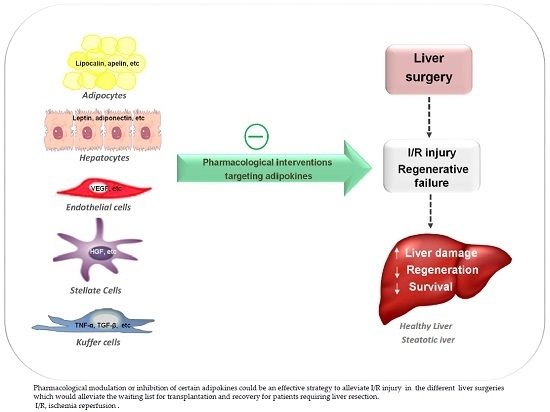
The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion
Abstract
1. Introduction
2. Partial Hepatic Resection
3. The Animal Models of PH
3.1. Gene-Specific Null Mutations (KnockoutModels)
3.2. Steatotic Livers
3.3. Relevance of Vascular Occlusion under Partial Hepatectomy
4. Animals Models of Partial Liver Transplantation
4.1. Gene-Specific Null Mutations (KnockoutModels)
4.2. Steatotic Livers
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LDLT | Living donor liver transplantation |
| I/R | Ischemia reperfusion |
| LT | Liver transplantation |
| SFS | Liver resection and small-for-size |
| SFSLT | Liver resection and small-for-size liver transplantation |
| LCN | Lipocalin |
| PH | Partial hepatectomy |
| KO | Knockout |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| EGF | Epidermal growth factor |
| Ang | Angiotensin |
| MM9-protein | Matrix metallopeptidase 9 |
| min | Minutes |
| HGF | Hepatocyte growth factor |
| Akt | Protein kinase B |
| ErK | Extracellular signal–regulated kinase |
| mRNA | Messenger ribonucleic acid |
| VEGF | Vascular endothelial growth factor |
| MSCs | Mesenquimal stem cells |
| TGF-β | Tumor growth factor-β |
| PDGF | Platelet-derived growth factor |
| HB-EGF | Heparin binding epidermal growth factor |
| FGF | Fibroblast growth factor |
| BMP-9 | Bone morphogenetic protein 9 |
| NO | Nitric oxide |
| IL-6 | Interleukin 6 |
| MCL-1 | Myeloid cell leukemia1 |
| Bcl-2 | B-cell lymphoma 2 |
| JAK | JAK kinase |
| CREB | cAMP response-element-binding |
| PAI-1 | Phosphoribosylanthranilateisomerase 1 |
| TNF-α | Tumor necrosis factor alpha |
| Gclm | Glutamate-cysteine ligase |
| mRNA | Messenger ribonucleic acid |
| DKL-1 | Delta like-1 homologue |
| IGF | Insulin growth factor |
| NGF | Nerve growth factor |
| HIF-2 α | Hypoxia-inducible factor alpha |
| NAD | Nicotinamide adenine dinucleotide |
References
- Mitchell, C.; Willenbring, H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 2008, 3, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Sander, F.; Miller, C.M. Live Donor Liver Transplantation. Liver Transplant. 2006, 12, 499–510. [Google Scholar]
- Jiménez-Castro, M.B.; Elias-Miró, M.; Peralta, C. Expanding the donor pool in liver transplantation Extended criteria donors. In Organ Donation and Organ Donor; Saidi, R.F., Ed.; Nova Science Publisher Inc.: New York, NY, USA, 2013; pp. 41–82. ISBN 978-1-626618. [Google Scholar]
- Pan, N.; Lv, X.; Liang, R.; Wang, L.; Liu, Q. Suppression of graft regeneration, not ischemia/reperfusion injury, is the primary cause of small-for-size syndrome after partial liver transplantation in mice. PLoS ONE 2014, 9, e93636. [Google Scholar] [CrossRef] [PubMed]
- Grąt, M.; Wronka, K.M.; Patkowski, W.; Stypułkowski, J.; Grąt, K.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Krawczyk, M. Effects of donor age and cold ischemia on liver transplantation outcomes according to the severity of recipient status. Dig. Dis. Sci. 2016, 61, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Massip-Salcedo, M.; Roselló-Catafau, J.; Prieto, J.; Avíla, M.A.; Peralta, C. The response of the hepatocyte to ischemia. Liver Int. 2007, 27, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Zhang, W.G.; Jiang, G.X.; Zhao, J.Y.; Li, H.; Wang, D.Z.; Cui, Y.F. Ischemia/Reperfusion in clamped lobes facilitates liver regeneration of non-clamped lobes after selective portal vein ligation. Dig. Dis. Sci. 2012, 57, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Casillas-Ramírez, A.; Peralta, C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: A 2015 update. Clin. Sci. 2015, 129, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J.; Büchler, C. Mechanisms of disease: Adipocytokines and visceral adipose tissue—Emerging role in nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shook, B.; Rivera Gonzalez, G.; Ebmeier, S.; Grisotti, G.; Zwick, R.; Horsley, V. The role of adipocytes in tissue regeneration and stem cell niches. Annu. Rev. Cell Dev. Biol. 2016, 32, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Bertolani, C. Adipokines in liver diseases. Hepatology 2009, 50, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the proinflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Halabis, M.; Dziedzic, M.; Warchulinska, J.; Kaznowska-Bystryk, I.; Solski, J. Omentin—A new adipokine with many roles to play. Curr. Issues Pharm. Med. Sci. 2015, 28, 176–180. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Mellouk, N.; Froment, P.; Dupont, J. Adiponectin and resistin: Potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction 2017, 153, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Muto, G. TGF-β function in immune suppression. Curr. Top. Microbiol. Immunol. 2011, 350, 127–147. [Google Scholar] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Smekal, A.; Vaclavik, J. Adipokines and cardiovascular disease: A comprehensive review. Biomed. Pap. 2017, 161, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Kong, J.; Chen, W.; Wang, Y. The role of the apelin/APJ system in the regulation of liver disease. Front. Pharmacol. 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Haberl, E.M.; Rein-Fischboeck, L.; Aslanidis, C. Adipokines in liver cirrhosis. Int. J. Mol. Sci. 2017, 18, e1392. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Feng, D.; Wu, H.; Wang, H.; Chan, Y.; Kolls, J.; Borregaard, N.; Porse, B.; Berger, T.; Mak, T.W.; et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: A critical role for IL-6/STAT3. Hepatology 2015, 61, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kienzl-Wagner, K.; Moschen, A.R.; Geiger, S.; Bichler, A.; Aigner, F.; Brandacher, G.; Pratschke, J.; Tilg, H. The role of lipocalin-2 in liver regeneration. Liver Int. 2015, 35, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Elias-Miró, M.; Massip-Salcedo, M.; Raila, J.; Schweigert, F.; Mendes-Braz, M.; Ramalho, F.; Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Bermudo, R.; Rimola, A.; et al. Retinol binding protein 4 and retinol in steatotic and nonsteatotic rat livers in the setting of partial hepatectomy under ischemia/reperfusion. Liver Transplant. 2012, 18, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Peng, W.; Xiao, S.; Li, H.; Jin, J.; Qin, L.; Dong, Y.; Su, Q. Role of serum vaspin in progression of type 2 diabetes: A 2-year cohort study. PLoS ONE 2014, 9, e94763. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Yilmaz, Y.; Eren, F.; Yonal, O.; Kurt, R.; Alahdab, Y.O.; Celikel, C.A.; Ozdogan, O.; Imeryuz, N.; Kalayci, C.; et al. Serum levels of vaspin, obestatin, and apelin-36 in patients with nonalcoholic fatty liver disease. Metabolism 2011, 60, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; de Luis, D.A.; Izaola, O.; Sagrado, M.G.; Conde, R.; Velasco, M.C.; Alvarez, T.; Pacheco, D.; González, J.M. Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease. Dig. Dis. Sci. 2009, 54, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.S.; Lin, W.H.; Lai, S.L.; Lin, H.Y.; Hsu, W.M.; Chou, C.H.; Lee, P.H. Interleukin-6 mediates angiotensinogen gene expression during liver regeneration. PLoS ONE 2013, 8, e67868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Asahina, K.; Wang, J.; Ueno, A.; Lazaro, R.; Miyaoka, Y.; Tsukamoto, H. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J. Biol. Chem. 2012, 287, 10355–10367. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Khai, N.C.; Wang, Y.; Irie, R.; Takamatsu, H.; Matsufuji, H.; Kosai, K.I. Heparin-binding epidermal growth factor-like growth factor and hepatocyte growth factor inhibit cholestatic liver injury in mice through different mechanisms. Int. J. Mol. Med. 2016, 38, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nakamura, T. HGF-MET cascade, a key target for inhibiting cancer metastasis: The impact of NK4 discovery on cancer biology and therapeutics. Int. J. Mol. Sci. 2013, 14, 888–919. [Google Scholar] [CrossRef] [PubMed]
- Delafontaine, P.; Song, Y.; Li, Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Amoras, S.; Gomes, S.T.; Freitas, F.B.; Santana, B.B.; Ishak, G.; de Araújo, M.T.; Demachki, S.; da Silva, S.R.; de Oliveira, I.M.; Ishak, R.; et al. NGF and P75NTR gene expression is associated with the hepatic fibrosis stage due to viral and non-viral causes. PLoS ONE 2015, 10, e0121754. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.; LeCouter, J. The biology of VEGF and its receptors. Nat. Rev. Gastroenterol. Hepatol. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Elias-Miro, M.; Massip-Salcedo, M.; Jimenez-Castro, M.; Peralta, C. Does adiponectin benefit steatotic liver transplantation? Liver Transplant. 2011, 17, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of liver. I. Restoration of liver of white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. Hepatology 2006, 43, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Miyake, Y.; Umeda, Y.; Matsushita, H.; Matsuda, H.; Takaki, A.; Sadamori, H.; Nouso, K.; Yagi, T.; Fujiwara, T.; et al. Serial changes of serum growth factor levels and liver regeneration after partial hepatectomy in healthy humans. Int. J. Mol. Sci. 2013, 14, 20877–20889. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G. Liver regeneration after partial hepatectomy. Am. J. Pathol. 2010, 176, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Massip-Salcedo, M.; Zaouali, M.A.; Padrissa-Altés, S.; Casillas-Ramirez, A.; Rodés, J.; Roselló-Catafau, J.; Peralta, C. Activation of peroxisome proliferator-activated receptor-α inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia-reperfusion. Hepatology 2008, 47, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramírez, A.; Mosbah, I.; Ramalho, F.; Roselló-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramírez, A.; Zaouali, A.; Padrissa-Altés, S.; Ben Mosbah, I.; Pertosa, A.; Alfany-Fernández, I.; Bintanel-Morcillo, M.; Xaus, C.; Rimola, A.; Rodés, J.; et al. Insulin-Like growth factor and epidermal growth factor treatment: New approaches to protecting steatotic livers against ischemia-reperfusion injury. Endocrinology 2009, 150, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramírez, A.; Alfany-Fernández, I.; Massip-Salcedo, M.; Juan, M.E.; Planas, J.M.; Serafín, A.; Pallàs, M.; Rimola, A.; Rodés, J.; Peralta, C. Retinol-Binding protein 4 and peroxisome proliferator-activated receptor-gamma in steatotic liver transplantation. J. Pharmacol. Exp. Ther. 2011, 338, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Mendes-Braz, M.; Massip-Salcedo, M.; Gracia-Sancho, J.; Elias-Miró, M.; Rodés, J.; Peralta, C. Adiponectin and resistin protect steatotic livers undergoing transplantation. J. Hepatol. 2013, 59, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Veldt, B.J.; Poterucha, J.J.; Watt, K.D.; Wiesner, R.H.; Hay, J.E.; Rosen, C.B.; Heimbach, J.K.; Janssen, H.L.; Charlton, M.R. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am. J. Transpl. 2009, 9, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.X.; Fan, J.G. Editorial: Fatty liver disease: A growing public health problem worldwide. J. Dig. Dis. 2011, 12, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fingas, C.; Beste, M.; Penndorf, V.; Sydor, S.; Nadalin, S.; Bechmann, L.; Paul, A.; Gerken, G.; Canbay, A.; Jochum, C. Liver regeneration-related cytokine profiles in donors and recipients before and after living-donor liver transplant. Exp. Clin. Transplant. 2018. [Google Scholar] [CrossRef]
- Stoot, J.; Coelen, R.S.; Vugt, J.; Dejong, C. General Introduction. In Hepatic Surgery; Abdeldayem, H., Allam, N., Eds.; InTech: London, UK, 2013; pp. 1–40. ISBN 978-953-51-0016-4. [Google Scholar]
- Saidi, R.F. Utilization of expanded criteria donors in liver transplantation. Int. J. Organ Transplant. Med. 2013, 4, 46–59. [Google Scholar] [PubMed]
- Kholodenko, I.V.; Yarygin, K.N. Cellular mechanisms of liver regeneration and cell-based therapies of liver diseases. BioMed Res. Int. 2017, 2017, 8910821. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tamai, M.; Tagawa, Y. Nitric oxide is critical for avoiding hepatic lipid overloading via IL-6 induction during liver regeneration after partial hepatectomy in mice. Exp. Anim. 2017, 66, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lanton, T.; Shriki, A.; Nechemia-Arbely, Y.; Abramovitch, R.; Levkovitch, O.; Adar, R.; Rosenberg, N.; Paldor, M.; Goldenberg, D.; Sonnenblick, A.; et al. Interleukin 6-dependent genomic instability heralds accelerated carcinogenesis following liver regeneration on a background of chronic hepatitis. Hepatology 2017, 65, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.G.; Studer, P.; Skroch, M.; Mahiou, J.; Minussi, D.C.; Peterson, C.R.; Wilson, S.W.; Patel, V.I.; Ma, A.; Csizmadia, E.; et al. A20 promotes liver regeneration by decreasing SOCS3 expression to enhance IL6/STAT3 proliferative signals. Hepatology 2013, 57, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Humar, B.; Gupta, A.; Maurizio, E.; Borgeaud, N.; Graf, R.; Clavien, P.A.; Tian, Y. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J. Pineal Res. 2018, 65, e12486. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.; Lai, S.; Chen, C.; Lee, P.; Peng, F.; Kuo, M.; Lai, H.S. IL-6 regulates Mcl-1L expression through the JAK/PI3K/Akt/CREB signaling pathway in hepatocytes: Implication of an anti-apoptotic role during liver regeneration. PLoS ONE 2013, 8, e66268. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Ma, S.F.; Qu, J.F.; Tian, D.H. Effects of Kupffer cell inactivation on graft survival and liver regeneration after partial liver transplantation in rats. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 56–62. [Google Scholar] [CrossRef]
- Beier, J.; Guo, L.; Ritzenthaler, J.; Joshi-Barve, S.; Roman, J.; Arteel, G. Fibrin-Mediated integrin signaling plays a critical role in hepatic regeneration after partial hepatectomy in mice. Ann. Hepatol. 2016, 15, 762–772. [Google Scholar] [PubMed]
- Elias-Miró, M.; Mendes-Braz, M.; Cereijo, R.; Villarroya, F.; Jiménez-Castro, M.; Gracia-Sancho, J.; Guixé-Muntet, S.; Massip-Salcedo, M.; Domingo, J.C.; Bermudo, R.; et al. Resistin and visfatin in steatotic and non-steatotic livers in the setting of partial hepatectomy under ischemia-reperfusion. J. Hepatol. 2014, 60, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Jeong, E.; Lee, K.; Seo, J.; Lee, W.; Choi, Y. Knockout of krupeppel-like factor 10 suppresses hepatic cell proliferation in a partially hepatectomized mouse model. Oncol. Lett. 2017, 13, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, H.; Hayashi, H.; Nakagawa, S.; Sakamoto, K.; Higashi, T.; Nitta, H.; Hashimoto, D.; Chikamoto, A.; Beppu, T.; Baba, H. Effect of LSKL peptide on thrombospondin 1-mediated transforming growth factor β signal activation and liver regeneration after hepatectomy in an experimental model. Br. J. Surg. 2015, 102, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf-Heinlein, K.; Meyer, C.; König, C.; Gaitantzi, H.; Addante, A.; Thomas, M.; Wiercinska, E.; Cai, C.; Li, Q.; Wan, F.; et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 2017, 66, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L.; Zhang, Y.; Sun, C.; Chen, X.; Wang, Y. Adipose-derived mesenchymal stem cells promote liver regeneration and suppress rejection in small-for-size liver allograft. Transpl. Immunol. 2017, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McMahan, R.; Riehle, K.J.; Fausto, N.; Campbell, J. A disintegrin and metalloproteinase 17 regulates TNF and TNFR1 levels in inflammation and liver regeneration in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Riehle, K.; Haque, J.; McMahan, R.; Kavanagh, T.; Fausto, N.; Campbell, J. Sustained glutathione deficiency interferes with the liver response to TNF-α and liver regeneration after partial hepatectomy in mice. J. Liver Dis. Transplant. 2013, 1, 1000105. [Google Scholar] [CrossRef]
- Qi, Q.A.; Yang, Z.Y.; Ma, K.S.; Lu, Q.; Wang, S.G.; Li, X.W.; Xia, F.; Liu, W.; Bie, P. Impact of cold ischemia on cytokines after partial liver transplantation in rats. Genet. Mol. Res. 2013, 12, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Correnti, J.; Cook, D.; Aksamitiene, E.; Swarup, A.; Ogunnaike, B.; Vadigepalli, R.; Hoek, J.B. Adiponectin fine-tuning of liver regeneration dynamics revealed through cellular network modelling. J. Physiol. 2015, 593, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Yoshiya, S.; Shirabe, K.; Imai, D.; Toshima, T.; Yamashita, Y.I.; Ikegami, T.; Okano, S.; Yoshizumi, T.; Kawanaka, H.; Maehara, Y. Blockade of the apelin–APJ system promotes mouse liver regeneration by activating Kupffer cells after partial hepatectomy. J. Gastroenterol. 2015, 50, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Cilekar, M.; Uysal, O.; Bal, C.; Turel, S.; Yılmaz, S. Leptin increases mitotic index and regeneration ratio in hepatectomized rats. Med. Sci. Monit. Basic Res. 2013, 19, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ng, K.T.; Xu, A.; Li, C.X.; Liu, X.B.; Guo, D.Y.; Poon, R.T.; Fan, S.T.; Lo, C.M.; Man, K. The roles of lipocalin-2 in small-for-size fatty liver graft injury. Ann. Surg. 2014, 260, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Wu, Y.; Lai, S.; Lin, W. Lipocalin-2 gene expression during liver regeneration after partial hepatectomy in rats. Int. J. Surg. 2013, 11, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Ager, E.; Costa, P.; Malcontenti-Wilson, C.; Muralidharan, V.; Christophi, C. Blockade of the renin-angiotensin system inhibits growth of colorectal cancer liver metastases in the regenerating liver. Clin. Exp. Metastasis 2014, 31, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Ager, E.; Malcontenti-Wilson, C.; Muralidharan, V.; Christophi, C. Blockade of the renin-angiotensin system improves the early stages of liver regeneration and liver function. J. Surg. Res. 2013, 179, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Arteaga, M.; Chitsike, L.; Wang, F.; Viswakarma, N.; Breslin, P.; Qiu, W. FAK deletion accelerates liver regeneration after two-thirds partial hepatectomy. Sci. Rep. 2016, 6, 34316. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.; de Oliveira, A.; Tobar, N.; Saad, M.; Moreira, L.; Reis, E.; Nicola, E.M.; de Jorge, G.L.; dos Tártaro, R.R.; Boin, I.F.; et al. Liver regeneration following partial hepatectomy is improved by enhancing the HGF/Met axis and Akt and Erk pathways after low-power laser irradiation in rats. Lasers Med. Sci. 2013, 28, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Chen, J.; Li, X.; Wang, L.; Wang, B.; Wang, B.; Peng, W.; Yang, C.; Li, Z.; et al. Pericentral hepatocytes produce insulin-like growth factor-2 to promote liver regeneration during selected injuries in mice. Hepatology 2017, 66, 2002–2015. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Linecker, M.; Limani, P.; Schlegel, A.; Kambakamba, P.; Lehn, J.M.; Nicolau, C.; Graf, R.; Humar, B.; Clavien, P.A. Hypoxia-driven Hif2a coordinates mouse liver regeneration by coupling parenchymal growth to vascular expansion. Hepatology 2016, 64, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Sowa, J.; Paul, A.; Gerken, G.; Schlaak, J. Vascular endothelial growth factor improves liver regeneration and survival after 90% hepatectomy in a rat model of diet-induced steatosis. Digestion 2013, 88, 235–242. [Google Scholar] [CrossRef] [PubMed]
- DeLeve, L.; Wang, X.; Wang, L. VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Taira, Z.; Ueda, Y.; Monmasu, H.; Yamase, D.; Miyake, S.; Shiraishi, M. Characteristics of intracellular Ca2+ signals consisting of two successive peaks in hepatocytes during liver regeneration after 70% partial hepatectomy in rats. J. Exp. Pharmacol. 2016, 8, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Adas, G.; Koc, B.; Adas, M.; Duruksu, G.; Subasi, C.; Kemik, O.; Sakiz, D.; Kalayci, M.; Purisa, S.; Unal, S.; et al. Effects of mesenchymal stem cells and VEGF on liver regeneration following major resection. Langenbeck’s Arch. Surg. 2016, 401, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C.; Dahrenmoller, C.; Marique, L.; Jabbour, N.; Gianello, P.; Leclercq, I. Hepatic regeneration in a rat model is impaired by chemotherapy agents used in metastatic colorectal cancer. Eur. J. Surg. Oncol. 2015, 41, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wei, C.; Cheng, K.; Han, B.; Yan, J.; Zhang, M.; Peng, C.; Liu, Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J. Surg. Res. 2013, 183, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Pascucci, M.; Ahmad, S.; Malik, I.A.; Bianchi, A.; Ramadori, P.; Ahmad, G.; Ramadori, G. LIPOCALIN-2 is a major acute-phase protein in a rat and mouse model of sterile abscess. Shock 2012, 37, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Behrns, K.E.; Tsiotos, G.G.; DeSouza, N.F.; Krishna, M.K.; Ludwig, J.; Nagorney, D.M. Hepatic steatosis as a potential risk factor for major hepatic. J. Gastrointest. Surg. 1998, 2, 292–298. [Google Scholar] [CrossRef]
- Mendes-Braz, M.; Elias-Miró, M.; Kleuser, B.; Fayyaz, S.; Jiménez-Castro, M.B.; Massip-Salcedo, M.; Gracia-Sancho, J.; Ramalho, F.S.; Rodes, J.; Peralta, C. The effects of glucose and lipids in steatotic and non-steatotic livers in conditions of partial hepatectomy under ischaemia-reperfusion. Liver Int. 2014, 34, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Cornide-Petronio, M.E.; Bujaldon, E.; Mendes-Braz, M.; Avalos de León, C.G.; Jiménez-Castro, M.B.; Álvarez-Mercado, A.I.; Gracia-Sancho, J.; Rodés, J.; Peralta, C. The impact of cortisol in steatotic and non-steatotic liver surgery. J. Cell. Mol. Med. 2017, 21, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and non-alcoholic fatty liver disease: Multiple interactions. Int. J. Mol. Sci. 2017, 18, e1649. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, C.; Marra, F. The role of adipokines in liver fibrosis. Pathophysiology 2008, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Braz, M.; Elias-Miró, M.; Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Ramalho, F.S.; Peralta, C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J. Biomed. Biotechnol. 2012, 2012, 298657. [Google Scholar] [CrossRef] [PubMed]
- Ohana, G.; Cohen, S.; Rath-Wolfson, L.; Fishman, P. Adenosine receptor agonist, CF102, protects against hepatic ischemia/reperfusion injury following partial hepatectomy. Mol. Med. Rep. 2016, 14, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Shteyer, E.; Liao, Y.; Muglia, L.J.; Hruz, P.W.; Rudnick, D.A. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 2004, 40, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Bönninghoff, R.; Schwenke, K.; Keese, M.; Magdeburg, R.; Bitter-Suermann, H.; Otto, M.; Hasenberg, T.; Post, S.; Sturm, J. Effect of different liver resection methods on liver damage and regeneration factors VEGF and FGF-2 in mice. Can. J. Surg. 2012, 55, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Castro, M.; Elias-Miro, M.; Casillas-Ramirez, A.; Peralta, C. Hepatic Surgery; Abdeldayem, H., Allam, N., Eds.; InTech: London, UK, 2013; pp. 121–166. ISBN 978-953-51-0016-4. [Google Scholar]
- García-Valdecasas, J.C.; Fuster, J.; Charco, R.; Bombuy, E.; Fondevila, C.; Ferrer, J.; Ayuso, C.; Taura, P. Changes in portal vein flow after adult living-donor liver transplantation: Does it influence postoperative liver function? Liver Transplant. 2003, 9, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Gracia-Sancho, J.; Peralta, C. Brain death and marginal grafts in liver transplantation. Cell Death Dis. 2015, 6, e1777. [Google Scholar] [CrossRef] [PubMed]
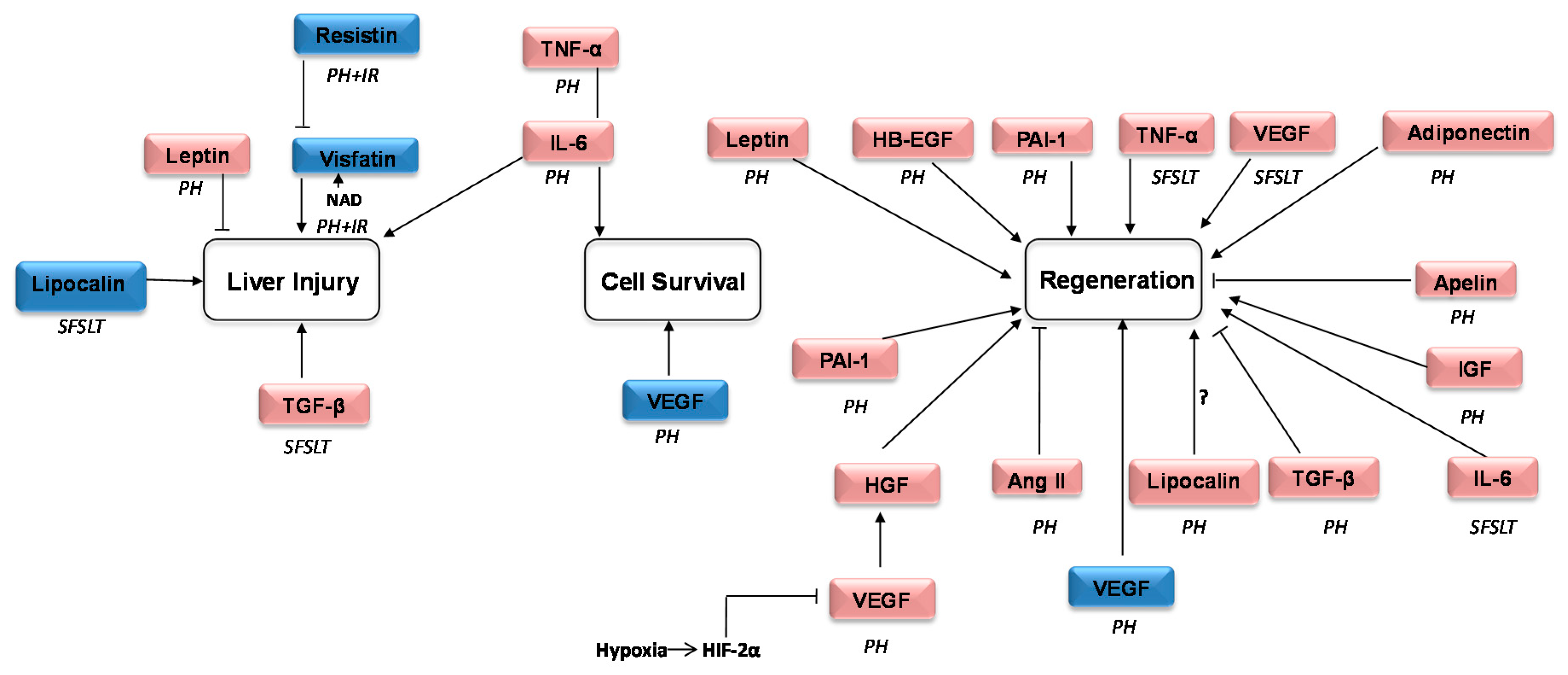
| Role | Name | Prevalent Described Action |
|---|---|---|
| Inflammation | Chemerin | Mediates inflammatory responses, serving as a chemo attractant to induce influx of macrophages and natural killer cells [12] |
| IL-6 | Regulator of both the immune and the nervous system as well in liver regeneration [13] | |
| Omentin | Inhibitor of vascular endothelial cells inflammation. Related to heart vasculature disease and insulin sensitivity [14] | |
| PAI-1 | Interacts with vascular cells. It has been related with angiogenesis and pro-inflammatory cytokines. Widely associated with insulin resistance and impaired immune response [15] | |
| Resistin | Involved in the pathogenesis of obesity, adipogenesis and insulin metabolism [16] | |
| TGF-β | Essential in establishing immunological tolerance. Pro-inflammatory roles in inflammatory responses [17] | |
| TNFα | As pro-inflammatory cytokine is involved in the development of many inflammatory diseases. “Master-regulator” of inflammatory [18] cytokines. Regulation of critical cell functions including cell proliferation, survival, differentiation, and apoptosis [10,18] | |
| Metabolic control | Adiponectin | Involved in the pathogenesis of diabetes mellitus, obesity, hypertension, renal failure and atherosclerosis [19] |
| Apelin | Takes part in the regulation of the physiology and pathophysiology of the circulatory system. Regulator of the metabolic balance, inflammation as well as cell proliferation and apoptosis [20] | |
| Leptin | Between other functions, regulates angiogenesis, hematopoiesis, carcinogenesis, satiety, energy expenditure and the immune system [21] | |
| Lipocalin | LCN2 in mainly produced by hepatocytes under acute-phase conditions. Considerable increased under stressed conditions like bacterial infection, surgical procedures or metabolic stress, plays an important role in suppressing bacterial infection by binding to bacterial catecholate-type ferric siderophores and consequent suppression of bacterial growth through the sequester of iron-laden siderophores [22]. LCN2 acts as immunomodulator and inhibitor of differentiation of erythroid progenitor cells and promotes apoptosis [23] | |
| Metabolic control | RBP4 | Retinol transportation in the circulation [24] |
| Vaspin | Potential insulin-sensitizing effects [25]. Related with non-alcoholic fatty liver disease [26] | |
| Vifastin | Control of energy balance and insulin sensitivity. Regulates lipid metabolism and fatty acid oxidation [27] | |
| Regeneration | Angiotensinogen | It is implied in the development of liver cirrhosis, portal hypertension, angiogenesis and apoptosis [28] |
| Dlk-1 | Adipogenesis, osteogenesis. Neuronal and neuroendocrine differentiation [29] | |
| HB-EGF | The soluble form induces mitogenic and regenerative activities [3] | |
| HGF | Proliferation, morphogenesis and anti-apoptosis [31] | |
| IGF | Both prenatal and postnatal development, including cell growth, differentiation, migration, and survival [32] | |
| NGF | Stimulation of growth, differentiation, survival and maintenance of neurons [33] | |
| VEGF | Regulator of angiogenesis also promotes collateral vessel growth [34] |
| Name | Experimental Model | Effect on Liver Function and Regeneration | Reference | ||
|---|---|---|---|---|---|
| Surgical Procedure | Specie | ||||
| PH | PartialLT | ||||
| IL-6 | 68% PH | Mouse | In NO KO mice, impairment of IL-6 induction provoked excess of hepatic lipid accumulation, increased ER stress and negatively affected hepatocyte proliferation after surgery | [51] | |
| 2/3 PH | Mouse | In multidrug resistance 2 knockout (Mdr2−/−) mice, pharmacological inhibition of IL-6 signaling inhibited tumorigenesis but did not affect survival or recovery of liver mass after PH | [52] | ||
| 78% PH | Mouse | A20 (an NF-κB inhibitory protein) promotes liver regeneration through enhance IL-6/STAT3 proliferative signals | [53] | ||
| 70% PH+I/R 1 h warm ischemia | Mouse | Melatonin protected from hepatic damage and promoted IL-6 and TNF-α and liver regeneration | [54] | ||
| 80% PH | Mouse | Melatonin-associated IL-6 increased liver microcirculation and survival | [54] | ||
| 70% PH | Rat | IL-6 regulated Mcl-1L (a member of the Bcl-2 family) expression through the JAK/PI3K/Akt/CREB signaling pathway. Mcl-1 inhibited apoptosis | [55] | ||
| SFSLT (30%) 1 h cold ischemia | Mouse | Melatonin activated the IL6/GP130-STAT3 pathway protecting SFS graft and promoted regeneration | [54] | ||
| SFSLT (30%) 1 h cold ischemia | Rat | The administration of Gadolinium chloride (GdCl3), a Kupffer cells inhibitor inhibited IL-6/p-STAT3 signal pathway, and thus in turn increased apoptosis and suppressed liver regeneration | [56] | ||
| PAI-1 | 70% PH | Mouse | Knocking out PAI-1 mice was associated with a decrease in hepatocyte proliferation | [57] | |
| Resistin | 70% PH+I/R 1 h warm ischemia | Rat: Steatotic and non-steatotic livers | Steatotic livers were more resistant to the overexpression of resistin after PH under I/R. Resisting originated in liver regulated the visfatin deleterious effects on inflammation and damage | [58] | |
| TGF-β | 2/3 PH | Mouse | Knockout of kupffel-like factor 10, an activator of the TGF-β/Smad signaling pathway suppressed hepatic cell proliferation | [59] | |
| 70% PH | Mouse | Leucine-serine-lysine-leucine peptide promoted liver regeneration by the inhibition of TGF-β | [60] | ||
| TGF-β | 2/3 PH | Mouse | BMP-9 (a member of the TGF-β family) disturbed the proliferative response and promoted fibrosis | [61] | |
| SFSLT (50%) 55–65 min cold ischemia | Rat | Administration of autologous adipose-derived mesenchymal stem cells increased IL-10 and TGF-β avoiding acute rejection and decreasing inflammatory responses | [62] | ||
| TNFα | 2/3 PH | Mouse | Hepatocyte expression of ADAM17 (a major regulator of TNF, TNFR1, and AR amphiregulin) was not essential for hepatocyte proliferation in ADAM17 KO mice | [63] | |
| 2/3 PH | Mouse | TNF-α injection exacerbates the regenerative failure in Gclm−/− mice | [64] | ||
| SFSLT (50%) 10 min or 10 h cold | Rat | TNFα expression was affected in a different way depending of the time of cold ischemia | [65] | ||
| Adiponectin | 2/3 PH | Mouse | Adiponectin regulated regeneration controlling cell cycle progression, cytokine signaling and growth factor bioavailability | [66] | |
| Apelin | 70% PH | Mouse | The blockade of the apelin-APJ system pharmacologically by F13A promoted cell-cycle progression and liver regeneration | [67] | |
| Leptin | 70% PH | Rat | Leptin administration increased regeneration, liver weight and reduced damage | [68] | |
| Lipocalin | 2/3 PH | Mouse | LCN2 was induced in mice after PH although increased expression of LCN2 had no effects in hepatocyte proliferation | [24] | |
| 2/3 PH | Mouse | In LCN2Hep−/− after treatment with IL-6, hepatocyte-derived LCN2 promoted liver regeneration | [23] | ||
| Major PH+I/R 20 min warm ischemia | Mouse: Steatotic and non-steatotic livers | Using wild type and mice over expressing LCN2, it was observed that LCN2 had deleterious effects in steatotic livers | [69] | ||
| 70% or 40% PH | Rat | Expression of the LCN2 mRNA was higher in 70% than in 40% PH | [70] | ||
| Lipocalin | SFSLT (55–70%). 40 min cold ischemia | Rat: Steatotic and non-steatotic livers | LCN2 is upregulated in steatotic small liver grafts. LCN2 exacerbated graft injury and promoted macrophage infiltration | [69] | |
| Vifastin | 70% PH 1 h warm ischemia | Rat: Steatotic and non-steatotic livers | Visfatin administration impaired damage and regenerative response in steatotic livers | [58] | |
| Angiotensinogen and Angiotensin | 70% PH | Mouse: Colorectal cancer liver metastases induction | Captopril (an inhibitor of renin–angiotensin system) did not impair liver regeneration Captopril exerted its effects on established tumors at only late stage acting as an angiogenic inhibitor, reducing widely tumor vessel density and enhancing tumor cell apoptosis | [71] | |
| 70% PH | Mouse | Captopril enhanced early liver regeneration, effect associated with increased hepatic stem cells and MMP-9 protein | [72] | ||
| HB-EGF | 2/3 PH | Mouse | Using focal adhesion kinase (FAK) KO mice, the authors show that Fakdeficiency enhanced liver regeneration modulating TNFα/HB-EGF axis | [73] | |
| HGF | 70% PH | Rat | Low-power laser irradiation enhanced the HGF/Met axis and Akt and Erk pathways improving liver regeneration | [74] | |
| IGF | 70% PH | Mouse | IGF-2 induced hepatocyte proliferation | [75] | |
| VEGF | 68% PH | Mouse | Hif2a-Vegf axed as a prime regulator of regenerative sinusoidal endothelial cells-hepatocyte crosstalk and revealed a crucial role for oxygen during liver regeneration | [76] | |
| 90% PH | Rat: Steatotic and non-steatotic livers | Treatment with VEGF improved survival and stimulated liver regeneration | [77] | ||
| VEGF | 90% PH | Rat | VEGF-sdf1 pathway in the liver is upregulated after PH, and this increases bone marrow production of progenitors of sinusoidal endothelial cells, which are required for liver regeneration | [78] | |
| 70% PH | Rat | The over-expression of VEGF following surgery promoted angiogenesis | [79] | ||
| 70% PH | Rat | After transplant stem cells and MSCs transfected with VEGF, an increment in proliferation of hepatocytes was observed. VEGF transected MSCs also promoted the secretion of several growth factors as HGF and PDGF. These effects supported liver function and regeneration | [80] | ||
| 70% PH | Rat | In rats exposed to chemotherapy, the treatment with Bevacizumab (Anti-VEGF-A) did not affect liver cells proliferation after surgery | [81] | ||
| SFSLT (50%) 55–65 min. cold ischemia | Rat | The over-expression of VEGF induced hepatocyte proliferation and neovascularization of the remnant liver | [82] | ||
| Studies Reported in the Last Five Years | |||
|---|---|---|---|
| Name | PH | Partial LT | Future Perspectives |
| IL-6 | 6 | 2 | Studies performed using different % of PH. IL-6 could be a potential target to promote hepatocyte proliferation and decrease damage. However we must to be cautious because the controversial results in the presence of tumorigenesis and the fact that any of the authors evaluated its effect in PH under I/R |
| PAI-1 | 1 | 0 | Only one reported study in an experimental mouse model of PH. PAI-1 was associated with a decrease in hepatocyte proliferation. More studies in the setting of PH under I/R, partial LT as well as considering steatotic status are required |
| Resistin | 1 | 0 | Its role in partial liver transplantation from steatotic and non-steatotic grafts has not been described. Further studies are required to consider its relevance |
| TGF-β | 3 | 1 | Deleterious effect in the hepatic proliferative response |
| TNFα | 2 | 1 | Although implied in hepatocyte proliferation, controversial results have been reported. Further studies are necessaries to elucidate the precise role of TNF-α in regeneration in surgical procedures as well as in the presence of steatosis |
| Adiponectin | 1 | 0 | As in the previous case, not many studies have been recently reported results and its modulation could beneficiate specially the outcome of hepatic resection in steatotic livers |
| Apelin | 1 | 0 | Only one study published make mandatory more research focused in this adipokine |
| Leptin | 1 | 0 | Not many works have been reported in the setting of PH, PH+I/R or partial LT. Since leptin deficiency impaired liver regeneration in obese mice, drugs aimed to modulate this adipokine would improve prognosis in liver transplantation from steatotic donors |
| Lipocalin | 4 | 1 | Controversial results even using the same experimental model of surgery. More studies are necessaries |
| Vifastin | 1 | 0 | Only one study published make mandatory more research focused in this adipokine |
| Angiotensinogen and Angiotensin | 2 | 0 | Promising results for cancer patients subjected to hepatic resection although more studies are necessaries |
| HB-EGF | 1 | 0 | Only one study published make mandatory more research focused in this adipokine |
| HGF | 1 | 0 | Only one study published make mandatory more research focused in this adipokine |
| IGF | 1 | 0 | Only one study published make mandatory more research focused in this adipokine |
| VEGF | 6 | 1 | Wide consensus in the published results even when different surgical procedures are compared. The benefits of VEGF for liver function and proliferation point that its pharmacological modulation would improve prognosis after surgery |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Mercado, A.I.; Bujaldon, E.; Gracia-Sancho, J.; Peralta, C. The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion. Int. J. Mol. Sci. 2018, 19, 3395. https://doi.org/10.3390/ijms19113395
Álvarez-Mercado AI, Bujaldon E, Gracia-Sancho J, Peralta C. The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion. International Journal of Molecular Sciences. 2018; 19(11):3395. https://doi.org/10.3390/ijms19113395
Chicago/Turabian StyleÁlvarez-Mercado, Ana Isabel, Esther Bujaldon, Jordi Gracia-Sancho, and Carmen Peralta. 2018. "The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion" International Journal of Molecular Sciences 19, no. 11: 3395. https://doi.org/10.3390/ijms19113395
APA StyleÁlvarez-Mercado, A. I., Bujaldon, E., Gracia-Sancho, J., & Peralta, C. (2018). The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion. International Journal of Molecular Sciences, 19(11), 3395. https://doi.org/10.3390/ijms19113395