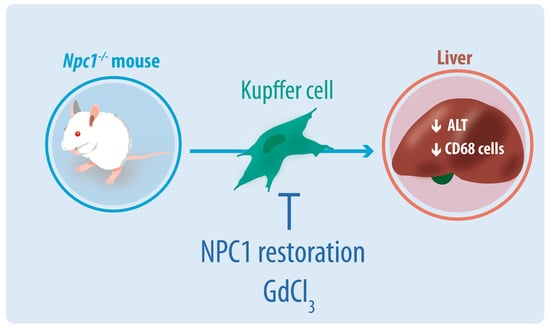
Gadolinium Chloride Rescues Niemann–Pick Type C Liver Damage
Abstract
1. Introduction
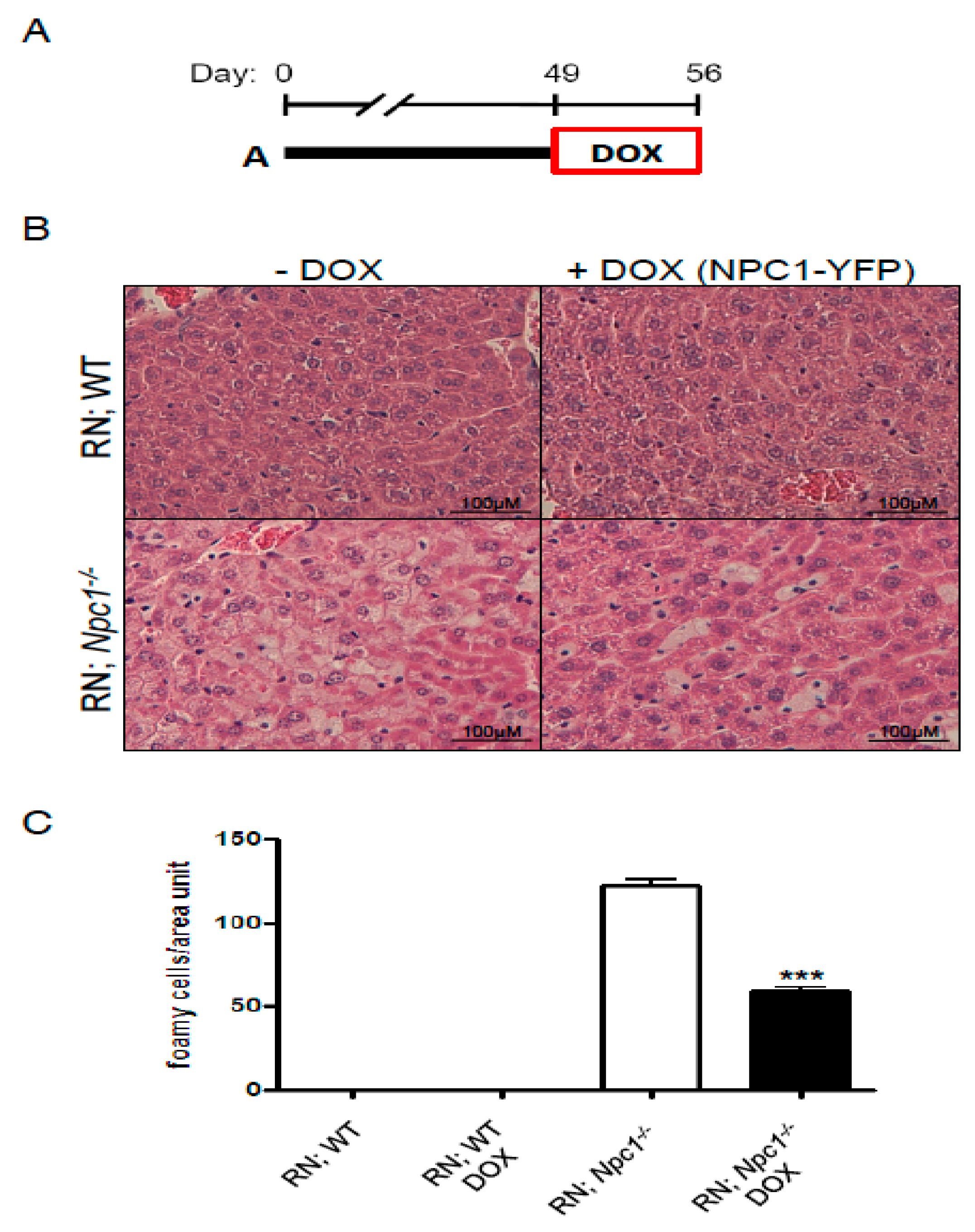
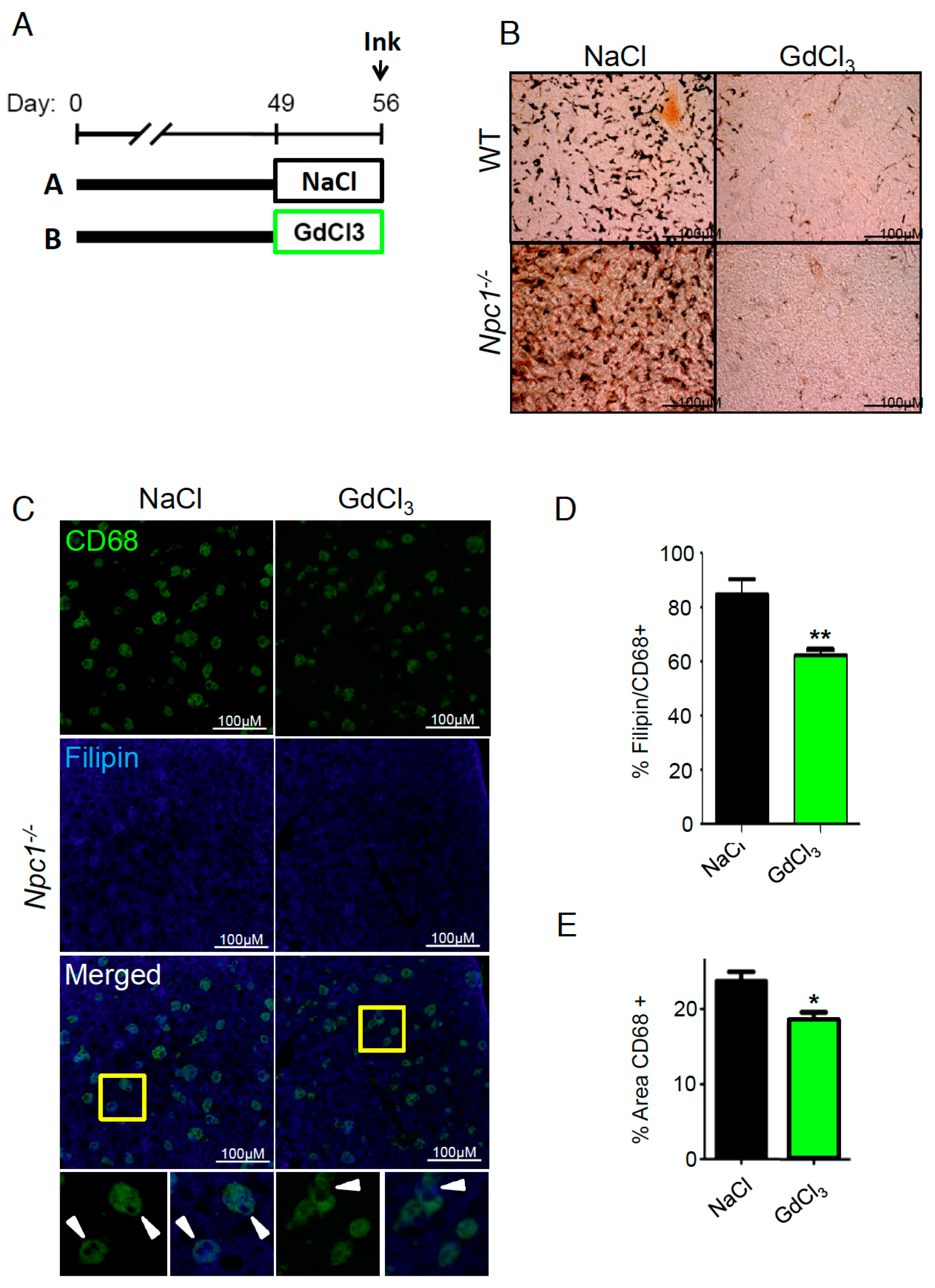
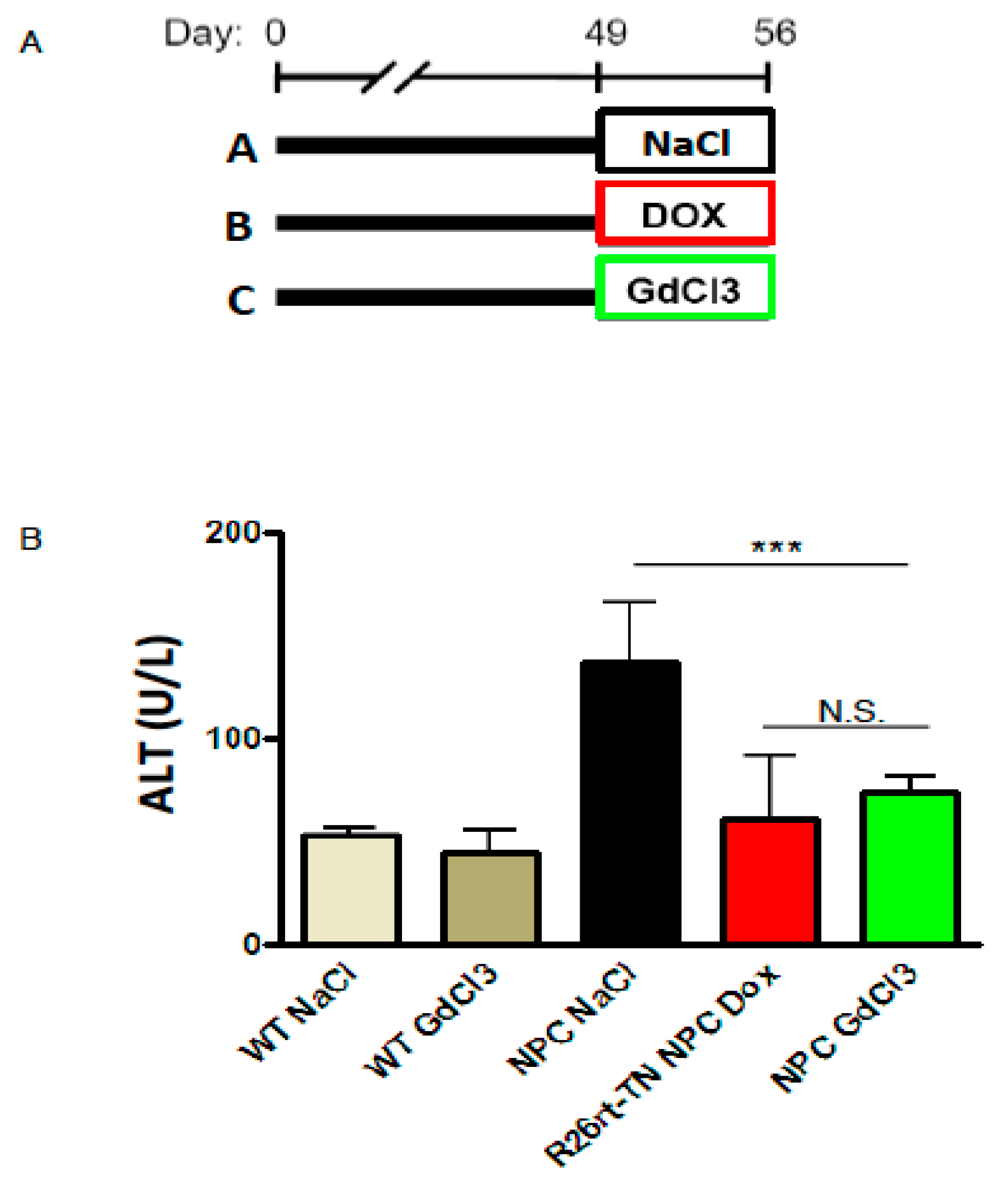
2. Results
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Treatments
4.3. Liver and Blood Sampling
4.4. Liver ALT
4.5. Histology
4.6. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-Terminal Domain of NPC1 Reveals Distinct Subdomains for Binding and Transfer of Cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Geberhiwot, T.; Moro, A.; Dardis, A.; Ramaswami, U.; Sirrs, S.; Marfa, M.P.; Vanier, M.T.; Walterfang, M.; Bolton, S.; Dawson, C.; et al. Consensus clinical management guidelines for Niemann–Pick disease type C. Orphanet J. Rare Dis. 2018, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.D.; Alvarez, A.; Zanlungo, S. The unique case of the Niemann–Pick type C cholesterol storage disorder. Pediatr. Endocrinol. Rev. 2014, 12, 166–175. [Google Scholar] [PubMed]
- Gumus, E.; Haliloglu, G.; Karhan, A.N.; Demir, H.; Gurakan, F.; Topcu, M.; Yuce, A. Niemann–Pick disease type C in the newborn period: A single-center experience. Eur. J. Pediatr. 2017, 176, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, B.; Sokol, R.J.; Narkewicz, M.R.; Smith, D.; Ashmead, J.W.; Wenger, D.A. Niemann–Pick disease type C in neonatal cholestasis at a North American Center. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tan, D.; Wang, Y.; Ye, J.; Han, L.; Qiu, W.; Gu, X.; Zhang, H. [Three Chinese children with Niemann–Pick disease type C with neonatal cholestasis as initial presentation]. Zhonghua er ke za zhi (Chin. J. Pediatr.) 2015, 53, 57–61. [Google Scholar]
- Kelly, D.A.; Portmann, B.; Mowat, A.P.; Sherlock, S.; Lake, B.D. Niemann–Pick disease type C: Diagnosis and outcome in children, with particular reference to liver disease. J. Pediatr. 1993, 123, 242–247. [Google Scholar] [CrossRef]
- Rimkunas, V.M.; Graham, M.J.; Crooke, R.M.; Liscum, L. In vivo antisense oligonucleotide reduction of NPC1 expression as a novel mouse model for Niemann Pick Type C- associated liver disease. Hepatology 2008, 47, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Rimkunas, V.M.; Graham, M.J.; Crooke, R.M.; Liscum, L. TNF-α plays a role in hepatocyte apoptosis in Niemann–Pick type C liver disease. J. Lipid Res. 2009, 50, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.C.; del Pozo, T.; Robledo, F.A.; Carrasco, G.; Pavez, L.; Olivares, F.; González, M.; Zanlungo, S. Alteration of gene expression profile in Niemann–Pick type C mice correlates with tissue damage and oxidative stress. PLoS ONE 2011, 6, e28777. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Niemann–Pick diseases. Handb. Clin. Neurol. 2013, 113, 1717–1721. [Google Scholar] [PubMed]
- Husztik, E.; Lázár, G.; Párducz, A. Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride. Br. J. Exp. Pathol. 1980, 61, 624–630. [Google Scholar] [PubMed]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Q.; Zhu, B.; Cui, Z.; Zhou, J. Protective Effect of Gadolinium Chloride on Early Warm Ischemia/Reperfusion Injury in Rat Bile Duct during Liver Transplantation. PLoS ONE 2013, 8, e52743. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Meyakno, E.; Akanji, M.A. Inhibition of Kupffer cell functions modulates arsenic intoxication in Wistar rats. Gen. Physiol. Biophys. 2017, 2, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Oda, S.; Tsuneyama, K.; Fukami, T.; Nakajima, M.; Yokoi, T. Establishment of a mouse model for amiodarone-induced liver injury and analyses of its hepatotoxic mechanism. J. Appl. Toxicol. 2016, 36, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, L.; Dong, C.; Li, L. The class D scavenger receptor CD68 contributes to mouse chronic liver injury. Immunol. Res. 2018, 3, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Ma, S.F.; Qu, J.F.; Tian, D.H. Effects of Kupffer cell inactivation on graft survival and liver regeneration after partial liver transplantation in rats. Hepatobiliary Pancreat. Dis. Int. 2015, 1, 56–62. [Google Scholar] [CrossRef]
- Zhu, R.; Guo, W.; Fang, H.; Cao, S.; Yan, B.; Chen, S.; Zhang, K.; Zhang, S. Kupffer cell depletion by gadolinium chloride aggravates liver injury after brain death in rats. Mol. Med. Rep. 2018, 17, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of human Macrophage M1-M2 Polarization Balance by hypoxia and the Triggering receptor expressed on Myeloid cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.M.; Caravan, P.; Rao, A.G.; McDonald, R.J.; Winfeld, M.; Fleck, R.J.; Gee, M.S. Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr. Radiol. 2017, 47, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Hardonk, M.J.; Dijkhuis, F.W.; Hulstaert, C.E.; Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 1992, 52, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.; Klein, A.D.; Hong, J.; Dimbil, U.J.; Scott, M.P. Neuronal and epithelial cell rescue resolves chronic systemic inflammation in the lipid storage disorder Niemann–Pick C. Hum. Mol. Genet. 2012, 21, 2946–2960. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.; Klein, A.D.; Dimbil, U.J.; Scott, M.P. Anatomically defined neuron-based rescue of neurodegenerative Niemann–Pick type C disorder. J. Neurosci. 2011, 31, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Mosher, B.; Dean, R.; Harkema, J.; Remick, D.; Palma, J.; Crockett, E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J. Surg. Res. 2001, 99, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, H.; Zhao, X.; Miao, Q.; Li, Y.; Hu, R. Selective depletion of hepatic kupffer cells significantly alleviated hepatosteatosis and intrahepatic inflammation induced by high fat diet. Hepatogastroenterology. 2012, 59, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.; Klein, A.D.; Scott, M.P. Complement is dispensable for neurodegeneration in Niemann–Pick disease type C. J. Neuroinflammation 2012, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Cougnoux, A.; Cluzeau, C.; Mitra, S.; Li, R.; Williams, I.; Burkert, K.; Xu, X.; Wassif, C.A.; Zheng, W.; Porter, F.D. Necroptosis in Niemann–Pick disease, type C1: A potential therapeutic target. Cell Death Dis. 2016, 7, e2147. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Wallom, K.L.; Williams, I.M.; Jeyakumar, M.; Platt, F.M. Beneficial effects of anti-inflammatory therapy in a mouse model of Niemann–Pick disease type C1. Neurobiol. Dis. 2009, 36, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.F.; Klein, A.D. Controversies on the potential therapeutic use of rapamycin for treating a lysosomal cholesterol storage disease. Mol. Genet. Metab. Reports 2018, 15, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Platt, N.; Speak, A.O.; Colaco, A.; Gray, J.; Smith, D.A.; Williams, I.M.; Wallom, K.L.; Platt, F.M. Immune dysfunction in Niemann–Pick disease type C. J. Neurochem. 2016, 136, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Koop, D.R.; Klopfenstein, B.; Iimuro, Y.; Thurman, R.G. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol. Pharmacol. 1997, 51, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.L.; Pumford, N.R.; Mayeux, P.R.; Niesman, M.R.; Hinson, J.A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999, 30, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, E.; Nadai, M.; Caretta, C.M.; Bergonzini, V.; Del Vecchio, C.; Ha, H.R.; Bigler, L.; Dal Zoppo, D.; Faggin, E.; Pettenazzo, A.; et al. Amiodarone impairs trafficking through late endosomes inducing a Niemann–Pick C-like phenotype. Biochem. Pharmacol. 2011, 82, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Wei, L.; Zhang, X.; Li, X.; Wu, H.; Wu, Y.; Ni, J.; Pei, F. Metabolic profiling of serum from gadolinium chloride-treated rats by1H NMR spectroscopy. Anal. Biochem. 2007, 364, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Napolitano, A.; Visconti, E.; Longo, D.; Romano, A.; Tomà, P.; Espagnet, M.C. R. Gadolinium-Based Contrast Agent-Related Toxicities. CNS Drugs 2018, 32, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.P.; Lequin, M.H.; Huisman, T.A.G.M. Safety of Contrast Material Use in Children. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Ramalho, M. Gadolinium Deposition and Chronic Toxicity. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 765–778. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.J.; Ayala, A.; Wang, P.; Ba, Z.F.; Morrison, M.H.; Schultze, A.E.; Reich, S.S.; Chaudry, I.H. Role of Kupffer cells in interleukin-6 release following trauma-hemorrhage and resuscitation. Shock 1994, 1, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Iimuro, Y.; Yamamoto, M.; Kohno, H.; Itakura, J.; Fujii, H.; Matsumoto, Y. Blockade of liver macrophages by gadolinium chloride reduces lethality in endotoxemic rats—Analysis of mechanisms of lethality in endotoxemia. J. Leukoc. Biol. 1994, 55, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.; Rüttinger, D.; Wanner, G.A.; Leiderer, R.; Menger, M.D. Modulation of Kupffer cell activity by gadolinium chloride in endotoxemic rats. Shock 1996, 6, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Nakafusa, Y.; Flye, M.W. In vitro analysis of gadolinium chloride abrogation of the systemic tolerance induced by portal venous administration of ultraviolet B-irradiated donor cells. In Transplantation; 1997, 64, 1684–1688. [Google Scholar] [CrossRef]
- Vincent, M.; Sayre, N.L.; Graham, M.J.; Crooke, R.M.; Shealy, D.J.; Liscum, L. Evaluation of an anti-tumor necrosis factor therapeutic in a mouse model of Niemann–Pick C liver disease. PLoS ONE 2010, 5, e12941. [Google Scholar] [CrossRef] [PubMed]
- Pelled, D.; Trajkovic-Bodennec, S.; Lloyd-Evans, E.; Sidransky, E.; Schiffmann, R.; Futerman, A.H. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol. Dis. 2005, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M. Emptying the stores: Lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 2018, 17, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, D.; Haberkant, P.; Aguilera-Romero, A.; Riezman, H.; Porter, F.D.; Platt, F.M.; Galione, A.; Schultz, C. Intracellular sphingosine releases calcium from lysosomes. Elife 2015, 4, e10616. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, X.; Li, X.; Zhang, X.; Yao, Z.; Dibble, S.; Dong, X.P.; Yu, T.; Lieberman, A.P.; Showalter, H.D.; et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012, 3, 731. [Google Scholar] [CrossRef] [PubMed]
- Lansman, J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J. Gen. Physiol. 1990, 95, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Ahlem, A.; Ali, E.H.; Leila, T. Lysosomes of the liver and the duodenal enterocytes, a site of handling of two rare earths. Ultrastructural and microanalytical study. Biol. Trace Elem. Res. 2008, 124, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, F.; Kawagoe, M.; Wang, J.S.; Arany, S.; Zhou, X.P.; Kumagai, A.; Koizumi, Y.; Koyota, S.; Sugiyama, T. Gadolinium chloride suppresses styrene-induced cytochrome P450s expression in rat liver. Biomed. Res 2007, 28, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Getz, M.; Haldar, K. Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann–Pick type C disease in a mouse model. Sci. Transl. Med. 2016, 8, 326ra23. [Google Scholar] [CrossRef] [PubMed]
- Ebner, L.; Gläser, A.; Bräuer, A.; Witt, M.; Wree, A.; Rolfs, A.; Frank, M.; Vollmar, B.; Kuhla, A. Evaluation of two liver treatment strategies in a mouse model of niemann–pick-disease type C1. Int. J. Mol. Sci. 2018, 19, 972. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Mendoza, H.; Castro, J.; Quiones, V.; Miquel, J.F.; Zanlungo, S. Relevance of Niemann–Pick type C1 protein expression in controlling plasma cholesterol and biliary lipid secretion in mice. Hepatology 2002, 36, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Janzen, E.G.; Chen, J.Z.; Reinke, L.A.; Yamashiro, S. Inhibitory effect of phenyl N-terf-butyl nitrone on Kupffer cell phagocytosis. Redox Rep. 1996, 2, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.D.; Ferreira, N.-S.; Ben-Dor, S.; Duan, J.; Hardy, J.; Cox, T.M.; Merrill, A.H.; Futerman, A.H. Identification of Modifier Genes in a Mouse Model of Gaucher Disease. Cell Rep. 2016, 16, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.D.; Oyarzún, J.E.; Cortez, C.; Zanlungo, S. Gadolinium Chloride Rescues Niemann–Pick Type C Liver Damage. Int. J. Mol. Sci. 2018, 19, 3599. https://doi.org/10.3390/ijms19113599
Klein AD, Oyarzún JE, Cortez C, Zanlungo S. Gadolinium Chloride Rescues Niemann–Pick Type C Liver Damage. International Journal of Molecular Sciences. 2018; 19(11):3599. https://doi.org/10.3390/ijms19113599
Chicago/Turabian StyleKlein, Andrés D., Juan Esteban Oyarzún, Cristian Cortez, and Silvana Zanlungo. 2018. "Gadolinium Chloride Rescues Niemann–Pick Type C Liver Damage" International Journal of Molecular Sciences 19, no. 11: 3599. https://doi.org/10.3390/ijms19113599
APA StyleKlein, A. D., Oyarzún, J. E., Cortez, C., & Zanlungo, S. (2018). Gadolinium Chloride Rescues Niemann–Pick Type C Liver Damage. International Journal of Molecular Sciences, 19(11), 3599. https://doi.org/10.3390/ijms19113599