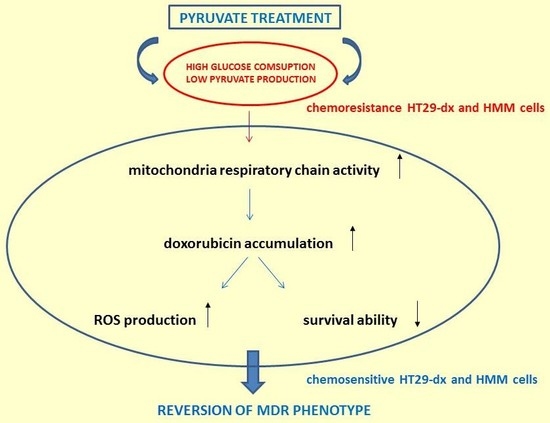
Pyruvate Treatment Restores the Effectiveness of Chemotherapeutic Agents in Human Colon Adenocarcinoma and Pleural Mesothelioma Cells
Abstract
1. Introduction
2. Results
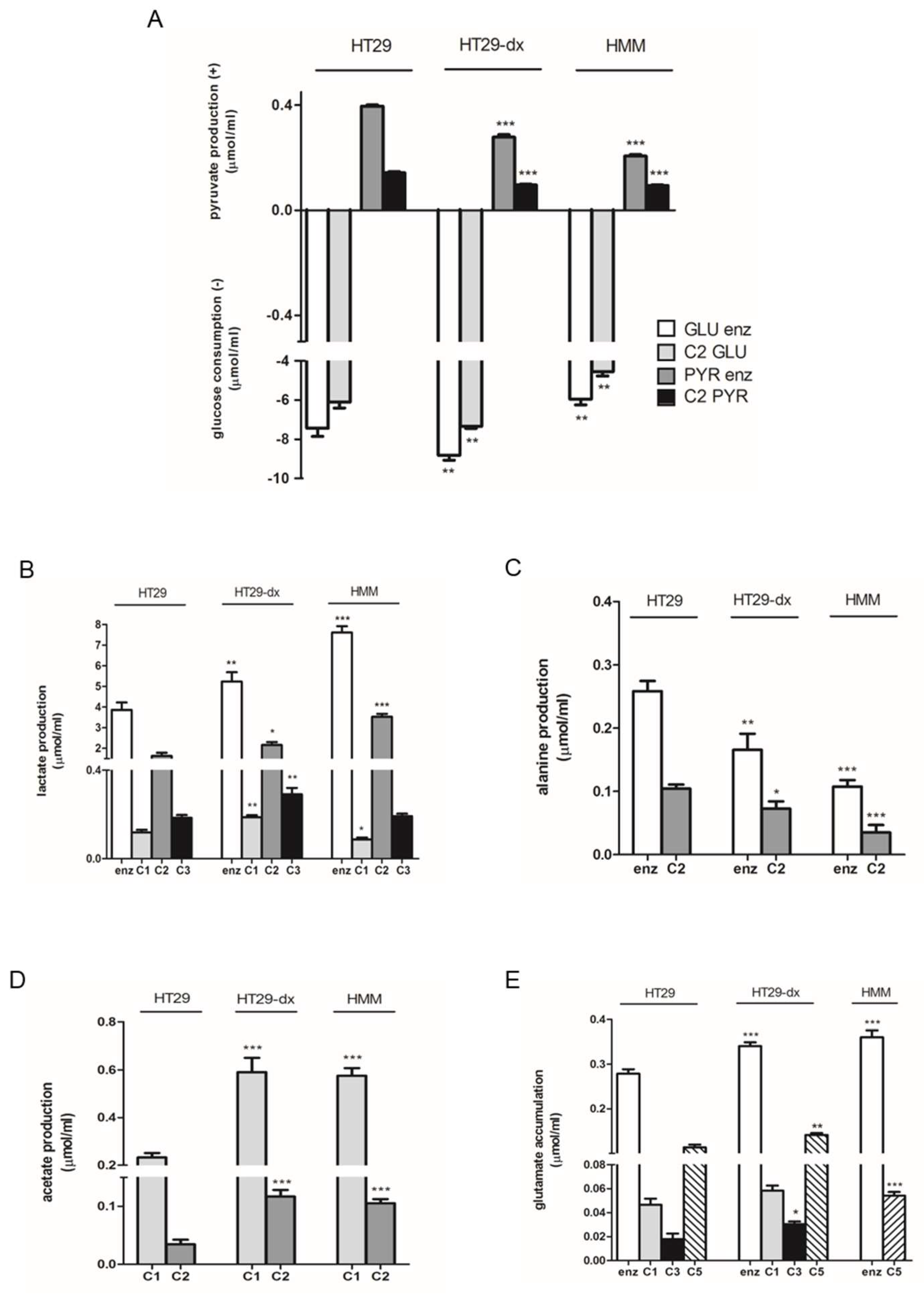
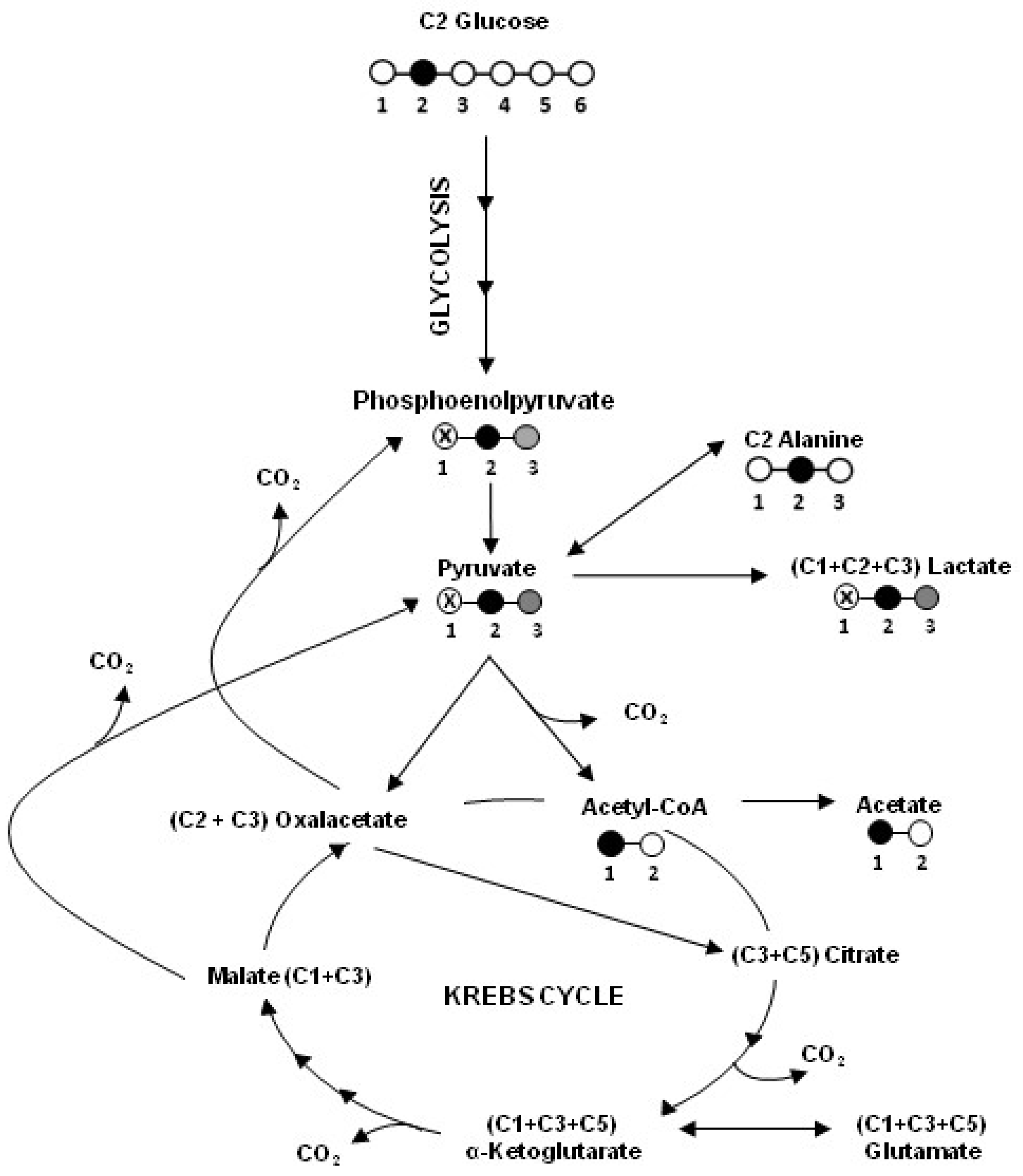
2.1. Human Colon Adenocarcinoma Cells (HT29), HT29-dx and Human Malignant Mesothelioma Cells (HMM) Had a Different Carbon Metabolism
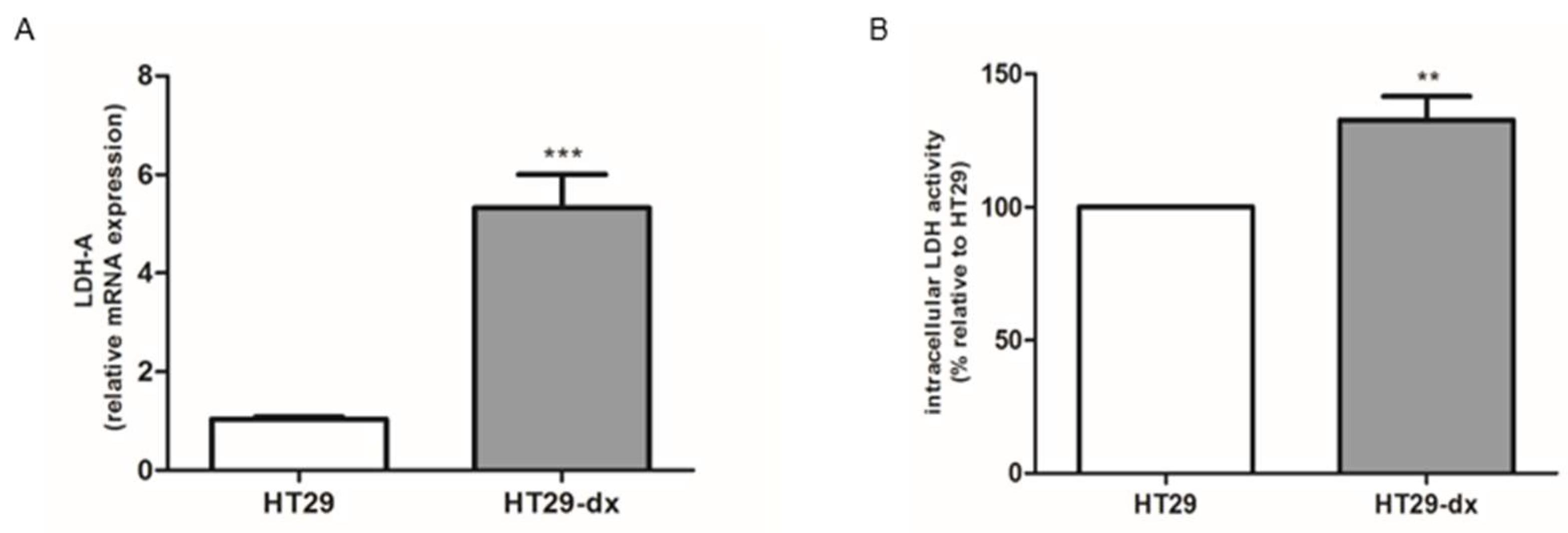
2.2. Phosphoenolpyruvate Carboxykinase 2 (PCK2), Lactate Dehydrogenase (LDH-A) mRNA Expression and LDH Activity Were Higher in HT29-dx Cells
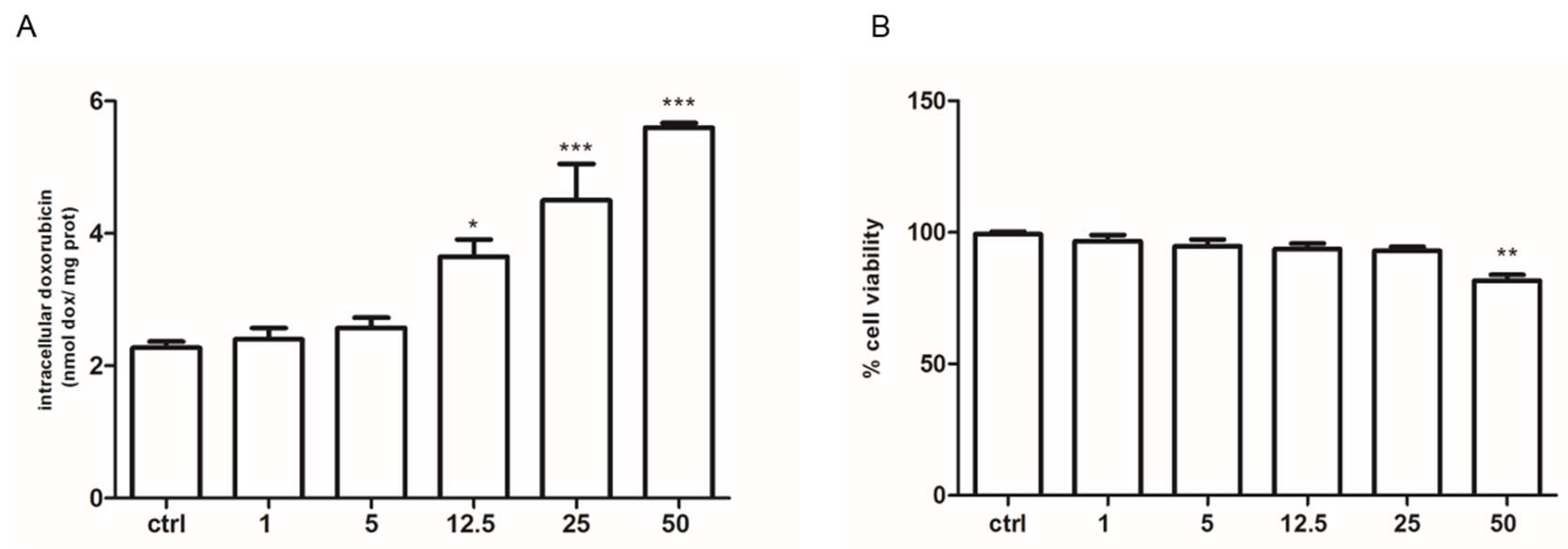
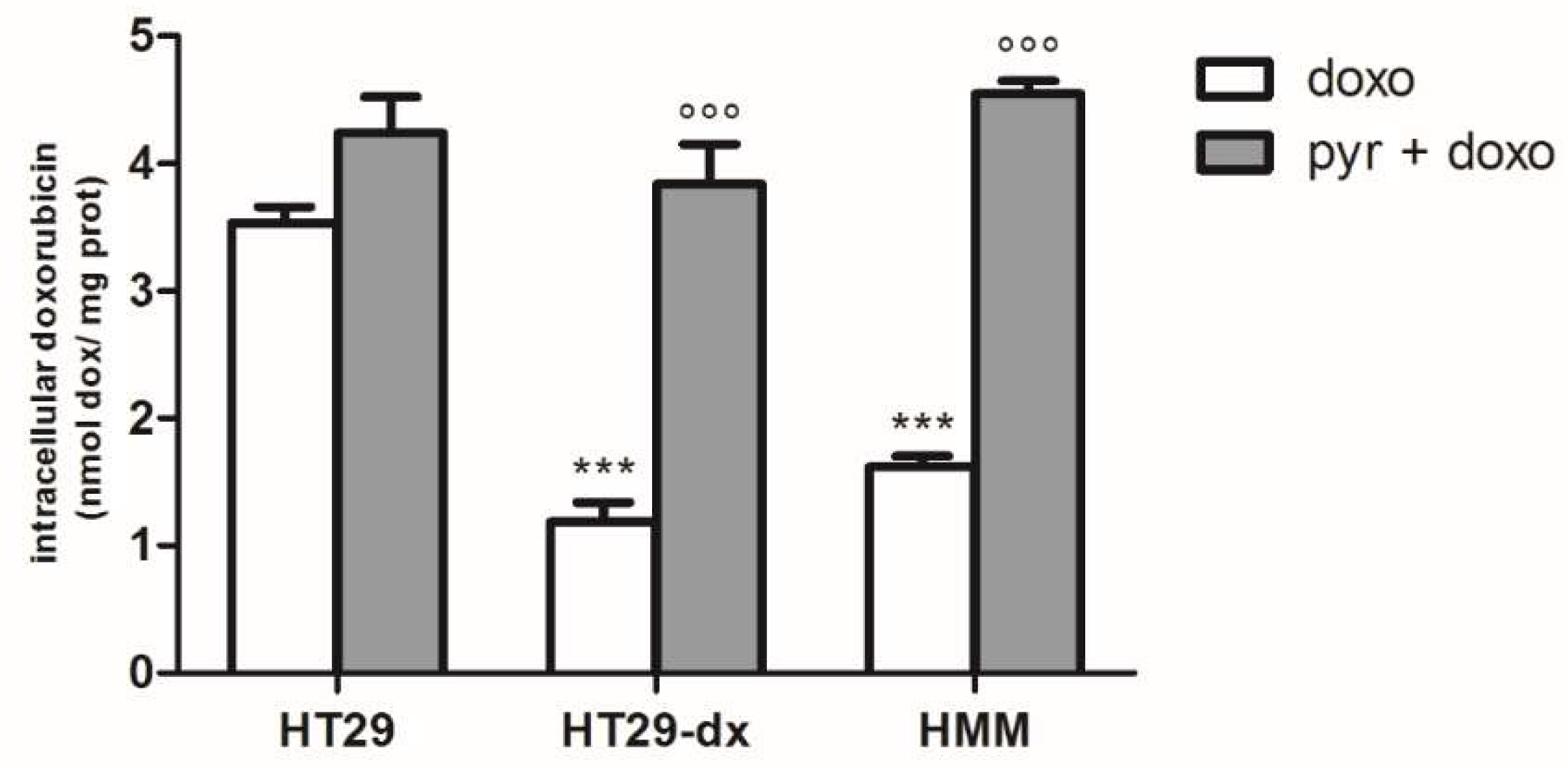
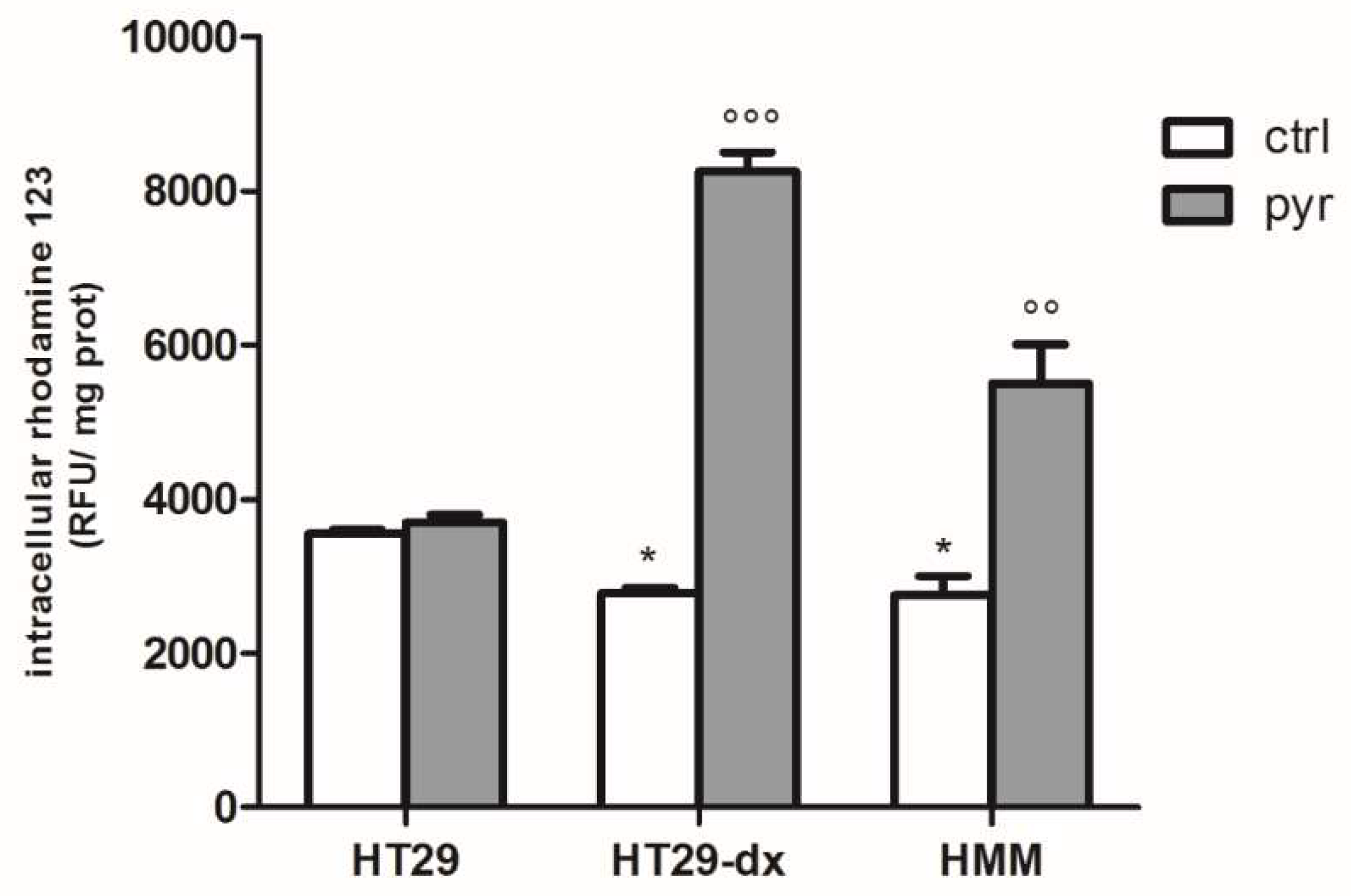
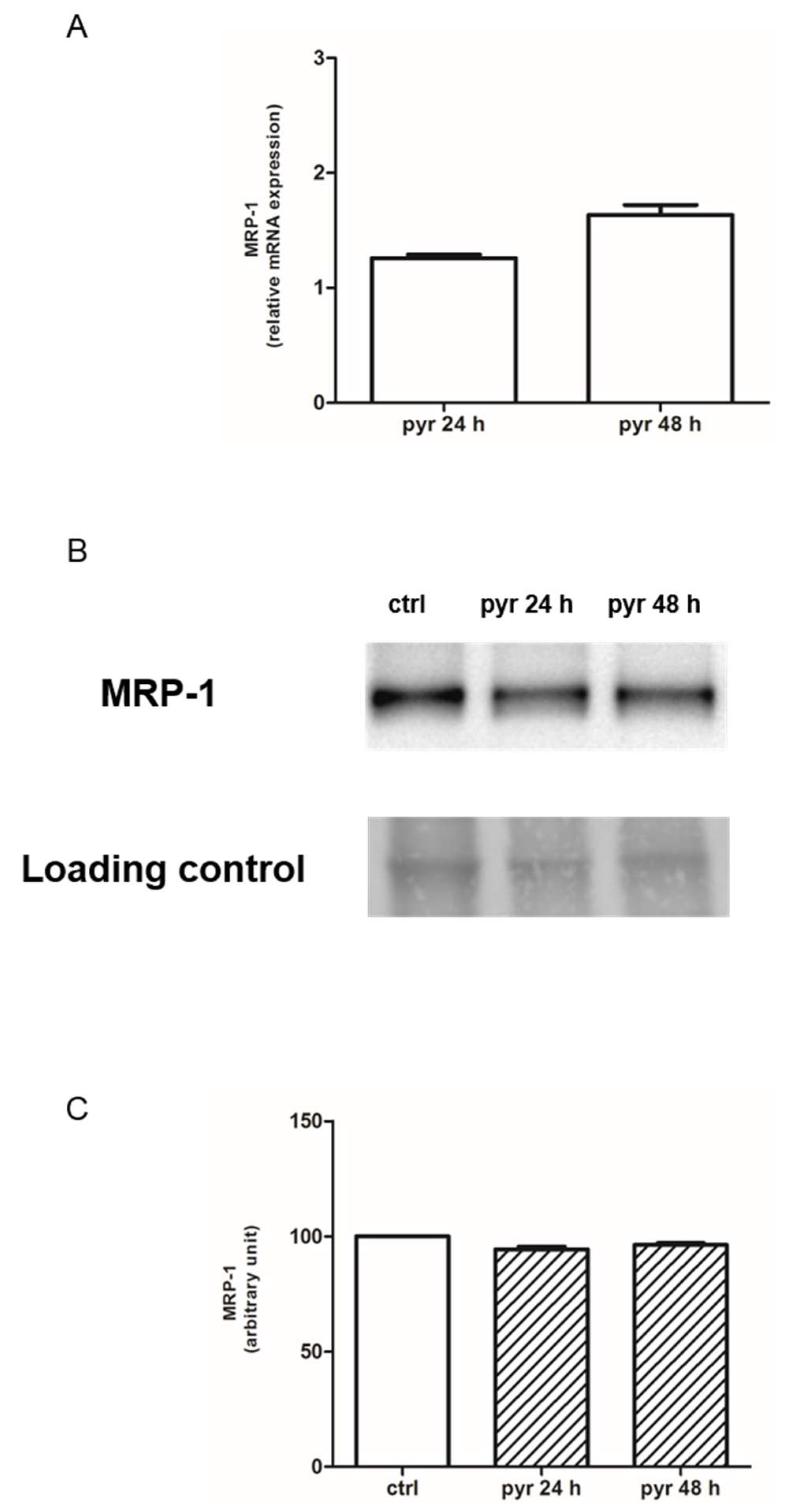
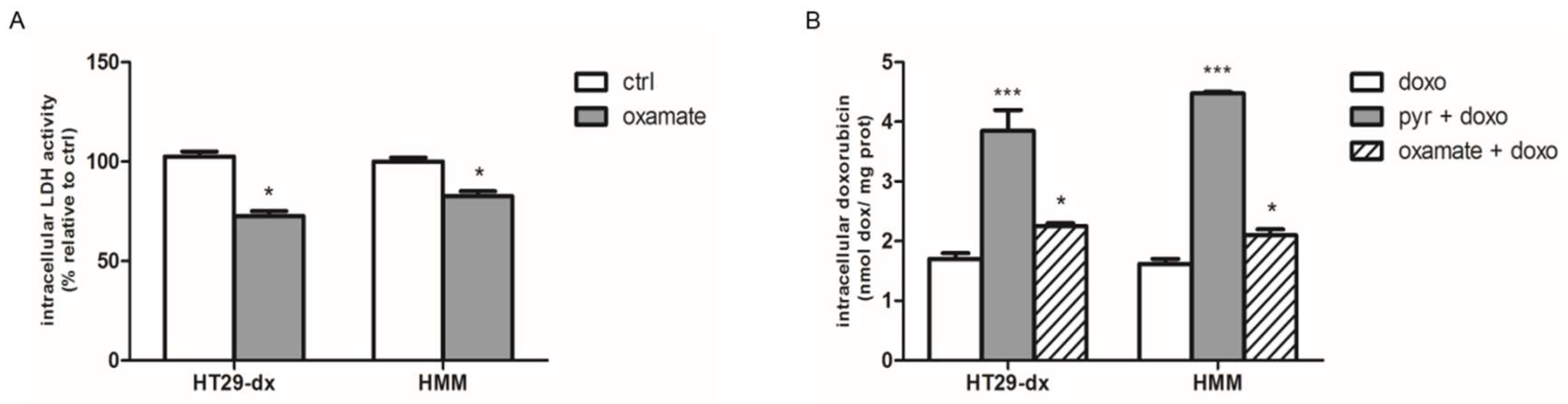
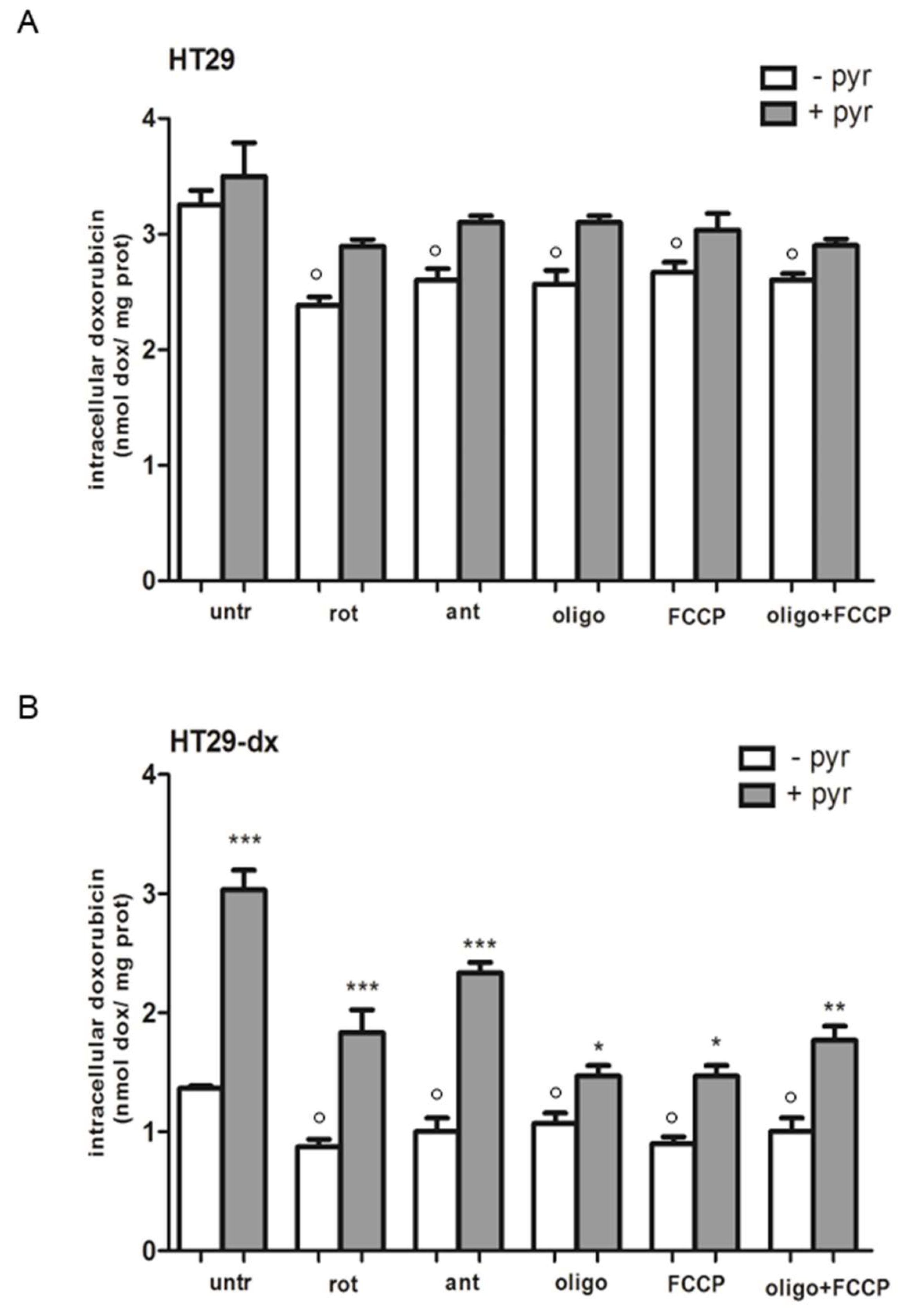
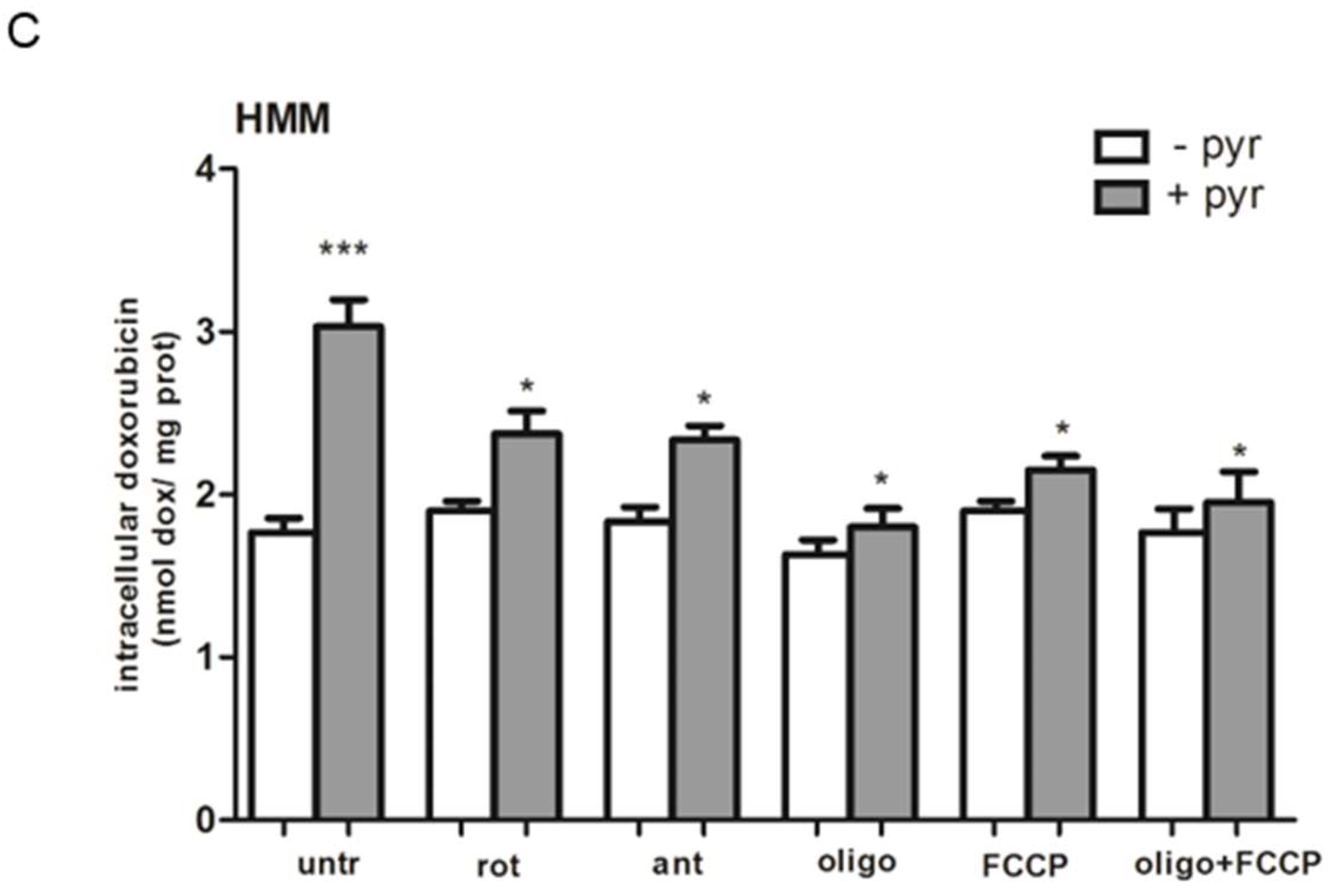
2.3. Pyruvate Restored the Sensibility to Doxorubicin in Both HT29-dx and HMM Cells, Modifying the Multidrug Resistance Protein 1 (MRP1) Activity
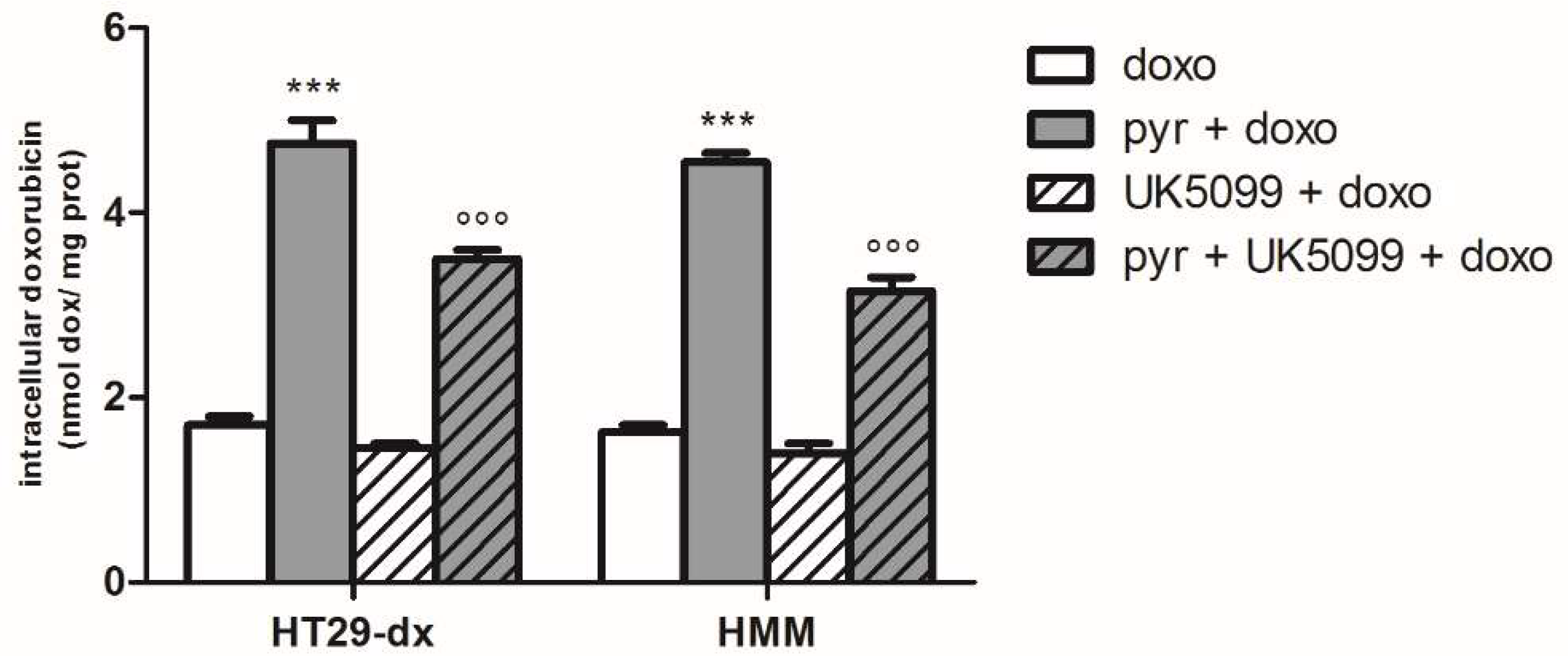
2.4. Sodium Oxamate and UK5099 Partially Concurred to the Effect of Pyruvate in Restore the Sensibility to Doxorubicin in HT29-dx and HMM
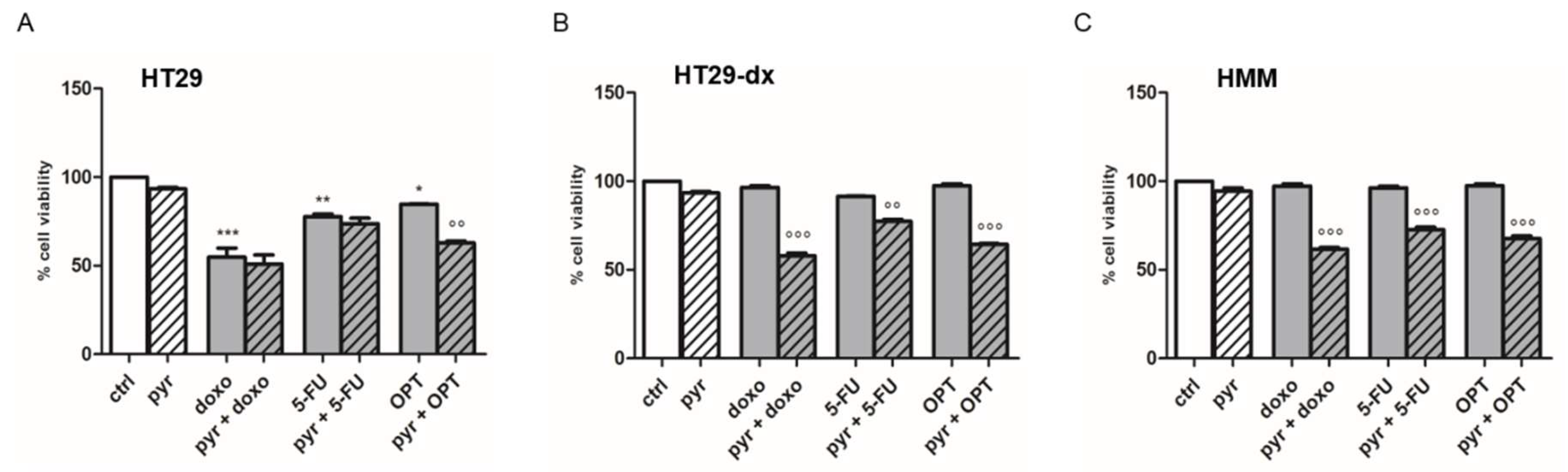
2.5. Pyruvate Restored Drugs Cytotoxicity in Both HT29-dx and HMM Cells
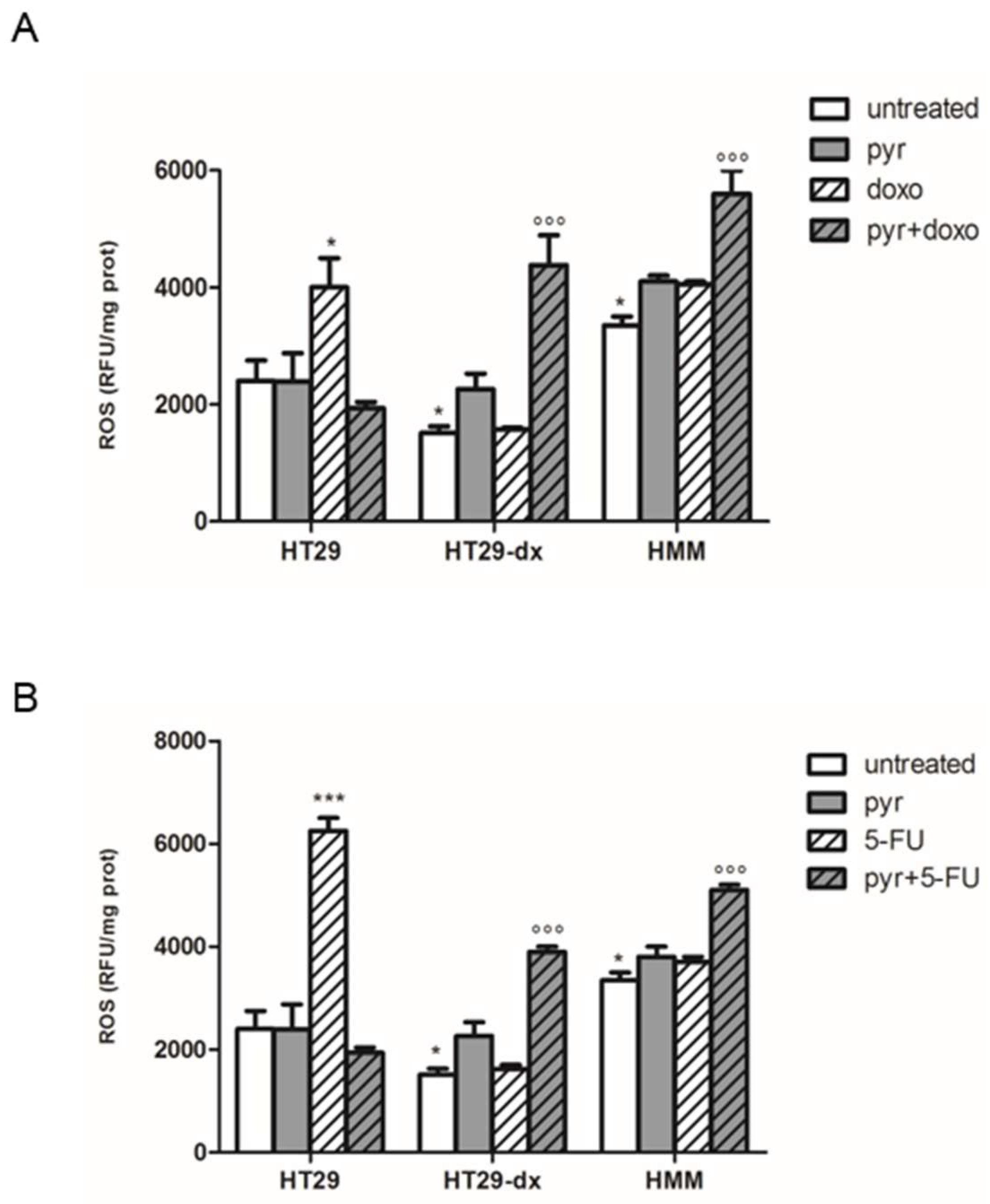
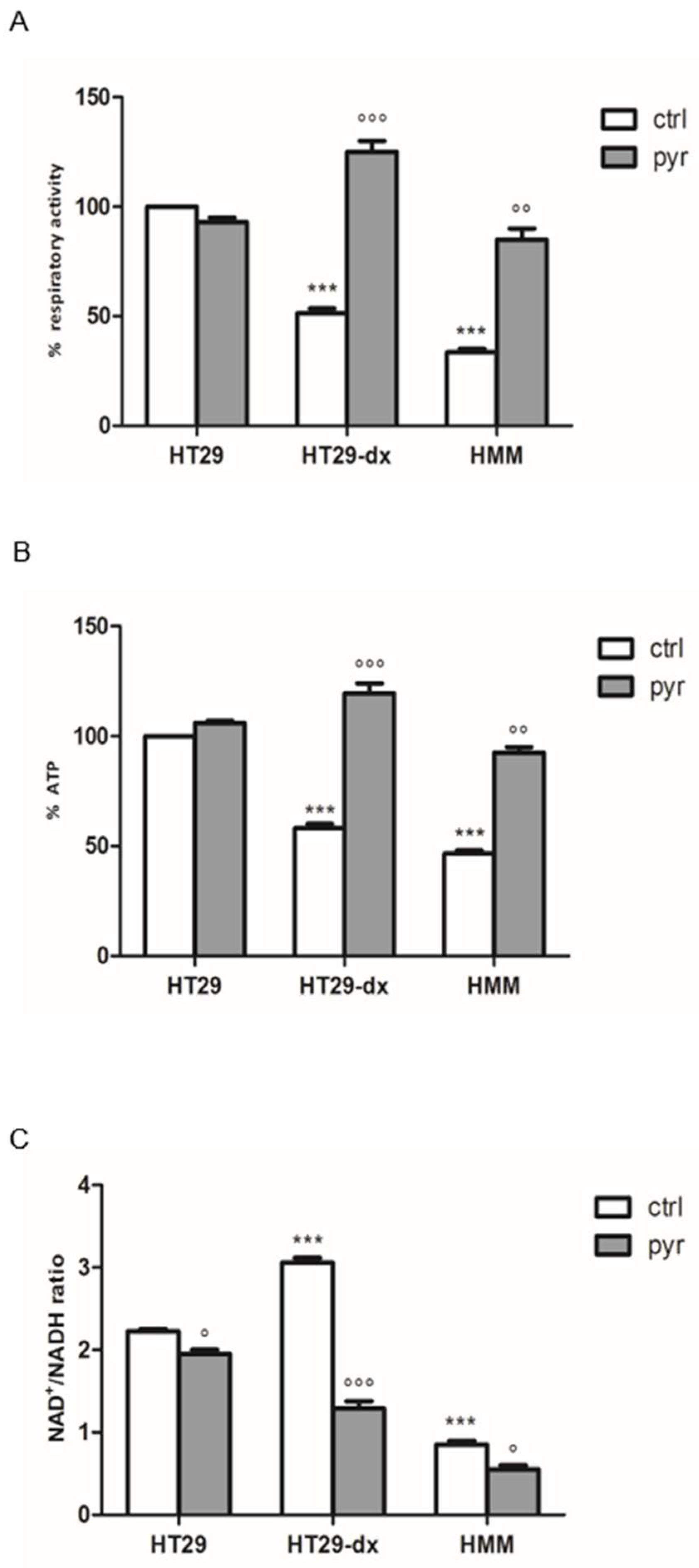
2.6. Pyruvate Restored Both Respiratory Activity and ATP Production in Both HT29-dx and HMM Cells
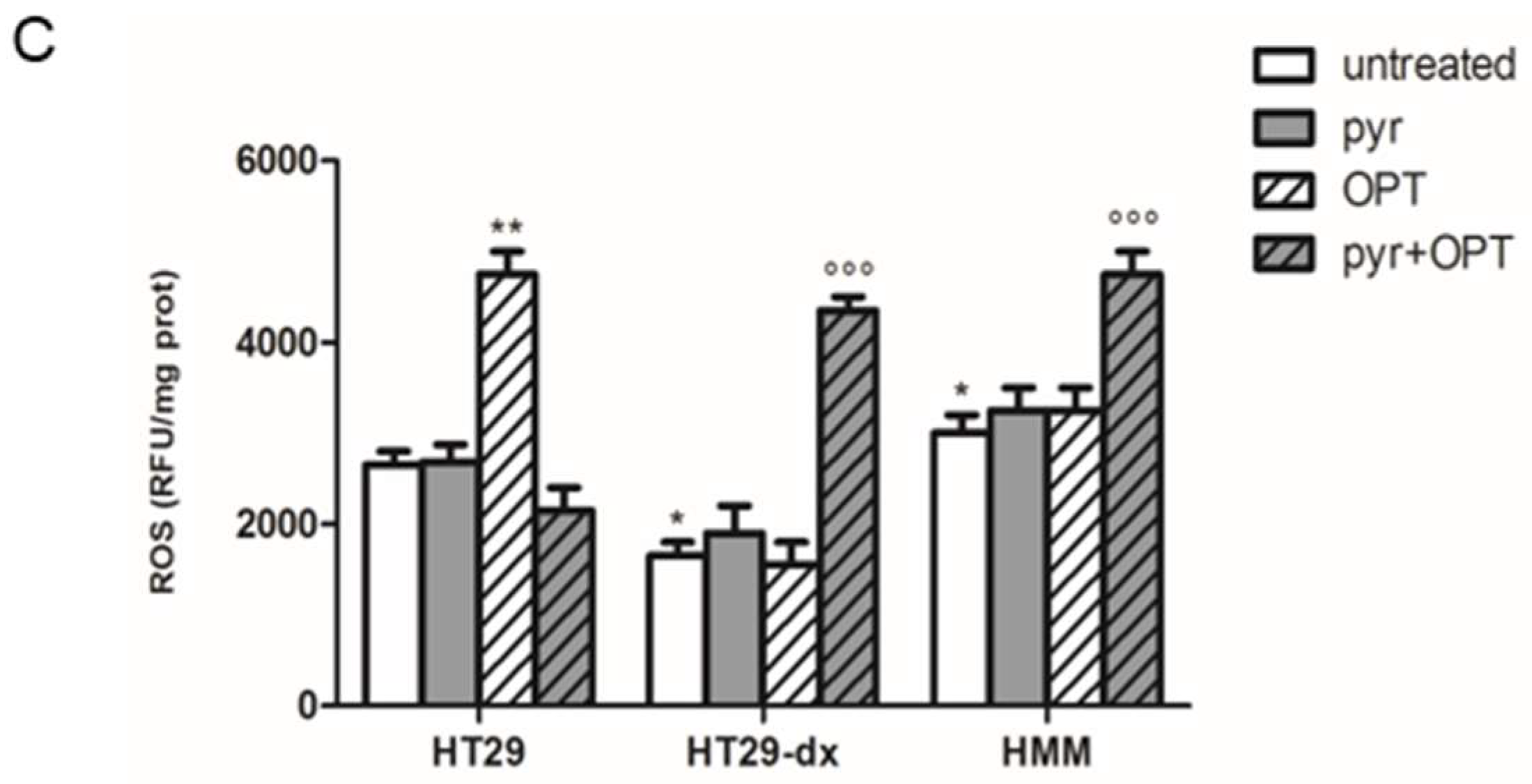
2.7. A Functional Mitochondrial Respiratory Chain Was Crucial for the Reversion of MDR Phenotype in HT29-dx and HMM Cells
3. Discussion
4. Material and Methods
4.1. Cells and Reagents
4.2. Metabolite Assays and 13C NMR Technique
4.3. Glycolysis and Gluconeogenesis Array
4.4. Quantitative Real-Time PCR (RT-PCR)
4.5. Measurement of Lactate Dehydrogenase LDH Activity
4.6. Doxorubicin Accumulation
4.7. Rhodamine 123 Accumulation
4.8. Western Blot Analysis
4.9. Cell Viability
4.10. Measurement of Total Cellular ROS Production
4.11. Isolation of Mitochondria and Measurement of Complex I–III Activity
4.12. ATP Assay
4.13. NAD+/NADH Assay
4.14. Measurement of Mitochondrial Pyruvate Concentration
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HT29 | human colon adenocarcinoma cell line |
| HT29-dx | human chemoresistant counterpart colon adenocarcinoma cell line |
| HMM | human malignant mesothelioma cell line |
| MDR | multidrug resistance |
| ROS | reactive oxygen species |
| MRP1 | multidrug resistance-associated protein 1 |
| PCK2 | phosphoenolpyruvate carboxykinase 2 |
| LDH | lactate dehydrogenase |
| FBP1 | fructose-1,6-bisphosphatase 1 |
| SLC2A4 | solute carrier family 2, member 4 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Fennell, D.; Giovannini, M.; Gaudino, G.; Mutti, L. Malignant pleural mesothelioma: Current treatments and emerging drugs. Expert Opin. Emerg. Drugs 2009, 14, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.M.; Schindler, M. Cell biological mechanisms of multidrug resistance in tumors. Proc. Natl. Acad. Sci. USA 1994, 91, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Doublier, S.; Aldieri, E.; Orecchia, S.; Betta, P.G.; Gazzano, E.; Ghigo, D.; Bosia, A. Asbestos induces doxorubicin resistance in MM98 mesothelioma cells via HIF-1α. Eur. Respir. J. 2008, 32, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet. Med. Off. J. Am. Coll. Med. Genet. 2008, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Zhang, J.-T. A new mechanism of drug resistance in breast cancer cells: Fatty acid synthase overexpression-mediated palmitate overproduction. Mol. Cancer Ther. 2008, 7, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation. Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar] [PubMed]
- Fujita, Y.; Ito, M.; Kojima, T.; Yatsuga, S.; Koga, Y.; Tanaka, M. GDF15 is a novel biomarker to evaluate efficacy of pyruvate therapy for mitochondrial diseases. Mitochondrion 2015, 20, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, G.; Corsetto, P.A.; Campia, I.; Montorfano, G.; Kopecka, J.; Castella, B.; Gazzano, E.; Ghigo, D.; Rizzo, A.M.; Riganti, C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol. Cancer 2013, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, X.; Yu, D.; Li, X.; Li, Y.; Long, Y.; Yuan, Y.; Ji, Z.; Zhang, M.; Wen, J.-G.; Nesland, J.M.; Suo, Z. Application of mitochondrial pyruvate carrier blocker UK5099 creates metabolic reprogram and greater stem-like properties in LnCap prostate cancer cells in vitro. Oncotarget 2015, 6, 37758–37769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, G.; Li, X.; Kan, Q.; Fan, Z.; Li, Y.; Ji, Y.; Zhao, J.; Zhang, M.; Grigalavicius, M.; et al. Mitochondrial pyruvate carrier function determines cell stemness and metabolic reprogramming in cancer cells. Oncotarget 2017, 8, 46363–46380. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 360, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.-C.; Cox, J.E.; Cardon, C.M.; van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Hill, B.G.; Benavides, G.A.; Dranka, B.P.; Darley-Usmar, V.M. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 2010, 428, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Verduzco, D.; Rauch, C.; Muddathir, A.K.; Adil, H.H.B.; Elhassan, G.O.; Ibrahim, M.E.; David Polo Orozco, J.; Cardone, R.A.; Reshkin, S.J.; et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 2014, 1, 777–802. [Google Scholar] [CrossRef] [PubMed]
- Duckwall, C.S.; Murphy, T.A.; Young, J.D. Mapping cancer cell metabolism with 13C flux analysis: Recent progress and future challenges. J. Carcinog. 2013, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, J.; Fan, T.W.M.; Hou, S.X. Glycolytic reprogramming through PCK2 regulates tumor initiation of prostate cancer cells. Oncotarget 2017, 8, 83602–83618. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.; Jansen, G.; Giovannetti, E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit. Rev. Oncol. Hematol. 2017, 114, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhao, Y.; Ding, Y.; Liu, H.; Liu, Z.; Fodstad, O.; Riker, A.I.; Kamarajugadda, S.; Lu, J.; Owen, L.B.; Ledoux, S.P.; Tan, M. Warburg effect in chemosensitivity: Targeting lactate dehydrogenase-A re-sensitizes Taxol-resistant cancer cells to Taxol. Mol. Cancer 2010, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.C.; Olson, K.A.; Jiang, L.; Hawkins, A.J.; van Vranken, J.G.; Xie, J.; Egnatchik, R.A.; Earl, E.G.; DeBerardinis, R.J.; Rutter, J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 2014, 56, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, M.; Qin, J.; Lin, S.-C.; Lee, H.-J.; Tsai, S.Y.; Tsai, M.-J. MPC1, a key gene in cancer metabolism, is regulated by COUPTFII in human prostate cancer. Oncotarget 2016, 7, 14673–14683. [Google Scholar] [CrossRef] [PubMed]
- Carlisi, D.; de Blasio, A.; Drago-Ferrante, R.; di Fiore, R.; Buttitta, G.; Morreale, M.; Scerri, C.; Vento, R.; Tesoriere, G. Parthenolide prevents resistance of MDA-MB231 cells to doxorubicin and mitoxantrone: The role of Nrf2. Cell Death Discov. 2017, 3, 17078. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lv, G.; Li, Y.; Li, E.; Li, H.; Zhou, Q.; Yang, B.; Cao, W. Enhancement of anti-tumor effects of 5-fluorouracil on hepatocellular carcinoma by low-intensity ultrasound. J. Exp. Clin. Cancer Res. 2016, 35, 71. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, B.; Yu, P.-W.; Tang, B.; Hao, Y.-X.; Lei, X.; Luo, H.-X.; Zeng, D.-Z. Autophagy protects against oxaliplatin-induced cell death via ER stress and ROS in Caco-2 cells. PLoS ONE 2012, 7, e51076. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Vander Heiden, M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015, 162, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Campia, I.; Kopecka, J.; Gazzano, E.; Orecchia, S.; Ghigo, D.; Riganti, C. Zoledronic acid overcomes chemoresistance and immunosuppression of malignant mesothelioma. Oncotarget 2015, 6, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Isobe, H.; Wellham, L.; Sauerteig, A.; Sridhar, K.S.; Ramachandran, C.; Krishan, A. Doxorubicin retention and chemoresistance in human mesothelioma cell lines. Int. J. Cancer 1994, 57, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Järvinen, K.; Kaarteenaho-Wiik, R.; Kinnula, V. The expression of P-glycoprotein and multidrug resistance proteins 1 and 2 (MRP1 and MRP2) in human malignant mesothelioma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12, 1239–1245. [Google Scholar] [CrossRef]
- Faiz, H.; Conjard-Duplany, A.; Boghossian, M.; Martin, G.; Baverel, G.; Ferrier, B. Cadmium chloride inhibits lactate gluconeogenesis in isolated human renal proximal tubules: A cellular metabolomic approach with 13C-NMR. Arch. Toxicol. 2011, 85, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- El Hage, M.; Ferrier, B.; Baverel, G.; Martin, G. Rat brain slices oxidize glucose at high rates: A 13C NMR study. Neurochem. Int. 2011, 59, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Knouzy, B.; Dubourg, L.; Baverel, G.; Michoudet, C. Ifosfamide metabolite chloroacetaldehyde inhibits cell proliferation and glucose metabolism without decreasing cellular ATP content in human breast cancer cells MCF-7. J. Appl. Toxicol. JAT 2010, 30, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Marbach, E.P.; Weil, M.H. Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin. Chem. 1967, 13, 314–325. [Google Scholar] [PubMed]
- Van den Bossche, D.; Schiettecatte, J.; Vekens, E.; de Smet, D.; Gorus, F.K.; Martens, G.A. Enzymatic pyruvate measurement by Cobas 6000 open channel assay. Clin. Lab. 2012, 58, 1091–1095. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungo, E.; Bergandi, L.; Salaroglio, I.C.; Doublier, S. Pyruvate Treatment Restores the Effectiveness of Chemotherapeutic Agents in Human Colon Adenocarcinoma and Pleural Mesothelioma Cells. Int. J. Mol. Sci. 2018, 19, 3550. https://doi.org/10.3390/ijms19113550
Mungo E, Bergandi L, Salaroglio IC, Doublier S. Pyruvate Treatment Restores the Effectiveness of Chemotherapeutic Agents in Human Colon Adenocarcinoma and Pleural Mesothelioma Cells. International Journal of Molecular Sciences. 2018; 19(11):3550. https://doi.org/10.3390/ijms19113550
Chicago/Turabian StyleMungo, Eleonora, Loredana Bergandi, Iris Chiara Salaroglio, and Sophie Doublier. 2018. "Pyruvate Treatment Restores the Effectiveness of Chemotherapeutic Agents in Human Colon Adenocarcinoma and Pleural Mesothelioma Cells" International Journal of Molecular Sciences 19, no. 11: 3550. https://doi.org/10.3390/ijms19113550
APA StyleMungo, E., Bergandi, L., Salaroglio, I. C., & Doublier, S. (2018). Pyruvate Treatment Restores the Effectiveness of Chemotherapeutic Agents in Human Colon Adenocarcinoma and Pleural Mesothelioma Cells. International Journal of Molecular Sciences, 19(11), 3550. https://doi.org/10.3390/ijms19113550