NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect
Abstract
1. Introduction
2. Results
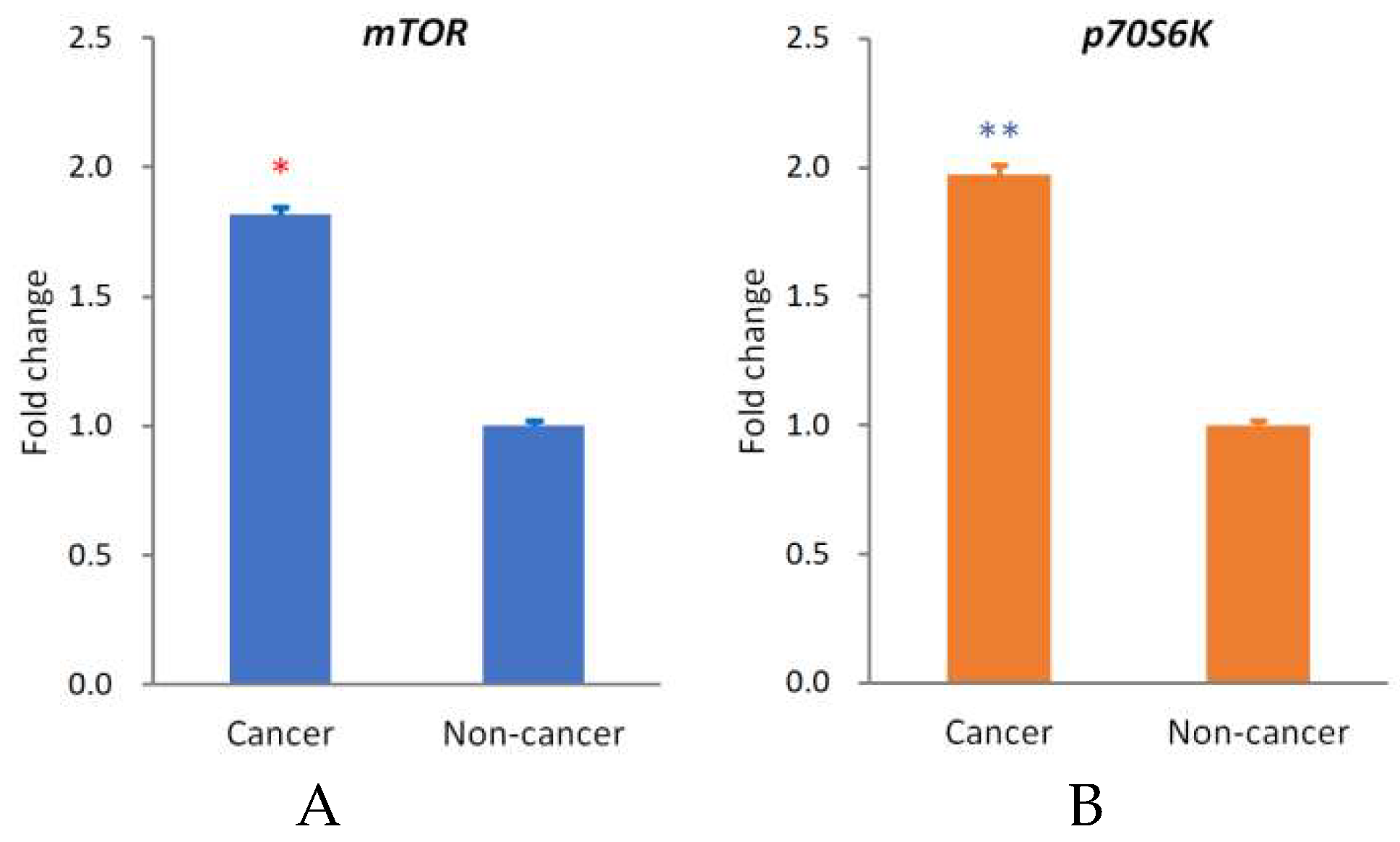
2.1. Analysis of mTOR Expression in OSCC Tissue Using Real-Time Quantitative Reverse Transcriptase—Polymerase Chain Reaction (qRT-PCR)
2.2. NVP-BEZ235 Inhibited Cell Proliferation and Downregulated the PI3K/AKT/mTOR-Signaling Pathway of OSCC Cells, Resulting in the Suppression of Phospho-p70S6K
2.3. NVP-BEZ235 Inhibited the Migratory and Invasion Abilities of SCC-4 and SCC-25 Cells
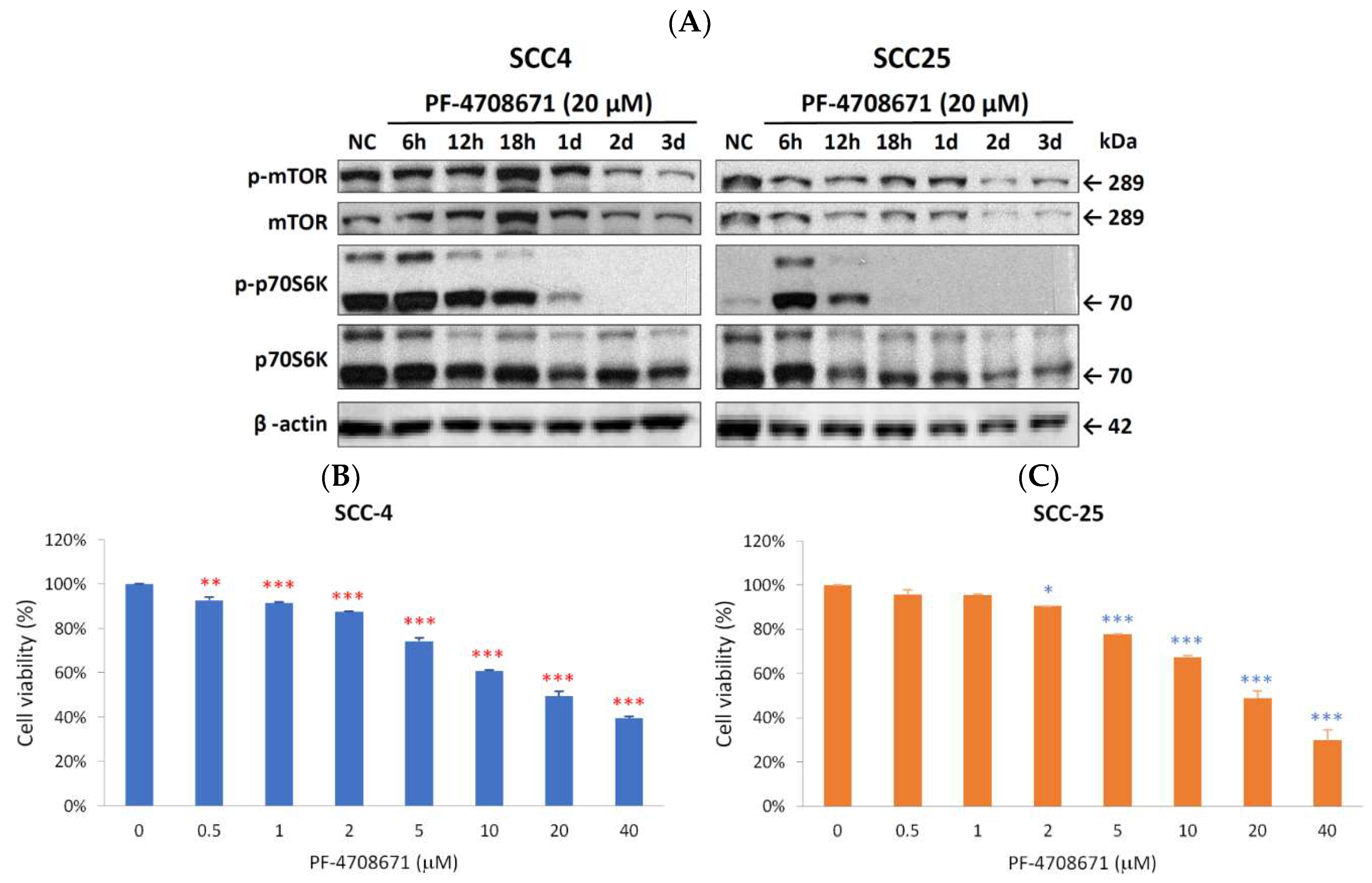
2.4. Phospho-p70S6K Inhibitor 2-((4-(5-Ethylpyrimidin-4-yl)piperazin-1-yl)methyl)-5-(trifluoromethyl)-1H-benzo[d]imidazole (PF-4708671) Suppressed Proliferation and Inhibited the Expression of Phospho-mTOR and Phospho-p70S6K in SCC-4 and SCC-25 Cells
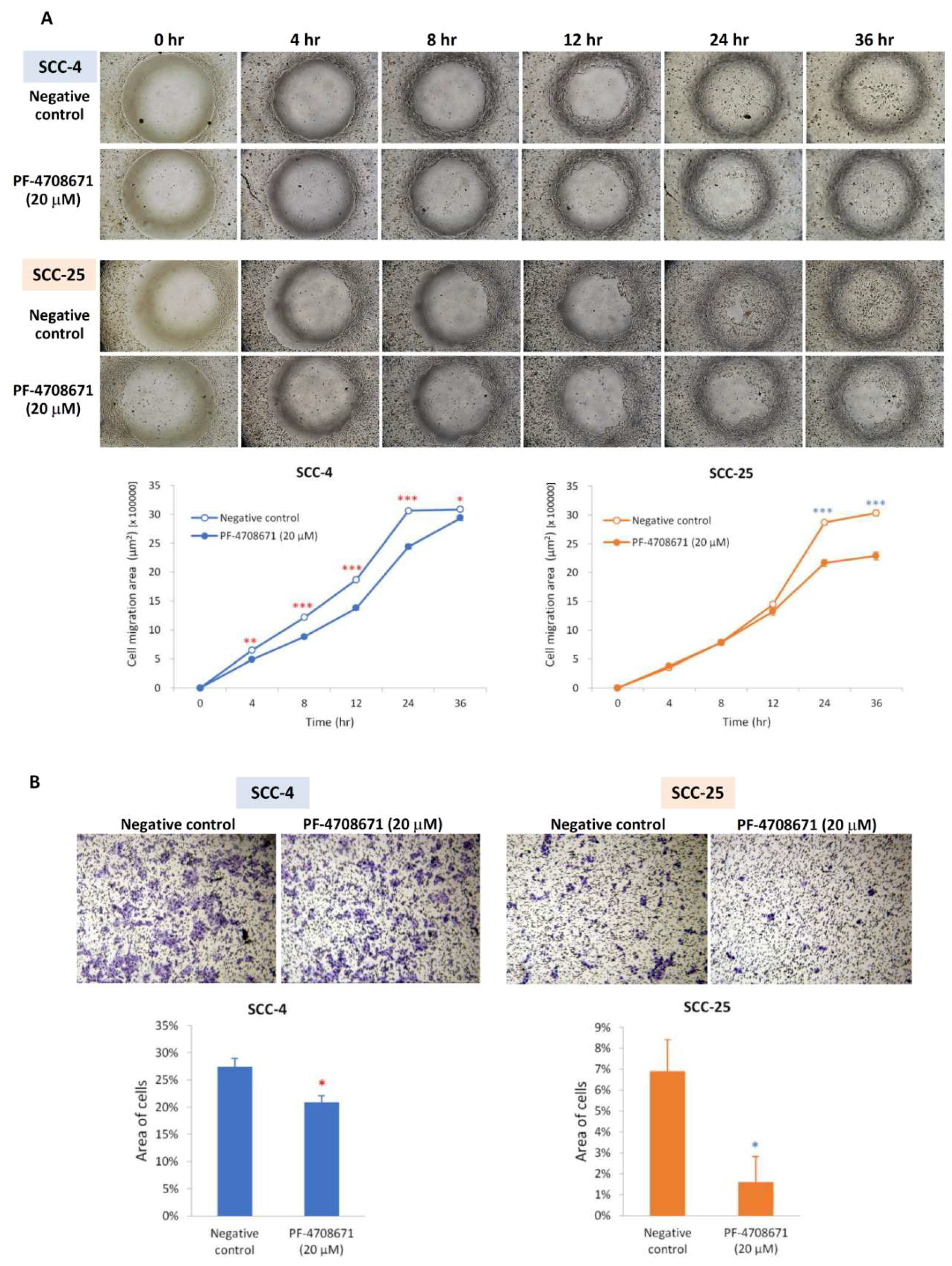
2.5. Phospho-p70S6K Inhibitor (PF-4708671) also Suppressed Migration and Invasion as an NVP-BEZ235 in SCC-4 and SCC-25 Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Real-time Quantitative Reverse-transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
4.3. Cell Culture
4.4. MTT Assay
4.5. Western Blotting
4.6. Wound-Healing Assay
4.7. Transwell Assays
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDDP | Cis-diamminedichloridoplatinum |
| cDNA | Complementary DNA |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| OSCC | Oral cavity squamous cell carcinoma |
| p70S6K | 70-kDa ribosomal S6 kinase |
| PBS | Phosphate-buffered saline |
| PI3K | Phosphoinositide 3-kinase |
| qRT-PCR | Real-time quantitative reverse transcriptase–polymerase chain reaction |
| S6K | S6 kinase proteins |
| SCCHN | Squamous cell carcinoma of the head and neck |
References
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.T.; Chen, H.N.; Wen, Y.W.; Lee, S.R.; Ng, S.H.; Liu, T.W.; Tsai, S.T.; Tsai, M.H.; Lin, J.C.; Lou, P.J.; et al. Association between the diagnosis-to-treatment interval and overall survival in Taiwanese patients with oral cavity squamous cell carcinoma. Eur. J. Cancer 2017, 72, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.T.; Lin, C.Y.; Fan, K.H.; Wang, H.M. The Optimal Treatment Modality for Taiwan Oral Cavity Cancer Patients-Experience of a Medical Center. J. Cancer Res. Pract. 2015, 2, 113–116. [Google Scholar]
- Tangthongkum, M.; Kirtsreesakul, V.; Supanimitjaroenporn, P.; Leelasawatsuk, P. Treatment outcome of advance staged oral cavity cancer: Concurrent chemoradiotherapy compared with primary surgery. Eur. Arch. Otorhinolaryngol. 2017, 274, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Denaro, N.; Russi, E.G.; Adamo, V.; Merlano, M.C. State-of-the-art and emerging treatment options in the management of head and neck cancer: News from 2013. Oncology 2014, 86, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Peyrade, F.; Krauss, J.; Mesía, R.; Remenar, E.; Gauler, T.C.; Keilholz, U.; Delord, J.P.; Schafhausen, P.; Erfán, J.; et al. Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: Results of the randomized phase I/II ADVANTAGE trial (phase II part). Ann. Oncol. 2014, 25, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, M.; Yang, A.; Feng, J.; Zhu, X.; Choi, Y.J.; Hu, G.; Pan, J.; Hu, C.; Luo, R.; et al. Platinum-based chemotherapy plus cetuximab first-line for Asian patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: Results of an open-label, single-arm, multicenter trial. Head Neck 2015, 37, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Sosa, A.E.; Grau, J.J.; Feliz, L.; Pereira, V.; Alcaraz, D.; Muñoz-García, C.; Caballero, M. Outcome of patients treated with palliative weekly paclitaxel plus cetuximab in recurrent head and neck cancer after failure of platinum-based therapy. Eur. Arch. Otorhinolaryngol. 2014, 271, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Péron, J.; Ceruse, P.; Lavergne, E.; Buiret, G.; Pham, B.N.; Chabaud, S.; Favier, B.; Girodet, D.; Zrounba, P.; Ramade, A.; et al. Paclitaxel and cetuximab combination efficiency after the failure of a platinum-based chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Anticancer Drugs 2012, 23, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Darr, O.A.; Peters, R.D.; Prince, M.E. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Front. Endocrinol. (Lausanne) 2012, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.G.; Lee, S.E.; Oh, M.M.; Lee, S.C.; Jeong, S.J.; Hong, S.K.; Yoon, C.Y.; Byun, S.S.; Park, H.S.; Cheon, J. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int. J. Oncol. 2014, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, A.; Gasparri, A.; Gallo-Stampino, C.; Toma, S.; Curnis, F.; Corti, A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin. Cancer Res. 2006, 12, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, A.; Xiao, C.; Chisholm, C.; Chen, W.; Xu, X.; Lahusen, T.J.; Bewley, C.; Deng, C.X. Synergistic therapeutic effect of cisplatin and phosphatidylinositol 3-kinase (PI3K) inhibitors in cancer growth and metastasis of Brca1 mutant tumors. J. Biol. Chem. 2014, 289, 24202–24214. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.R.; Mell, L.K.; Cohen, E.E. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015, 51, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gobin, B.; Battaglia, S.; Lanel, R.; Chesneau, J.; Amiaud, J.; Rédini, F.; Ory, B.; Heymann, D. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014, 344, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Herrera, V.A.; Zeindl-Eberhart, E.; Jung, A.; Huber, R.M.; Bergner, A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011, 31, 849–854. [Google Scholar] [PubMed]
- Xu, C.X.; Li, Y.; Yue, P.; Owonikoko, T.K.; Ramalingam, S.S.; Khuri, F.R.; Sun, S.Y. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS ONE 2011, 6, e20899. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Long, L.M.; Yang, N.; Zhang, Q.Q.; Ji, W.J.; Zhao, J.H.; Qin, Z.H.; Wang, Z.; Chen, G.; Liang, Z.Q. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol. Sin. 2013, 34, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Kuger, S.; Corek, E.; Polat, B.; Kammerer, U.; Flentje, M.; Djuzenova, C.S. Novel PI3K and mTOR Inhibitor NVP-BEZ235 Radiosensitizes Breast Cancer Cell Lines under Normoxic and Hypoxic Conditions. Breast Cancer (Auckl) 2014, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.; Kim, J.E.; Rewcastle, G.W.; Finlay, G.J.; Baguley, B.C. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol. Ther. 2011, 11, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Sznol, J.A.; Jilaveanu, L.B.; Kluger, H.M. Studies of NVP-BEZ235 in melanoma. Curr. Cancer Drug Targets 2013, 13, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Venkannagari, S.; Fiskus, W.; Peth, K.; Atadja, P.; Hidalgo, M.; Maitra, A.; Bhalla, K.N. Superior efficacy of co-treatment with dual PI3K/mTOR inhibitor NVP-BEZ235 and pan-histone deacetylase inhibitor against human pancreatic cancer. Oncotarget 2012, 3, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Yen, P.L.; Schwarz, M.A.; Schwarz, R.E. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J. Cell Biochem. 2012, 113, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.C.; Nicoletti, G.; Zambelli, D.; Ventura, S.; Guerzoni, C.; Landuzzi, L.; Lollini, P.L.; Maira, S.M.; García-Echeverría, C.; Mercuri, M.; et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin. Cancer Res. 2010, 16, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.B.; Lui, V.W.; Hui, C.W.; Lau, C.P.; Wong, C.H.; Hui, E.P.; Ng, M.H.; Cheng, S.H.; Tsao, S.W.; Tsang, C.M.; et al. Preclinical evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal cancer models. Cancer Lett. 2014, 343, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qian, X.J.; Qin, W.; Deng, R.; Wu, X.Q.; Qin, J.; Feng, G.K.; Zhu, X.F. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e59879. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Shimomura, M.; Kobayashi, K.; Kojima, S.; Nakatsura, T. Growth inhibition by NVP-BEZ235, a dual PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol. Rep. 2011, 26, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Shi, G.; Jin, J.; Guo, H.; Guo, X.; Luo, F.; Song, Y.; Jia, X. Dual PI3K/mTOR inhibitor NVP-BEZ235- induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int. J. Mol. Med. 2013, 31, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, M.M.; Boukouris, A.E.; Pothiraju, D.; Buitrago-Molina, L.E.; Marhenke, S.; Schütt, J.; Orlik, J.; Kühnel, F.; Hegermann, J.; Manns, M.P.; et al. Activity of the mTOR inhibitor RAD001, the dual mTOR and PI3-kinase inhibitor BEZ235 and the PI3-kinase inhibitor BKM120 in hepatocellular carcinoma. Liver Int. 2013, 33, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Yoon, S.O.; Kubota, K.; Mendoza, M.C.; Gygi, S.P.; Blenis, J. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J. Biol. Chem. 2009, 284, 14939–14948. [Google Scholar] [CrossRef] [PubMed]
- Berven, L.A.; Willard, F.S.; Crouch, M.F. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp. Cell Res. 2004, 296, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Moorthy, G.; Salkeni, M.A.; Karim, N.A.; Thomas, H.E.; Mercer, C.A.; Beg, M.S.; O’Gara, S.; Olowokure, O.; Fathallah, H.; et al. A phase Ib study of the dual PI3K/mTOR inhibitor Dactolisib (BEZ235) combined with Everolimus in patients with advanced solid malignancies. Target Oncol. 2017, 12, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Broek, R.V.; Mohan, S.; Eytan, D.F.; Chen, Z.; Van Waes, C. The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 2015, 21, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Sugimoto, N.; Yamamoto, N.; Shirai, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Igarashi, K.; Yachie, A.; et al. Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res. 2012, 32, 3643–3649. [Google Scholar] [PubMed]
- Tomioka, H.; Mukohara, T.; Kataoka, Y.; Ekyalongo, R.C.; Funakoshi, Y.; Imai, Y.; Kiyota, N.; Fujiwara, Y.; Minami, H. Inhibition of the mTOR/S6K signal is necessary to enhance fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Int. J. Oncol. 2012, 41, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Aslan, J.E.; Tormoen, G.W.; Loren, C.P.; Pang, J.; McCarty, O.J. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011, 118, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.I.; Molina, A.M.; Lakhman, Y.; Patil, S.; Woo, K.; DeLuca, J.; Lee, C.H.; Hsieh, J.J.; Feldman, D.R.; Motzer, R.J.; et al. A phase Ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist 2016, 21, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Pongas, G.; Fojo, T. BEZ235: When promising science meets clinical reality. Oncologist 2016, 21, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J. Transwell(®) invasion assays. Methods Mol. Biol. 2011, 769, 97–110. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Number of Patients |
|---|---|
| Sex | |
| Male | 27 |
| Female | 1 |
| Median age year (range) | 53.23 (31–75) |
| Staging 1 | |
| I | 6 |
| II | 6 |
| III | 7 |
| IV | 9 |
| Site | |
| Bucca | 7 |
| Gum | 6 |
| Palate | 1 |
| Tongue | 12 |
| Trigone | 2 |
| N stage 1 | |
| N0 | 20 |
| N1 | 7 |
| N2a | 0 |
| N2b | 1 |
| N2c | 0 |
| N3 | 0 |
| Survival | |
| Expired | 10 2 |
| Survived | 18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-M.; Lin, P.-M.; Lin, H.-C.; Tsai, Y.-T.; Tsai, M.-S.; Li, S.-H.; Wu, C.-Y.; Yang, Y.-H.; Lin, S.-F.; Yang, M.-Y. NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect. Int. J. Mol. Sci. 2018, 19, 3546. https://doi.org/10.3390/ijms19113546
Hsu C-M, Lin P-M, Lin H-C, Tsai Y-T, Tsai M-S, Li S-H, Wu C-Y, Yang Y-H, Lin S-F, Yang M-Y. NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect. International Journal of Molecular Sciences. 2018; 19(11):3546. https://doi.org/10.3390/ijms19113546
Chicago/Turabian StyleHsu, Cheng-Ming, Pai-Mei Lin, Hsin-Ching Lin, Yao-Te Tsai, Ming-Shao Tsai, Shau-Hsuan Li, Ching-Yuan Wu, Yao-Hsu Yang, Sheng-Fung Lin, and Ming-Yu Yang. 2018. "NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect" International Journal of Molecular Sciences 19, no. 11: 3546. https://doi.org/10.3390/ijms19113546
APA StyleHsu, C.-M., Lin, P.-M., Lin, H.-C., Tsai, Y.-T., Tsai, M.-S., Li, S.-H., Wu, C.-Y., Yang, Y.-H., Lin, S.-F., & Yang, M.-Y. (2018). NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect. International Journal of Molecular Sciences, 19(11), 3546. https://doi.org/10.3390/ijms19113546






