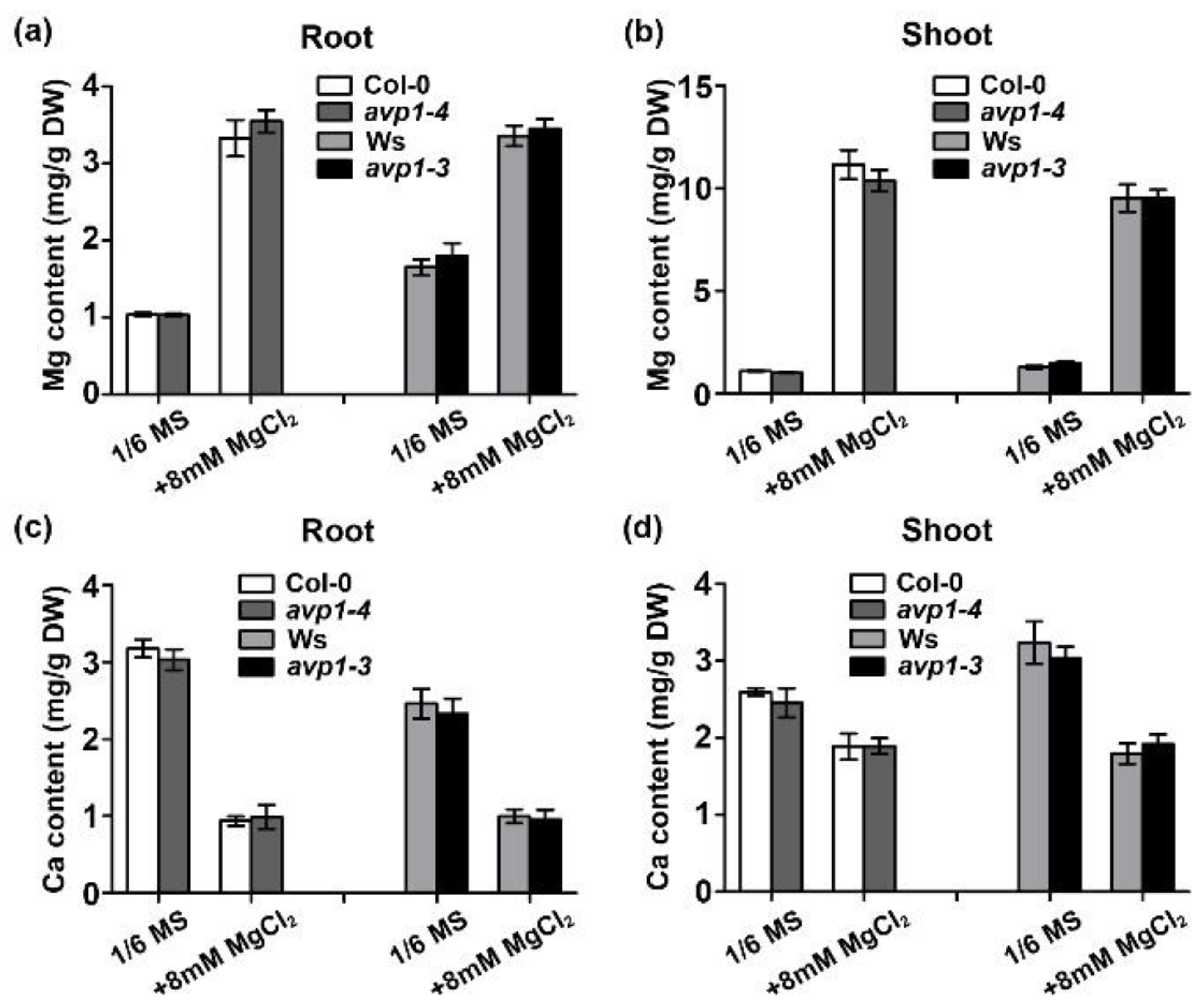
2.3. The avp1 Mutant Is Not Compromised in Mg2+ Homeostasis
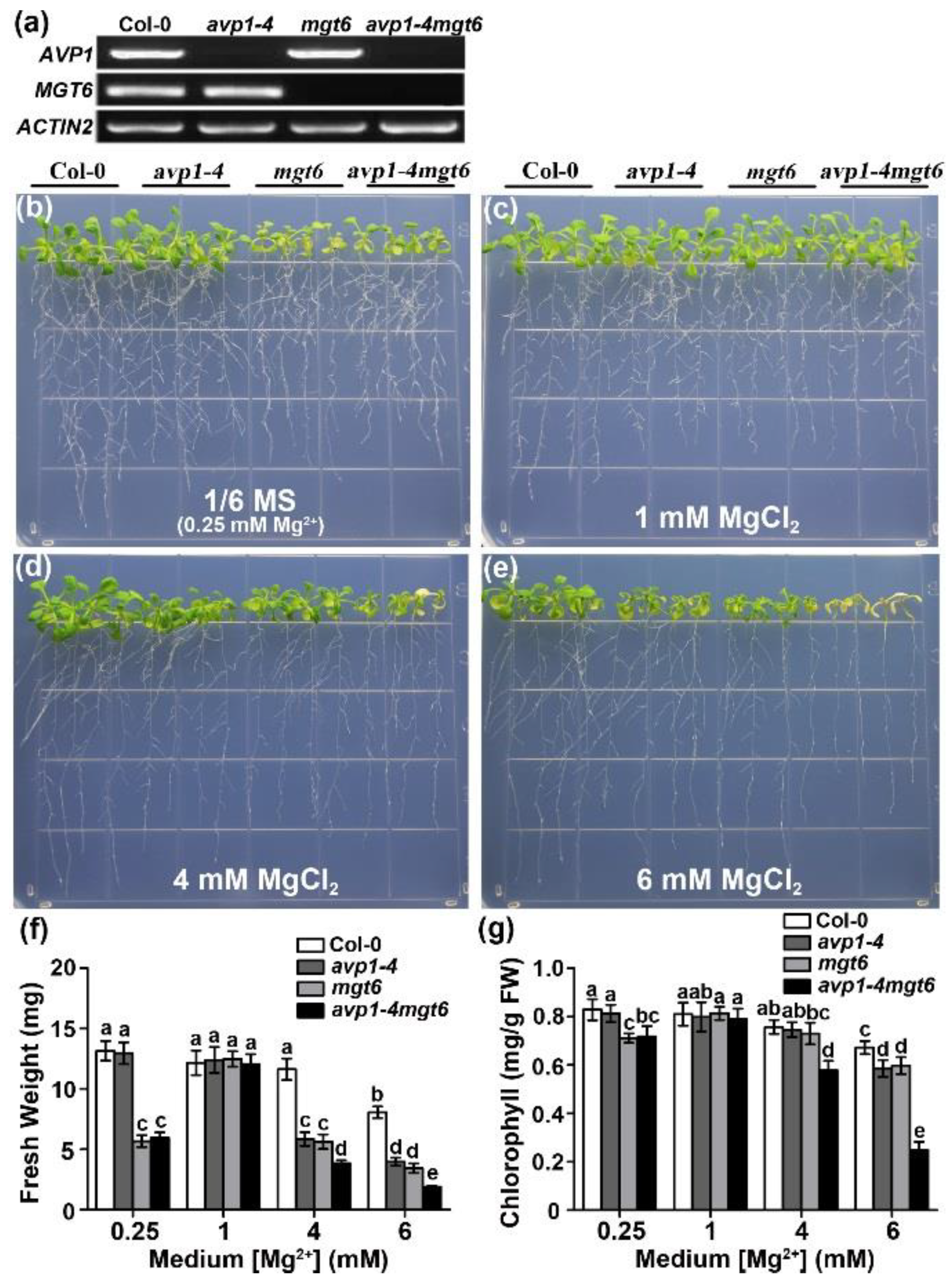
2.4. AVP1 and MGT6 Function Independently in High-Mg Tolerance in Arabidopsis
2.5. Hypersensitivity of avp1-4 cbl2 cbl3 Triple Mutant to High External Mg2+ Concentrations
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phenotypic Analysis
4.3. Crude Membrane Preparation and Enzymatic Activity Assays
4.4. Quantification of Pyrophosphate in Plants
4.5. Measurements of Mg and Ca Content
4.6. Statistical Analysis of the Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maeshima, M. Vacuolar H+-pyrophosphatase. Biochim. Biophys. Acta 2000, 1465, 37–51. [Google Scholar] [CrossRef]
- George, G.M.; van der Merwe, M.J.; Nunes-Nesi, A.; Bauer, R.; Fernie, A.R.; Kossmann, J.; Lloyd, J.R. Virus-induced gene silencing of plastidial soluble inorganic pyrophosphatase impairs essential leaf anabolic pathways and reduced drought stress tolerance in Nicotiana benthamiana. Plant Physiol. 2010, 154, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lόpez-Marqués, R.L.; Pérez-Castiñeira, J.R.; Losada, M.; Serrano, A. Differential regulation of soluble and membrane-bound inorganic pyrophosphatases in the photosynthetic bacterium Rhodospirillum rubrum provides insights into pyrophosphate-based stress bioenergetics. J. Bacteriol. 2004, 186, 5418–5426. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, A.; Kawade, K.; Asaoka, M.; Oikawa, A.; Okada, T.; Mochizuki, A.; Maeshima, M.; Hirai, M.Y.; Saito, K.; Tsukaya, H. Pyrophosphate inhibits gluconeogenesis by restricting UDP-glucose formation in vivo. Sci. Rep. 2018, 8, 14696–14705. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.; Mant, A.; Kossmann, J.; Lloyd, J.R. Identification of an Arabidopsis inorganic pyrophosphatase capable of being imported into chloroplasts. FEBS Lett. 2004, 565, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Navarro-De la Sancha, E.; Coello-Coutiño, M.P.; Valencia-Turcotte, L.G.; Hernández-Domínguez, E.E.; Trejo-Yepes, G.; Rodríguez-Sotres, R. Characterization of two soluble inorganic pyrophosphatases from Arabidopsis thaliana. Plant Sci. 2007, 172, 796–807. [Google Scholar] [CrossRef]
- Chen, J.; Brevet, A.; Fromant, M.; Lévêque, F.; Schmitter, J.M.; Blanquet, S.; Plateau, P. Pyrophosphatase is essential for growth of Escherichia coli. J. Bacteriol. 1990, 172, 5686–5689. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castiñeira, J.R.; Lόpez-Marqués, R.L.; Villalba, J.M.; Losada, M.; Serrano, A. Functional complementation of yeast cytosolic pyrophosphatase by bacterial and plant H+-translocating pyrophosphatases. Proc. Natl. Acad. Sci. USA 2002, 99, 15914–15919. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Bueno, G.; Hernández, A.; López-Lluch, G.; Pérez-Castiñeira, J.R.; Navas, P.; Serrano, A. Inorganic pyrophosphatase defects lead to cell cycle arrest and autophagic cell death through NAD+ depletion in fermenting yeast. J. Biol. Chem. 2013, 288, 13082–13092. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, V.; Kim, Y.; Poole, R.J.; Rea, P.A. Molecular cloning and sequence of cDNA encoding the pyrophosphate-energized vacuolar membrane proton pump of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1992, 89, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Rocha Façanha, A.; de Meis, L. Reversibility of H+-ATPase and H+ pyrophosphatase in tonoplast vesicles from maize coleoptiles and seeds. Plant Physiol. 1998, 116, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Gonzalez, P.; Echeverría, E. PPi formation by reversal of the tonoplast-bound H+-pyrophosphatase from Valencia orange juice cells. J. Am. Soc. Hortic. Sci. 2000, 125, 420–424. [Google Scholar]
- Ferjani, A.; Segami, S.; Horiguchi, G.; Muto, Y.; Maeshima, M.; Tsukaya, H. Keep an eye on PPi: The vacuolar-type H+-pyrophosphatase regulates postgerminative development in Arabidopsis. Plant Cell 2011, 23, 2895–2908. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Regmi, K.; Paez-Valencia, J.; Pizzio, G.; Zhang, S. Plant H+-PPases: Reversible enzymes with contrasting functions dependent on membrane environment. Mol. Plant 2015, 9, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Morimoto, R.; Tabeta, H.; Asaoka, M.; Ishida, M.; Maeshima, M.; Tsukaya, H.; Ferjani, A. Compensated Cell Enlargement in fugu5 is Specifically Triggered by Lowered Sucrose Production from Seed Storage Lipids. Plant Cell Physiol. 2017, 58, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Segami, S.; Tomoyama, T.; Sakamoto, S.; Gunji, S.; Fukuda, M.; Kinoshita, S.; Mitsuda, N.; Ferjani, A.; Maeshima, M. Vacuolar H+-Pyrophosphatase and Cytosolic Soluble Pyrophosphatases Cooperatively Regulate Pyrophosphate Levels in Arabidopsis thaliana. Plant Cell 2018, 30, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Paez-Valencia, J.; Patron-Soberano, A.; Rodriguez-Leviz, A.; Sanchez-Lares, J.; Sanchez-Gomez, C.; Valencia-Mayoral, P.; Diaz-Rosas, G.; Gaxiola, R. Plasma membrane localization of the type I H+-PPase AVP1 in sieve element–companion cell complexes from Arabidopsis thaliana. Plant Sci. 2011, 181, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Sanchez, C.A.; Paez-Valencia, J.; Ayre, B.G.; Elser, J.J. Genetic manipulation of a “vacuolar” H+-PPase: From salt tolerance to yield enhancement under phosphorus-deficient soils. Plant Physiol. 2012, 159, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A.; Paez-Valencia, J.; Khadilkar, A.S.; Regmi, K.; Patron-Soberano, A.; Zhang, S.; Sanchez-Lares, J.; Furstenau, T.; Li, J.; Sanchez-Gomez, C.; et al. Arabidopsis type I proton-pumping pyrophosphatase expresses strongly in phloem, where it is required for pyrophosphate metabolism and photosynthate partitioning. Plant Physiol. 2015, 167, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.S.; Yadav, U.P.; Salazar, C.; Shulaev, V.; Paez-Valencia, J.; Pizzio, G.A.; Gaxiola, R.A.; Ayre, B.G. Constitutive and companion cell-specific overexpression of AVP1, encoding a proton-pumping pyrophosphatase, enhances biomass accumulation, phloem loading and long-distance transport. Plant Physiol. 2015, 170, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, M. Tonoplast transporters: Organization and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.; Andrés, Z.; Medzihradszky, A.; Krüger, F.; Scholl, S.; Delang, S.; Patir-Nebioglu, M.G.; Gute, G.; Yang, H.; Murphy, A.S.; et al. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 2015, 27, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Li, J.; Undurraga, S.; Dang, L.M.; Allen, G.J.; Alper, S.L.; Fink, G.R. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA 2001, 98, 11444–11449. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Knapp, J.; Koirala, P.; Rajagopal, D.; Peer, W.A.; Silbart, L.K.; Murphy, A.; Gaxiola, R.A. Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus-responsive type I H+-pyrophosphatase. Plant Biotechnol. J. 2007, 5, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Paez-Valencia, J.; Sanchez-Lares, J.; Marsh, E.; Dorneles, L.T.; Santos, M.P.; Sanchez, D.; Winter, A.; Murphy, S.; Cox, J.; Trzaska, M.; et al. Enhanced proton translocating pyrophosphatase activity improves nitrogen use efficiency in romaine lettuce. Plant Physiol. 2013, 161, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Schilling, R.K.; Tester, M.; Marschner, P.; Plett, D.C.; Roy, S.J. AVP1: One Protein, Many Roles. Trends Plant Sci. 2017, 22, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Tutone, A.F.; Drummond, R.S.M.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef] [PubMed]
- Schock, I.; Gregan, J.; Steinhauser, S.; Schweyen, R.; Brennicke, A.; Knoop, V. A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J. 2000, 24, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Gebert, M.; Meschenmoser, K.; Svidová, S.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Sokolov, L.N.; Yang, Y.H.; Li, D.P.; Ting, J.; Pandy, G.K.; Luan, S. A mitochondrial magnesium transporter functions in Arabidopsis pollen development. Mol. Plant 2008, 1, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Tan, H.; Yang, X.; Tian, L.; Luan, S.; Chen, L.; Li, D. An endoplasmic reticulum magnesium transporter is essential for pollen development in Arabidopsis. Plant Sci. 2015, 231, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.G.; Liu, Z.H.; Yuan, Y.J.; Guo, L.L.; Mao, D.D.; Tian, L.F.; Chen, L.B.; Luan, S.; Li, D.P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009, 19, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Wang, B.; Lou, Y.; Han, W.J.; Lu, J.Y.; Li, D.D.; Li, L.G.; Zhu, J.; Yang, Z.N. Magnesium transporter 5 plays an important role in Mg transport for male gametophyte development in Arabidopsis. Plant J. 2015, 84, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.W.; Mao, D.D.; Yang, L.; Qi, J.L.; Zhang, X.X.; Tang, Q.L.; Li, Y.P.; Tang, R.J.; Luan, S. Magnesium transporter MGT6 plays an essential role in maintaining magnesium homeostasis and regulating high magnesium tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Kamiya, T.; Shikanai, Y.; Shigenobu, S.; Yamaguchi, K.; Fujiwara, T. The Arabidopsis Mg transporter, MRS2-4, is essential for Mg homeostasis under both low and high Mg conditions. Plant Cell Physiol. 2016, 57, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Peer, W.A.; Richter, G.; Blakeslee, J.; Bandyopadhyay, A.; Titapiwantakun, B.; Undurraga, S.; Khodakovskaya, M.; Richards, E.L.; et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005, 310, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Segami, S.; Makino, S.; Miyake, A.; Asaoka, M.; Maeshima, M. Dynamics of vacuoles and H+-pyrophosphatase visualized by monomeric green fluorescent protein in Arabidopsis: Artifactual bulbs and native intravacuolar spherical structures. Plant Cell 2014, 26, 3416–3434. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef]
- Li, Z.G.; Baldwin, C.M.; Hu, Q.; Liu, H.; Luo, H. Heterologous expression of Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ. 2010, 33, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Schilling, R.K.; Marschner, P.; Shavrukov, Y.; Berger, B.; Tester, M.; Roy, S.J.; Plett, D.C. Expression of the Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) improves the shoot biomass of transgenic barley and increases grain yield in a saline field. Plant Biotechnol. J. 2014, 12, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Liu, H.; Yang, Y.; Yang, L.; Gao, X.S.; Garcia, V.J.; Luan, S.; Zhang, H.X. Tonoplast calcium sensors CBL2 and CBL3 control plant growth and ion homeostasis through regulating V-ATPase activity in Arabidopsis. Cell Res. 2012, 22, 1650–1665. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Segami, S.; Tomoyama, T.; Asaoka, M.; Nakanishi, Y.; Gunji, S.; Ferjani, A.; Maeshima, M. Lack of H+-pyrophosphatase prompts developmental damage in Arabidopsis leaves on ammonia-free culture medium. Front. Plant Sci. 2016, 7, 819. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tang, R.-J.; Mu, B.; Ferjani, A.; Shi, J.; Zhang, H.; Zhao, F.; Lan, W.-Z.; Luan, S. Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3617. https://doi.org/10.3390/ijms19113617
Yang Y, Tang R-J, Mu B, Ferjani A, Shi J, Zhang H, Zhao F, Lan W-Z, Luan S. Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis. International Journal of Molecular Sciences. 2018; 19(11):3617. https://doi.org/10.3390/ijms19113617
Chicago/Turabian StyleYang, Yang, Ren-Jie Tang, Baicong Mu, Ali Ferjani, Jisen Shi, Hongxia Zhang, Fugeng Zhao, Wen-Zhi Lan, and Sheng Luan. 2018. "Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis" International Journal of Molecular Sciences 19, no. 11: 3617. https://doi.org/10.3390/ijms19113617
APA StyleYang, Y., Tang, R.-J., Mu, B., Ferjani, A., Shi, J., Zhang, H., Zhao, F., Lan, W.-Z., & Luan, S. (2018). Vacuolar Proton Pyrophosphatase Is Required for High Magnesium Tolerance in Arabidopsis. International Journal of Molecular Sciences, 19(11), 3617. https://doi.org/10.3390/ijms19113617





