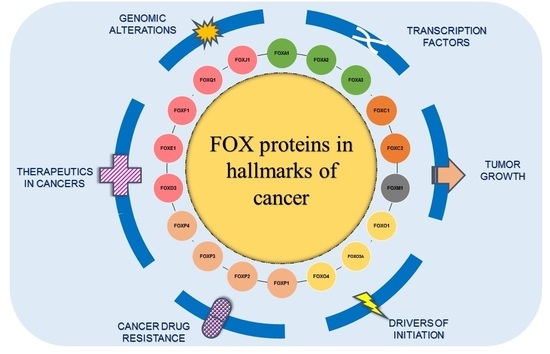
The Dominant Role of Forkhead Box Proteins in Cancer
Abstract
1. Introduction
2. An Overview of Recent Insights on FOXs in Cancer
2.1. Forkhead Box A (FOXA) in Cancer
2.2. FOXC in Cancer
2.3. FOXM1 in Cancer
2.4. FOXO in Cancer
2.5. FOXP in Cancer
2.6. Other Important FOX Transcription Factors in Cancer
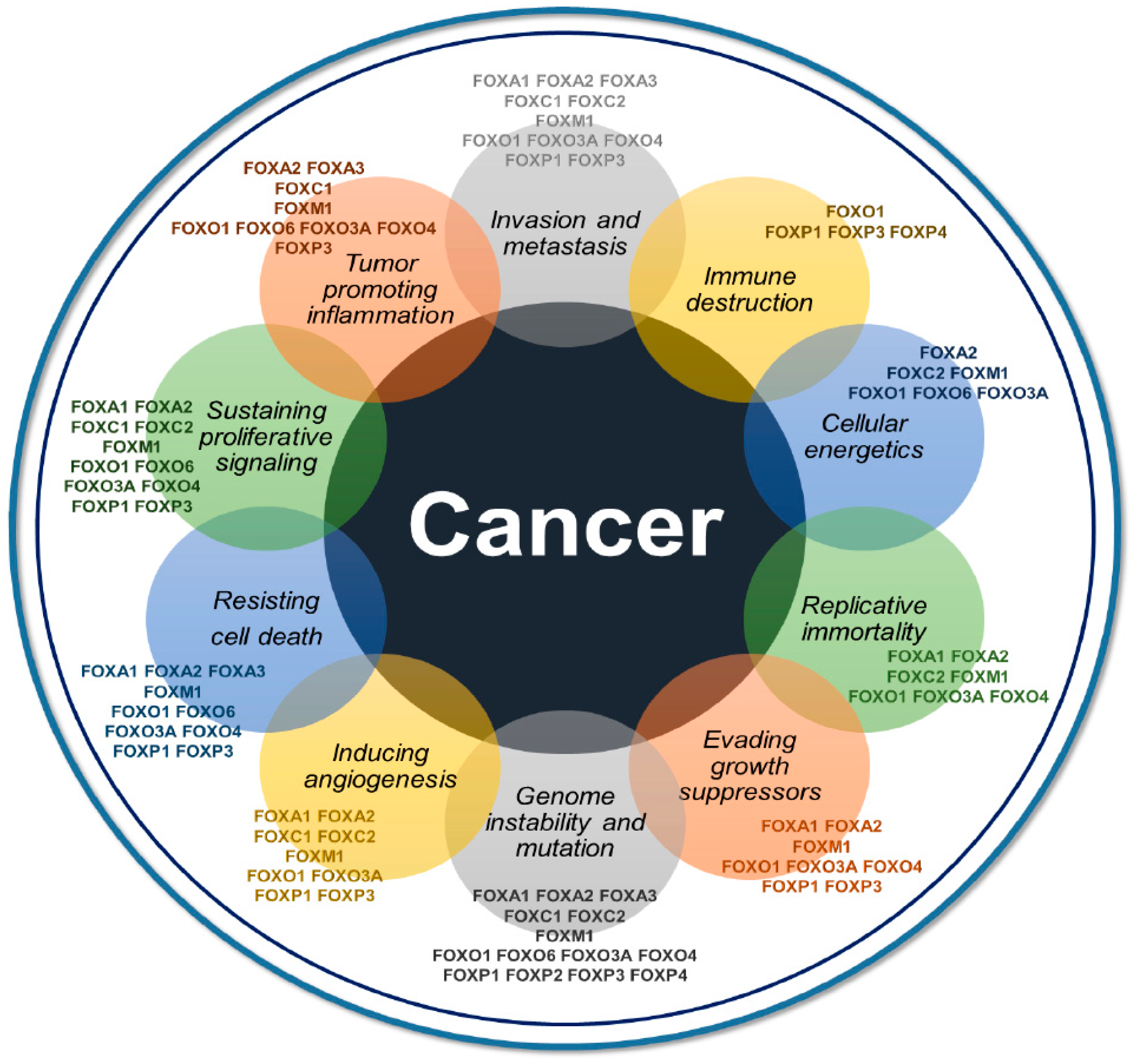
3. Major Areas of Focus on FOXs in Cancer
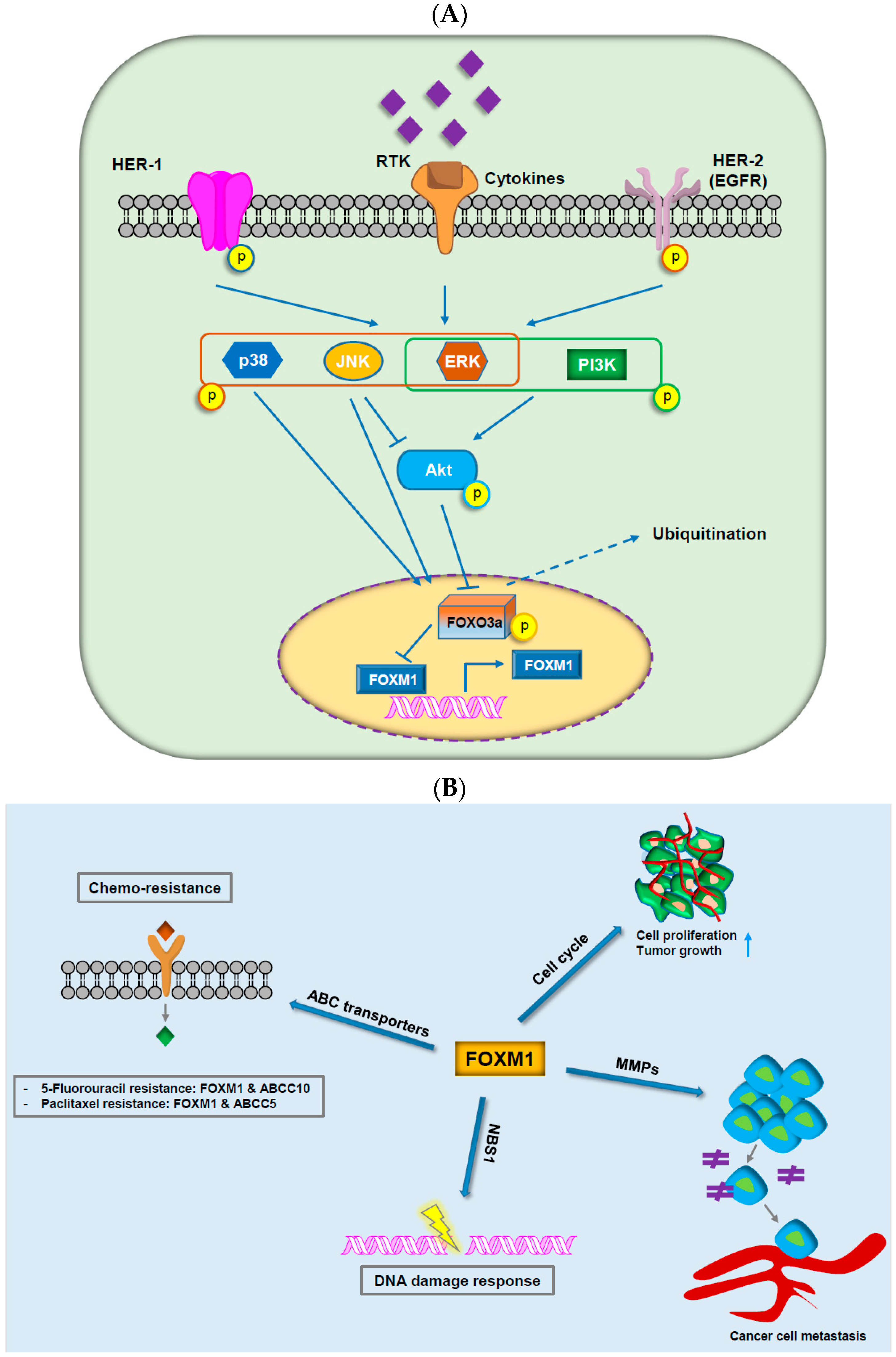
3.1. FOX Proteins in Cancer Drug Resistance
3.2. FOX Proteins and Genomic Alterations
3.3. FOXM1
3.4. FOXO Subfamily Genes
3.5. Other FOX Genes
4. Negative Modulation of FOX Proteins by miRNAs
5. Targeting FOX Proteins as Potential Therapeutics in Cancers
5.1. FOX Proteins-Targeting RNAi
5.2. Proteasome Inhibitors
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| CIN | chromosomal instability |
| DBD | DNA-binding domain |
| EMT | epithelial-mesenchymal transition |
| FASLG | Fas ligand |
| GSK | glycogen synthase kinase 3 |
| NSCLC | non-small cell lung cancer |
| RNAi | RNA interference |
| YAP | Yes-Associated Protein |
References
- Lam, E.W.F.; Brosens, J.J.; Gomes, A.R.; Koo, C.-Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer 2013, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Coffer, P.J.; Burgering, B.M.T. Forkhead-box transcription factors and their role in the immune system. Nat. Rev. Immunol. 2004, 4, 889. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Peng, S.L. Forkhead transcription factors in immunology. Cell Mol. Life Sci. 2005, 62, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, O.J.; Sowden, J.C.; Carlsson, P.; Jordan, T.; Bhattacharya, S.S. FOX’s in development and disease. Trends Genet. 2003, 19, 339–344. [Google Scholar] [CrossRef]
- Jackson, B.C.; Carpenter, C.; Nebert, D.W.; Vasiliou, V. Update of human and mouse forkhead box (FOX) gene families. Hum. Genom. 2010, 4, 345–352. [Google Scholar]
- Choi, E.J.; Seo, E.J.; Kim, D.K.; Lee, S.I.; Kwon, Y.W.; Jang, I.H.; Kim, K.H.; Suh, D.S.; Kim, J.H. FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells. Oncotarget 2016, 7, 3506–3519. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Bao, W.; Wang, J.; Yang, T.; He, X.; Liao, Y.; Wan, X. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.-F.; Gomes, A.R. Forkhead box transcription factors in cancer initiation, progression and chemotherapeutic drug response. Front. Oncol. 2014, 4, 305. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.G.; Cotter, T.G. Chapter Six—New insight into the role of reactive oxygen species (ROS) in cellular signal-transduction processes. Int. Rev. Cell Mol. Biol. 2015, 319, 221–254. [Google Scholar] [PubMed]
- Obsil, T.; Obsilova, V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008, 27, 2263. [Google Scholar] [CrossRef] [PubMed]
- Essaghir, A.; Dif, N.; Marbehant, C.Y.; Coffer, P.J.; Demoulin, J.B. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J. Biol. Chem. 2009, 284, 10334–10342. [Google Scholar] [CrossRef] [PubMed]
- Myatt, S.S.; Lam, E.W.F. The emerging roles of forkhead box (FOX) proteins in cancer. Nat. Rev. Cancer 2007, 7, 847. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.-Y.; Muir, K.W.; Lam, E.W.F. FOXM1: From cancer initiation to progression and treatment. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Brosens, J.J.; Schwenen, H.D.; Lam, E.W. FOXO and FOXM1 in cancer: The FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr. Drug Targets 2011, 12, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Elian, F.A.; Yan, E.; Walter, M.A. FOXC1, the new player in the cancer sandbox. Oncotarget 2018, 9, 8165–8178. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Z.; Gao, X.; He, W.; Cai, Y.; Chen, H.; Xu, R. FOXC1 induces cancer stem cell-like properties through upregulation of beta-catenin in NSCLC. J. Exp. Clin. Cancer Res. 2018, 37, 220. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Igarashi, M.; Fukuda, H.; Nakagama, H.; Katoh, M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013, 328, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Ali, I.; Silins, I.; Pyysalo, S.; Guo, Y.; Hogberg, J.; Stenius, U.; Korhonen, A. Cancer Hallmarks Analytics Tool (CHAT): A text mining approach to organize and evaluate scientific literature on cancer. Bioinformatics 2017, 33, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Gartel, A.L. FOX(M1) News—It Is Cancer. Mol. Cancer Ther. 2013, 12, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Kaestner, K.H. The FOXA family of transcription factors in development and metabolism. Cell Mol. Life Sci. 2006, 63, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Jägle, S.; Busch, H.; Freihen, V.; Beyes, S.; Schrempp, M.; Boerries, M.; Hecht, A. SNAIL1-mediated downregulation of FOXA proteins facilitates the inactivation of transcriptional enhancer elements at key epithelial genes in colorectal cancer cells. PLoS Genet. 2017, 13, e1007109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Y.; Liu, Y.; Kao, L.-P.; Wang, X.; Skerry, B.; Li, Z. FOXA1 defines cancer cell specificity. Sci. Adv. 2016, 2, e1501473. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Miller, C.T.; Contreras, J.I.; Prescott, M.S.; Dagenais, S.L.; Wu, R.; Yee, J.; Orringer, M.B.; Misek, D.E.; Hanash, S.M.; et al. The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1), on chromosome band 14q13 is amplified and overexpressed in esophageal and lung adenocarcinomas. Cancer Res. 2002, 62, 5273. [Google Scholar] [PubMed]
- Mirosevich, J.; Gao, N.; Gupta, A.; Shappell, S.B.; Jove, R.; Matusik, R.J. Expression and role of FOXA proteins in prostate cancer. Prostate 2005, 66, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z. Interplay of estrogen receptors and FOXA factors in the liver cancer. Mol. Cell Endocrinol. 2015, 418, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tuteja, G.; Schug, J.; Kaestner Klaus, H. FOXA1 and FOXA2 are essential for sexual dimorphism in liver cancer. Cell 2012, 148, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Laakso, M.; Ovaska, K.; Mirtti, T.; Lundin, J.; Rannikko, A.; Sankila, A.; Turunen, J.P.; Lundin, M.; Konsti, J.; et al. Dual role of FOXA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011, 30, 3962. [Google Scholar] [CrossRef] [PubMed]
- DeGraff, D.J.; Clark, P.E.; Cates, J.M.; Yamashita, H.; Robinson, V.L.; Yu, X.; Smolkin, M.E.; Chang, S.S.; Cookson, M.S.; Herrick, M.K.; et al. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PLoS ONE 2012, 7, e36669. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.R.; Chen, Y.Y.; Yokomori, K. Mechanisms of cohesin-mediated gene regulation and lessons learned from cohesinopathies. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; McEwan, M.; Horsfield, J.A. Gene regulation by cohesin in cancer: Is the ring an unexpected party to proliferation? Mol. Cancer Res. 2011, 9, 1587. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Bourriquen, G.; Lamaze, F.C.; Côté, M.C.; Fournier, É.; Joly-Beauparlant, C.; Caron, V.; Gobeil, S.; Droit, A.; Bilodeau, S. FOXA and master transcription factors recruit Mediator and Cohesin to the core transcriptional regulatory circuitry of cancer cells. Sci. Rep. 2016, 6, 34962. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Asilmaz, E.; Luca, E.; Friedman, J.M.; Stoffel, M. FOXA2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 2004, 432, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kume, T. The cooperative roles of FOXC1 and FOXC2 in cardiovascular development. Adv. Exp. Med. Biol. 2009, 665, 63–77. [Google Scholar] [PubMed]
- Papanicolaou, K.N.; Izumiya, Y.; Walsh, K. Forkhead transcription factors and cardiovascular biology. Circ. Res. 2008, 102, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Bhowmick, N.; Qu, Y.; Chung, S.; Giuliano, A.E.; Cui, X. FOXC1: An emerging marker and therapeutic target for cancer. Oncogene 2017, 36, 3957. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Camacho, F.A.; Levin, C.I.; Flores, K.; Clift, A.; Galvez, A.; Terres, M.; Rivera, S.; Kolli, S.N.; Dodderer, J.; et al. FOXC1 plays a crucial role in the growth of pancreatic cancer. Oncogenesis 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.S.; Wang, J.; Qu, Y.; Sim, M.-S.; Shamonki, J.; Bagaria, S.P.; Ye, X.; Liu, B.; Elashoff, D.; Hoon, D.S.; et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010, 70, 3870. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Huang, W.; Tian, D.; Zhu, H.; Qi, X.; Chen, Z.; Zhang, Y.; Hu, H.; Fan, D.; Nie, Y.; et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology 2012, 57, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, L.; Wang, Q.; Hu, Y.W. Emerging roles and mechanisms of FOXC2 in cancer. Clin. Chim. Acta 2018, 479, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Jiao, H.L.; Ye, Y.P.; Chen, C.M.; Wang, J.X.; Tang, N.; Li, T.T.; Lin, J.; Qi, L.; Wu, P.; et al. FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene 2015, 34, 4379–4390. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Qu, Y.; Jin, Y.; Yu, Y.; Deng, N.; Wawrowsky, K.; Zhang, X.; Li, N.; Bose, S.; Wang, Q.; et al. FOXC1 activates smoothened-independent Hedgehog signaling in basal-like breast cancer. Cell Rep. 2015, 13, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Hollier, B.G.; Tinnirello, A.A.; Werden, S.J.; Evans, K.W.; Taube, J.H.; Sarkar, T.R.; Sphyris, N.; Shariati, M.; Kumar, S.V.; Battula, V.L.; et al. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013, 73, 1981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, B.; Liu, F.; Zhang, M.; Wang, Q.; Liu, Y.; Yao, Y.; Li, D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Mol. Cancer 2017, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, H.; Liao, W.; Luo, X.; Li, Y.; Chen, T.; Zhang, X. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp. Cell Res. 2017, 357, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, P.; Park, H.J. FOXM1: A master regulator of tumor metastasis. Cancer Res. 2011, 71, 4329. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, D.; Yu, Q.; Li, L.; Wu, P. Prognostic value of FOXM1 in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 32298–32308. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Carr, J.R.; Wang, Z.; Nogueira, V.; Hay, N.; Tyner, A.L.; Lau, L.F.; Costa, R.H.; Raychaudhuri, P. FOXM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009, 28, 2908. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Gusarova, G.; Wang, Z.; Carr, J.R.; Li, J.; Kim, K.H.; Qiu, J.; Park, Y.D.; Williamson, P.R.; Hay, N.; et al. Deregulation of FOXM1B leads to tumour metastasis. EMBO Mol. Med. 2011, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Chen, Y.-J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell Biol. 2005, 25, 10875. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Wang, Z.; Ali, S.; Kong, D.; Banerjee, S.; Ahmad, A.; Li, Y.; Azmi, A.S.; Miele, L.; Sarkar, F.H. Over-expression of FOXM1 leads to epithelial–mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J. Cell Biochem. 2011, 112, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Zhao, F.; Lam, E.W.F. Role and regulation of the forkhead transcription factors FOXO3a and FOXM1 in carcinogenesis and drug resistance. Chin. J. Cancer 2013, 32, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Gartel, A.L. FOXM1: The Achilles’ heel of cancer? Nat. Rev. Cancer 2008, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Coomans de Brachène, A.; Demoulin, J.B. FOXO transcription factors in cancer development and therapy. Cell Mol. Life Sci. 2016, 73, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Rashid, A.J.; Colas, D.; de la Torre-Ubieta, L.; Zhu, R.P.; Morgan, A.A.; Santo, E.E.; Ucar, D.; Devarajan, K.; Cole, C.J.; et al. FOXO6 regulates memory consolidation and synaptic function. Genes Dev. 2012, 26, 2780–2801. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tindall, D.J. Dynamic FOXO transcription factors. J. Cell Sci. 2007, 120, 2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gan, B.; Liu, D.; Paik, J.H. FOXO family members in cancer. Cancer Biol. Ther. 2011, 12, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Brunet, A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2007, 192, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Hou, J. A “FOXO” in sight: Targeting FOXO proteins from conception to cancer. Med. Res. Rev. 2008, 29, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Kloet, D.E.A.; Burgering, B.M.T. The PKB/FOXO switch in aging and cancer. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, H. PI3K/Akt/FOXO: A novel participant in signal transduction in bone cells under mechanical stimulation. Cell Biol. Int. 2013, 36, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Hou, J. Clever cancer strategies with FOXO transcription factors. Cell Cycle 2008, 7, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-evaluating the role of FOXOs in cancer. Semin. Cancer Biol. 2018, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Smits, L.M.M.; Meerlo, M.; van Amersfoort, M.; Groot Koerkamp, M.J.A.; van Leenen, D.; Kloet, D.E.A.; Holstege, F.C.P.; Derksen, P.W.B.; Burgering, B.M.T.; et al. FOXO transcription factors both suppress and support breast cancer progression. Cancer Res. 2018, 78, 2356–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Duan, N.; Song, T.; Li, Z.; Zhang, C.; Chen, X. The emerging roles of forkhead box (FOX) proteins in osteosarcoma. J. Cancer 2017, 8, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Tan, P.; Xie, L.; Mi, B.; Fang, Z.; Li, J.; Yue, J.; Liao, H.; Li, F. FOXO1 inhibits osteosarcoma oncogenesis via Wnt/beta-catenin pathway suppression. Oncogenesis 2015, 4, e166. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Hung, M.-C. A new fork for clinical application: Targeting forkhead transcription factors in cancer. Clin. Cancer Res. 2009, 15, 752. [Google Scholar] [CrossRef] [PubMed]
- Van der Vos, K.E.; Coffer, P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 2011, 14, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Nho, R.S.; Hergert, P. FOXO3A and disease progression. World J. Biol. Chem. 2014, 5, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3A in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Chauhan, A.S.; Zhuang, L.; Gan, B. FOXO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018, 50, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Holder, D.D.; Pawel, B.R.; Zhang, C.; Barr, F.G. High expression of the PAX3-FKHR oncoprotein is required to promote tumorigenesis of human myoblasts. Am. J. Pathol. 2009, 175, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Sin, C.; Li, H.; Crawford, D.A. Transcriptional regulation by FOXP1, FOXP2, and FOXP4 dimerization. J. Mol. Neurosci. 2015, 55, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Meerschaut, I.; Rochefort, D.; Revençu, N.; Pètre, J.; Corsello, C.; Rouleau, G.A.; Hamdan, F.F.; Michaud, J.L.; Morton, J.; Radley, J.; et al. FOXP1-related intellectual disability syndrome: A recognisable entity. J. Med. Genet. 2017, 54, 613. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-H.; Cheng, M.; Tang, J.-P.; Liu, Q.; Pan, F.; Li, X.-P. FOXP3, regulatory T cell, and autoimmune diseases. Inflammation 2017, 40, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Ladoire, S.; Mignot, G.; Apetoh, L.; Ghiringhelli, F. Human FOXP3 and cancer. Oncogene 2010, 29, 4121. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.J.; Gitton, Y. The untold stories of the speech gene, the FOXP2 cancer gene. Genes Cancer 2018, 9, 11–38. [Google Scholar] [PubMed]
- Teufel, A.; Wong, E.A.; Mukhopadhyay, M.; Malik, N.; Westphal, H. FOXP4, a novel forkhead transcription factor. Biochim. Biophys. Acta 2003, 1627, 147–152. [Google Scholar] [CrossRef]
- Vernes, S.C.; Spiteri, E.; Nicod, J.; Groszer, M.; Taylor, J.M.; Davies, K.E.; Geschwind, D.H.; Fisher, S.E. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am. J. Hum. Genet. 2007, 81, 1232–1250. [Google Scholar] [CrossRef] [PubMed]
- DeLaughter, D.M.; Christodoulou, D.C.; Robinson, J.Y.; Seidman, C.E.; Baldwin, H.S.; Seidman, J.G.; Barnett, J.V. Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro. J. Mol. Cell Cardiol. 2013, 59, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Tang, Y.; Lei, X.H.; Zhao, S.C.; Wu, Z.Q. miR-618 inhibits prostate cancer migration and invasion by targeting FOXP2. J. Cancer 2017, 8, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Gascoyne, D.M.; Soilleux, E.J.; Lyne, L.; Spearman, H.; Roncador, G.; Pedersen, L.M.; Moller, M.B.; Green, T.M.; Banham, A.H. FOXP2-positive diffuse large B-cell lymphomas exhibit a poor response to R-CHOP therapy and distinct biological signatures. Oncotarget 2016, 7, 52940–52956. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Lyne, L.; Brown, P.J.; Launchbury, R.J.; Bignone, P.; Chi, J.; Roncador, G.; Lawrie, C.H.; Gatter, K.C.; Kusec, R.; et al. Aberrant expression of the neuronal transcription factor FOXP2 in neoplastic plasma cells. Br. J. Haematol. 2010, 149, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.B.; Ippolito, G.C.; Banham, A.H.; Tucker, P.W. FOXP1: A potential therapeutic target in cancer. Expert. Opin. Ther. Targets 2007, 11, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Sawant, D.V.; Vignali, D.A.A. Once a Treg, always a Treg? Immunol. Rev. 2014, 259, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Szylberg, L.; Karbownik, D.; Marszalek, A. The role of FOXP3 in human cancers. Anticancer Res. 2016, 36, 3789–3794. [Google Scholar] [PubMed]
- Li, X.; Gao, Y.; Li, J.; Zhang, K.; Han, J.; Li, W.; Hao, Q.; Zhang, W.; Wang, S.; Zeng, C.; et al. FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer. Cell Death Dis. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, H.; Thakur, A.; Chen, T.; Xue, J.; Li, D.; Chen, M. FOXP4 modulates tumor growth and independently associates with miR-138 in non-small cell lung cancer cells. Tumour Biol. 2015, 36, 8185–8191. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, X.; Wang, J.; Xu, Y.; Wei, D.; Zhang, Y.; Yang, K.; Wang, X.; Liang, S.; Chen, X.; et al. Association of FOXP4 gene with prostate cancer and the cumulative effects of rs4714476 and 8q24 in Chinese men. Clin. Lab. 2015, 61, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.D.; Blood, K.A.; Ng, B.L.; Beavis, J.C.; Chua, Y.; Cooke, S.L.; Raby, S.; Ichimura, K.; Collins, V.P.; Carter, N.P.; et al. Array painting reveals a high frequency of balanced translocations in breast cancer cell lines that break in cancer-relevant genes. Oncogene 2007, 27, 3345. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-H.; Zhao, C.L.; Ding, L.-B.; Zhou, X. FOXD3 suppresses tumor growth and angiogenesis in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2015, 466, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-L.; Zhao, H.-M.; Li, Y.; Chen, A.-X.; Sun, X.; Ge, J. FoxD3 deficiency promotes breast cancer progression by induction of epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2014, 446, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mei, H.; Qi, M.; Yang, D.; Zhao, X.; Xiang, X.; Pu, J.; Huang, K.; Zheng, L.; Tong, Q. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget 2013, 4, 2021–2044. [Google Scholar] [CrossRef] [PubMed]
- Mond, M.; Bullock, M.; Yao, Y.; Clifton-Bligh, R.J.; Gilfillan, C.; Fuller, P.J. Somatic mutations of FOXE1 in papillary thyroid cancer. Thyroid 2015, 25, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Penna-Martinez, M.; Epp, F.; Kahles, H.; Ramos-Lopez, E.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grunwald, F.; Holzer, K.; Bechstein, W.O.; et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid 2014, 24, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xi, Q.; Liu, L.; Wang, J.; Gu, M. Quantitative assessment of common genetic variants on FOXE1 and differentiated thyroid cancer risk. PLoS ONE 2014, 9, e87332. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Sasaki, Y.; Koyama, R.; Takeda, K.; Idogawa, M.; Tokino, T. Forkhead transcription factor FOXF1 is a novel target gene of the p53 family and regulates cancer cell migration and invasiveness. Oncogene 2013, 33, 4837. [Google Scholar] [CrossRef] [PubMed]
- Gialmanidis, I.P.; Bravou, V.; Petrou, I.; Kourea, H.; Mathioudakis, A.; Lilis, I.; Papadaki, H. Expression of Bmi1, FOXF1, Nanog, and γ-Catenin in relation to Hedgehog signaling pathway in human non-small-cell lung cancer. Lung 2013, 191, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Fulford, L.; Milewski, D.; Ustiyan, V.; Ravishankar, N.; Cai, Y.; Le, T.; Masineni, S.; Kasper, S.; Aronow, B.; Kalinichenko, V.V.; et al. The transcription factor FOXF1 promotes prostate cancer by stimulating the mitogen-activated protein kinase ERK5. Sci. Signal. 2016, 9, ra48. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.-K.; Lee, J.S.; Sukumar, S. The p53-p21(WAF1) checkpoint pathway plays a protective role in preventing DNA rereplication induced by abrogation of FOXF1 function. Cell Signal. 2012, 24, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.G.; Wang, D.Q.; Hu, D.F.; Li, Y.S.; Liu, S.H. Decreased FOXF1 promotes hepatocellular carcinoma tumorigenesis, invasion, and stemness and is associated with poor clinical outcome. OncoTargets Ther. 2016, 9, 1743–1752. [Google Scholar]
- Zhang, G.; He, P.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Yfantis, H.G.; Lee, D.H.; Hanna, N.; Alexander, H.R.; Hussain, S.P. FOXL1, a novel candidate tumor suppressor, inhibits tumor aggressiveness and predicts outcome in human pancreatic cancer. Cancer Res. 2013, 73, 5416–5425. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Yang, F.P.; Li, W.; Liu, M.; Wang, G.C.; Che, J.P.; Huang, J.H.; Zheng, J.H. FOXL1 inhibits tumor invasion and predicts outcome in human renal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 110–122. [Google Scholar] [PubMed]
- Qin, Y.; Gong, W.; Zhang, M.; Wang, J.; Tang, Z.; Quan, Z. Forkhead box L1 is frequently downregulated in gallbladder cancer and inhibits cell growth through apoptosis induction by mitochondrial dysfunction. PLoS ONE 2014, 9, e102084. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, M.; Ma, L.; Zhou, J.; Xiao, Y.; Zhou, X.; Zhang, C.; Wu, M. Inhibitory effects of forkhead box L1 gene on osteosarcoma growth through the induction of cell cycle arrest and apoptosis. Oncol. Rep. 2015, 34, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, P.; Liu, H.; He, F.; Ming, L. FOXQ1 promotes esophageal cancer proliferation and metastasis by negatively modulating CDH1. Biomed. Pharmacother. 2015, 74, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Speyer, C.L.; Zhang, B.; Zhao, Y.; Chen, W.; Gorski, D.H.; Miller, F.R.; Wu, G. PDGFRalpha and beta play critical roles in mediating FOXQ1-driven breast cancer stemness and chemoresistance. Cancer Res. 2015, 75, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Luo, Z.; Kang, Q.; Deng, D.; Wang, Q.; Peng, H.; Wang, S.; Wei, Z. FOXQ1 mediates the crosstalk between TGF-beta and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol. Ther. 2015, 16, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.X.; Xu, J.W.; Wang, L.; Wu, D.; Zhang, G.Y.; Hu, S.Y. FOXQ1 is a novel molecular target for pancreatic cancer and is associated with poor prognosis. Curr. Mol. Med. 2015, 15, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, J.; Guo, Q. Research progress on the regulation of tumor initiation and development by the forkhead box Q1 gene. J. Cancer Res. Ther. 2018, 14, 6–11. [Google Scholar] [PubMed]
- Chen, H.W.; Huang, X.D.; Li, H.C.; He, S.; Ni, R.Z.; Chen, C.H.; Peng, C.; Wu, G.; Wang, G.H.; Wang, Y.Y.; et al. Expression of FOXJ1 in hepatocellular carcinoma: Correlation with patients’ prognosis and tumor cell proliferation. Mol. Carcinog. 2012, 52, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Shang, D.; Kong, G.; Tian, Y. FOXJ1 promotes bladder cancer cell growth and regulates Warburg effect. Biochem. Biophys. Res. Commun. 2018, 495, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, X.; Xia, L.; Zhou, J.; Xin, J.; Liu, M.; Shang, X.; Liu, J.; Li, X.; Chen, Z.; et al. Decreased expression of FOXJ1 is a potential prognostic predictor for progression and poor survival of gastric cancer. Ann. Surg. Oncol. 2015, 22, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Meng, Y.H.; Wang, J.L.; Yang, B.B.; Zhang, F.; Tang, S.J. FOXL2 suppresses proliferation, invasion and promotes apoptosis of cervical cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 1534–1543. [Google Scholar] [PubMed]
- Rosario, R.; Cohen, P.A.; Shelling, A.N. The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumours. Gynecolog. Oncol. 2014, 133, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; Anglesio, M.S.; Mackenzie, R.; Yang, W.; Senz, J.; Ho, J.; Bell, L.; Lee, S.; Lorette, J.; Huntsman, D.G.; et al. FOXL2 molecular testing in ovarian neoplasms: Diagnostic approach and procedural guidelines. Mod. Pathol. 2013, 26, 860. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Fan, L.Y.; Lam, E.W. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer Biol. 2018, 50, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer 2017, 141, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Kim, D.; Bae, S.Y.; Kim, W.K.; Hong, J.-Y.; Lee, H.-J.; Rajasekaran, N.; Kwon, S.; Fan, Y.; Luu, T.-T.-T.; et al. Targeting nicotinamide n-methyltransferase and miR-449a in EGFR-TKI-resistant non-small-cell lung cancer cells. Mol. Ther. Nucleic Acids 2018, 11, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Lee, S.K. Long noncoding RNAs in cancer cells. Cancer Lett. 2018, 419, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Park, H.J.; Lee, S.K. The dual role of bone morphogenetic proteins in cancer. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Duc-Hiep, B.; Lee, S.K. The potential impacts of tylophora alkaloids and their derivatives in modulating inflammation, viral infections, and cancer. Curr. Med. Chem. 2018, 25, 1–16. [Google Scholar]
- Bach, D.H.; Luu, T.T.T.; Kim, D.; An, Y.J.; Park, H.J.; Park, S.; Lee, S.K. BMP4 upregulation is associated with acquired drug resistance and fatty acid metabolism in EGFR-mutant non-small cell lung cancer cells. Mol. Ther. Nucleic Acids 2018, 12, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, J.; Zhou, W.; Ren, Y.; Wang, X.; Chen, H.; Zhang, J.; Chen, J.; Sun, Y.; Cui, L.; et al. Activation of an AKT/FOXM1/STMN1 pathway drives resistance to tyrosine kinase inhibitors in lung cancer. Br. J. Cancer 2017, 117, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, G.L.; Chen, Y.; Teng, N.N.H. Targeting FOXM1 improves cytotoxicity of paclitaxel and cisplatinum in platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 2017, 27, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Wen, L.; Yang, H.; Wen, M.; Yun, Y.; Zhao, L.; Zhu, X.; Tian, L.; Luo, E.; et al. FOXM1 confers resistance to gefitinib in lung adenocarcinoma via a MET/AKT-dependent positive feedback loop. Oncotarget 2016, 7, 59245–59259. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Halasi, M.; Zia, M.F.; Gann, P.; Gaitonde, S.; Mahmud, N.; Gartel, A.L. Nuclear FOXM1 drives chemoresistance in AML. Leukemia 2017, 31, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Geng, J.; Wang, Y.; Wang, L.; Huang, M.; Chen, J.; Zhang, K.; Xue, L.; Liu, X.; Mao, X.; et al. FOXM1 evokes 5-fluorouracil resistance in colorectal cancer depending on ABCC10. Oncotarget 2017, 8, 8574–8589. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Han, X.; Zhang, C.L.; Ge, L.; Du, F.Y.; Jin, J.; Gong, A.H. FOXM1-mediated RFC5 expression promotes temozolomide resistance. Cell Biol. Toxicol. 2017, 33, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhu, Q.; Li, Z.; Peng, Y.; Yu, X.; Yuan, B.; Liu, Y.; Liu, Y.; Yin, L.; Peng, Y.; et al. The FOXM1-ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis. 2017, 8, e2659. [Google Scholar] [CrossRef] [PubMed]
- Tassi, R.A.; Todeschini, P.; Siegel, E.R.; Calza, S.; Cappella, P.; Ardighieri, L.; Cadei, M.; Bugatti, M.; Romani, C.; Bandiera, E.; et al. FOXM1 expression is significantly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients. J. Exp. Clin. Cancer Res. 2017, 36, 63. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.G.; Mun, M.H.; Jeong, M.S.; Kim, W.T.; Lee, S.R.; Chung, J.W.; Kim, S.I.; Kim, T.N.; Nam, J.K.; Leem, S.H. Drug resistance of bladder cancer cells through activation of ABCG2 by FOXM1. BMB Rep. 2018, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, P.; Karunarathna, U.; Khongkow, M.; Gong, C.; Gomes, A.R.; Yague, E.; Monteiro, L.J.; Kongsema, M.; Zona, S.; Man, E.P.; et al. FOXM1 targets NBS1 to regulate DNA damage-induced senescence and epirubicin resistance. Oncogene 2014, 33, 4144–4155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, W.; Liu, B.; Yao, R.; Liu, S.; Yao, Y.; Liang, J. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J. Transl. Med. 2013, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Z.; Shi, F.; Wang, J. Pin1 modulates chemo-resistance by up-regulating FOXM1 and the involvements of Wnt/beta-catenin signaling pathway in cervical cancer. Mol. Cell Biochem. 2016, 413, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Nestal de Moraes, G.; Delbue, D.; Silva, K.L.; Robaina, M.C.; Khongkow, P.; Gomes, A.R.; Zona, S.; Crocamo, S.; Mencalha, A.L.; Magalhaes, L.M.; et al. FOXM1 targets XIAP and Survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015, 27, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Consolaro, F.; Basso, G.; Ghaem-Magami, S.; Lam, E.W.; Viola, G. FOXM1 is overexpressed in B-acute lymphoblastic leukemia (B-ALL) and its inhibition sensitizes B-ALL cells to chemotherapeutic drugs. Int. J. Oncol. 2015, 47, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, U.; Kongsema, M.; Zona, S.; Gong, C.; Cabrera, E.; Gomes, A.R.; Man, E.P.; Khongkow, P.; Tsang, J.W.; Khoo, U.S.; et al. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene 2016, 35, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, P.; Gomes, A.R.; Gong, C.; Man, E.P.; Tsang, J.W.; Zhao, F.; Monteiro, L.J.; Coombes, R.C.; Medema, R.H.; Khoo, U.S.; et al. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene 2016, 35, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.T.; Huang, Y.F.; Tsai, H.Y.; Chen, C.C.; Chang, C.H.; Huang, S.C.; Hsu, K.F.; Chou, C.Y. FOXM1 confers to epithelial-mesenchymal transition, stemness and chemoresistance in epithelial ovarian carcinoma cells. Oncotarget 2015, 6, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Wang, B.; Tang, B.; Chen, B.J.; Xiao, Y.F.; Qin, Y.; Yong, X.; Luo, G.; Zhang, J.W.; Zhang, D.; et al. The FOXM1-induced resistance to oxaliplatin is partially mediated by its novel target gene Mcl-1 in gastric cancer cells. Biochim. Biophys. Acta 2015, 1849, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Siu, M.K.; Jiang, L.; Tam, K.F.; Ngan, H.Y.; Le, X.F.; Wong, O.G.; Wong, E.S.; Gomes, A.R.; Bella, L.; et al. Overexpression of forkhead box protein M1 (FOXM1) in ovarian cancer correlates with poor patient survival and contributes to paclitaxel resistance. PLoS ONE 2014, 9, e113478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Wang, Y.; Yin, X.; He, Y.; Chen, L.; Wang, W.; Liu, T.; Di, W. FOXM1 modulates cisplatin sensitivity by regulating EXO1 in ovarian cancer. PLoS ONE 2014, 9, e96989. [Google Scholar] [CrossRef] [PubMed]
- Yu-Rice, Y.; Jin, Y.; Han, B.; Qu, Y.; Johnson, J.; Watanabe, T.; Cheng, L.; Deng, N.; Tanaka, H.; Gao, B.; et al. FOXC1 is involved in ERalpha silencing by counteracting GATA3 binding and is implicated in endocrine resistance. Oncogene 2016, 35, 5400–5411. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, L.; Ni, S.; Gu, J.; Zhu, H.; Wang, H.; Zhang, S.; Zhang, W.; Huang, J. Involvement of FOXQ1 in NSCLC through regulating EMT and increasing chemosensitivity. Oncotarget 2014, 5, 9689–9702. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, H.; Tian, J.; Wu, L.; Wang, Y.; Xing, Y.; Chen, M. Forkhead box protein C2 promotes epithelial-mesenchymal transition, migration and invasion in cisplatin-resistant human ovarian cancer cell line (SKOV3/CDDP). Cell Physiol. Biochem. 2016, 39, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, H.; Tian, J.; Wu, L.; Wang, Y.; Xing, Y.; Chen, M. Forkhead Box Protein C2 (FOXC2) promotes the resistance of human ovarian cancer cells to cisplatin in vitro and in vivo. Cell Physiol. Biochem. 2016, 39, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Xie, B.; Wang, X.; Yang, X.; Ding, N.; Zhang, J.; Liu, Q.; Tan, G.; Feng, D.; et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015, 363, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhao, J.Y.; Yue, H.; Hu, K.S.; Shen, H.; Guo, Z.G.; Su, X.J. FOXD1 promotes breast cancer proliferation and chemotherapeutic drug resistance by targeting p27. Biochem. Biophys. Res. Commun. 2015, 456, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.F.; Chang, Y.W.; Kuo, K.T.; Shen, Y.S.; Liu, C.Y.; Yu, Y.H.; Cheng, C.C.; Lee, K.Y.; Chen, F.C.; Hsu, M.K.; et al. NF-kappaB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl. Acad. Sci. USA 2016, 113, E2526–E2535. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guo, M.; Wei, Y.; Yu, S.; Li, H.; Wang, Y.; Xu, X.; Cui, Y.; Tian, J.; Liang, L.; et al. FOXO3A confers cetuximab resistance in RAS wild-type metastatic colorectal cancer through c-Myc. Oncotarget 2016, 7, 80888–80900. [Google Scholar] [CrossRef] [PubMed]
- Aldonza, M.B.; Hong, J.Y.; Lee, S.K. Paclitaxel-resistant cancer cell-derived secretomes elicit ABCB1-associated docetaxel cross-resistance and escape from apoptosis through FOXO3a-driven glycolytic regulation. Exp. Mol. Med. 2017, 49, e286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Li, X.; Li, C.; Zhao, L.; Zhou, Y.; Hou, H. Butein sensitizes HeLa cells to cisplatin through the AKT and ERK/p38 MAPK pathways by targeting FOXO3A. Int. J. Mol. Med. 2015, 36, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Chen, Y.; Zhang, Q.; Guo, Y.; Huang, Z.; Dai, L.; Cao, S. 8bromo7methoxychrysin induces apoptosis by regulating Akt/FOXO3A pathway in cisplatinsensitive and resistant ovarian cancer cells. Mol. Med. Rep. 2015, 12, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Chen, S.L.; Cheng, Y.H.; Lin, T.K.; Tsai, C.Y.; Tsai, M.M.; Lin, Y.H.; Huang, Y.H.; Lin, K.H. Chemotherapy resistance and metastasis-promoting effects of thyroid hormone in hepatocarcinoma cells are mediated by suppression of FOXO1 and Bim pathway. Cell Death Dis. 2016, 7, e2324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, C.; Yue, J.; Jiang, Z.; Zhou, R.; Xie, R.; Wang, Y.; Wu, S. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFbeta1 signaling loop in esophageal squamous cell carcinoma. Mol. Carcinog. 2017, 56, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Ko, Y.S.; Kim, Y.; Pyo, J.S.; Jang, B.G.; Kim, M.A.; Lee, J.S.; Chang, M.S.; Park, J.W.; et al. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through met upregulation. Cancer Res. Treat. 2018, 50, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ko, Y.S.; Yoon, J.; Kim, M.A.; Park, J.W.; Kim, W.H.; Choi, Y.; Kim, J.H.; Cheon, Y.; Lee, B.L. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer 2014, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wagle, M.; Eiring, A.M.; Wongchenko, M.; Lu, S.; Guan, Y.; Wang, Y.; Lackner, M.; Amler, L.; Hampton, G.; Deininger, M.W.; et al. A role for FOXO1 in BCR-ABL1-independent tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Leukemia 2016, 30, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Shun, W.W.; Hang, J.B.; Gao, B.L.; Hu, J.A. Posttranslational modifications of FOXO1 regulate epidermal growth factor receptor tyrosine kinase inhibitor resistance for non-small cell lung cancer cells. Tumour Biol. 2015, 36, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.D.; Schinzel, A.C.; Cotter, M.B.; Lis, R.T.; Labella, K.; Lock, Y.J.; Izzo, F.; Guney, I.; Bowden, M.; Li, Y.Y.; et al. Castration resistance in prostate cancer is mediated by the kinase NEK6. Cancer Res. 2017, 77, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, R.; Ren, G.; Li, X.; Wang, J.; Sun, Y.; Liang, J.; Nie, Y.; Wu, K.; Feng, B.; et al. HMGA2-FOXL2 axis regulates metastases and epithelial-to-mesenchymal transition of chemoresistant gastric cancer. Clin. Cancer Res. 2017, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Prado, K.; Zhang, K.X.; Peek, E.M.; Lee, J.; Wang, X.; Huang, J.; Li, G.; Pellegrini, M.; Chin, A.I. Biased expression of the FOXP3Delta3 isoform in aggressive bladder cancer mediates differentiation and cisplatin chemotherapy resistance. Clin. Cancer Res. 2016, 22, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, L.; Jiang, R.; Wang, P.; Xue, H.; Zhan, Y.; Gai, X. Downregulation of FOXP3 inhibits cell proliferation and enhances chemosensitivity to cisplatin in human lung adenocarcinoma. Pathol. Res. Pract. 2017, 213, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Long, Z.J.; Xu, D.; Lv, S.S.; Liu, B.; Wang, C.L.; Xu, J.; Lam, E.W.; Liu, Q. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis 2017, 6, e298. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhu, L.; Gao, J.; Cai, M.; Tan, M.; Liu, J.; Lin, B. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumour Biol. 2015, 36, 7269–7275. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Nakayama, Y.; Yamaguchi, N. Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin-6. J. Biol. Chem. 2017, 292, 8136–8148. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Wade, M.; Nakjang, S.; Chaytor, L.; Grey, J.; Robson, C.N.; Gaughan, L. FOXA1 regulates androgen receptor variant activity in models of castrate-resistant prostate cancer. Oncotarget 2015, 6, 29782–29794. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Tian, A.X.; Wang, Q.S.; Kong, P.Z.; Du, X.; Li, X.Q.; Feng, Y.M. FOXF2 suppresses the FOXC2-mediated epithelial-mesenchymal transition and multidrug resistance of basal-like breast cancer. Cancer Lett. 2015, 367, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nestal de Moraes, G.; Bella, L.; Zona, S.; Burton, M.J.; Lam, E.W. Insights into a critical role of the FOXO3a-FOXM1 axis in DNA damage response and genotoxic drug resistance. Curr. Drug Targets 2016, 17, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.C.; Kashida, Y.; Kulp, S.K.; Wang, D.; Brueggemeier, R.W.; Shapiro, C.L.; Chen, C.S. Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol. Cancer Ther. 2008, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M. FOXO factors and breast cancer: Outfoxing endocrine resistance. Endocr. Relat. Cancer 2016, 23, R113–R130. [Google Scholar] [CrossRef] [PubMed]
- Hannenhalli, S.; Kaestner, K.H. The evolution of FOX genes and their role in development and disease. Nat. Rev. Genet. 2009, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Caburet, S.; Veitia, R.A. Forkhead transcription factors: Key players in health and disease. Trends Genet. 2011, 27, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Deshmukh, H.; Payton, J.E.; Dunham, C.; Scheithauer, B.W.; Tihan, T.; Prayson, R.A.; Guha, A.; Bridge, J.A.; Ferner, R.E.; et al. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin. Cancer Res. 2011, 17, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Laoukili, J.; Stahl, M.; Medema, R.H. FOXM1: At the crossroads of ageing and cancer. Biochim. Biophys. Acta 2007, 1775, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Laoukili, J.; Kooistra, M.R.; Bras, A.; Kauw, J.; Kerkhoven, R.M.; Morrison, A.; Clevers, H.; Medema, R.H. FOXM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Katoh, Y.; Matsumoto, M.; Sato, M.; Ebina, M.; Itoh-Nakadai, A.; Funayama, R.; Nakayama, K.; Unno, M.; Igarashi, K. Regulatory signatures of liver regeneration distilled by integrative analysis of mRNA, histone methylation, and proteomics. J. Biol. Chem. 2017, 292, 8019–8037. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Arceci, A.; Bird, K.; Mills, C.A.; Choudhury, R.; Kernan, J.L.; Zhou, C.; Bae-Jump, V.; Bowers, A.; Emanuele, M.J. VprBP/DCAF1 regulates the degradation and nonproteolytic activation of the cell cycle transcription factor FOXM1. Mol. Cell Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Bakhoum, S.F.; Compton, D.A. Mechanisms of chromosomal instability. Curr. Biol. 2010, 20, R285–R295. [Google Scholar] [CrossRef] [PubMed]
- Weiler, S.M.E.; Pinna, F.; Wolf, T.; Lutz, T.; Geldiyev, A.; Sticht, C.; Knaub, M.; Thomann, S.; Bissinger, M.; Wan, S.; et al. Induction of chromosome instability by activation of yes-associated protein and forkhead box M1 in liver cancer. Gastroenterology 2017, 152, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, R.; Freije, A.; de Pedro, I.; Stoll, S.W.; Elder, J.T.; Gandarillas, A. FOXM1 allows human keratinocytes to bypass the oncogene-induced differentiation checkpoint in response to gain of MYC or loss of p53. Oncogene 2016, 36, 956. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 2004, 25, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Dansen, T.B.; Burgering, B.M. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008, 18, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kano, F.; Murata, M. Translocation of forkhead box O1 to the nuclear periphery induces histone modifications that regulate transcriptional repression of PCK1 in HepG2 cells. Genes Cells 2015, 20, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Daitoku, H.; Sakamaki, J.-i.; Fukamizu, A. Regulation of FOXO transcription factors by acetylation and protein–protein interactions. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, J.M.; Kalimutho, M.; Johansson, P.; Cardenas, D.G.; Kumar, R.; Khanna, K.K. FBXO31 protects against genomic instability by capping FOXM1 levels at the G2/M transition. Oncogene 2017, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Li, B.; Yang, J.; Huen, M.S.; Pan, X.; Tsao, S.W.; Cheung, A.L. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). J. Biol. Chem. 2014, 289, 21508–21518. [Google Scholar] [CrossRef] [PubMed]
- Malonia, S.K.; Dutta, P.; Santra, M.K.; Green, M.R. F-box protein FBXO31 directs degradation of MDM2 to facilitate p53-mediated growth arrest following genotoxic stress. Proc. Natl. Acad. Sci. USA 2015, 112, 8632–8637. [Google Scholar] [CrossRef] [PubMed]
- Bigarella, C.L.; Li, J.; Rimmele, P.; Liang, R.; Sobol, R.W.; Ghaffari, S. FOXO3 Transcription Factor Is Essential for Protecting Hematopoietic Stem and Progenitor Cells from Oxidative DNA Damage. J. Biol. Chem. 2017, 292, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Charitou, P.; Burgering, B.M. Forkhead box(O) in control of reactive oxygen species and genomic stability to ensure healthy lifespan. Antioxid. Redox Signal. 2013, 19, 1400–1419. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Matkar, S.; He, X.; Hua, X. FOXO family in regulating cancer and metabolism. Semin. Cancer Biol. 2018, 50, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, I.; Zaret, K.S.; Santisteban, P. The forkhead factor FOXE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure. Mol. Cell Biol. 2007, 27, 7302–7314. [Google Scholar] [CrossRef] [PubMed]
- Santo, E.E.; Ebus, M.E.; Koster, J.; Schulte, J.H.; Lakeman, A.; van Sluis, P.; Vermeulen, J.; Gisselsson, D.; Ora, I.; Lindner, S.; et al. Oncogenic activation of FOXR1 by 11q23 intrachromosomal deletion-fusions in neuroblastoma. Oncogene 2012, 31, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Goatly, A.; Bacon, C.M.; Nakamura, S.; Ye, H.; Kim, I.; Brown, P.J.; Ruskone-Fourmestraux, A.; Cervera, P.; Streubel, B.; Banham, A.H.; et al. FOXP1 abnormalities in lymphoma: Translocation breakpoint mapping reveals insights into deregulated transcriptional control. Mod. Pathol. 2008, 21, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Okugawa, Y.; Toden, S.; Toiyama, Y.; Kusunoki, M.; Goel, A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive miR-342 in human colorectal cancer. Clin. Cancer Res. 2016, 22, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, X.; Bai, Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 12775–12783. [Google Scholar] [PubMed]
- Song, G.Q.; Zhao, Y. MicroRNA-211, a direct negative regulator of CDC25B expression, inhibits triple-negative breast cancer cells’ growth and migration. Tumour Biol. 2015, 36, 5001–5009. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Shen, G.Z.; Cao, L.Q.; Jiang, X.F.; Peng, H.P.; Shen, G.; Chen, D.; Xue, P. MicroRNA-1269 promotes proliferation in human hepatocellular carcinoma via downregulation of FOXO1. BMC cancer 2014, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Yang, T.; Yuan, S.; Wang, R.; Pan, Z.; Yang, Y.; Huang, G.; Gu, F.; Jiang, B.; et al. Double-negative feedback loop between microRNA-422a and forkhead box (FOX)G1/Q1/E1 regulates hepatocellular carcinoma tumor growth and metastasis. Hepatology 2015, 61, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Batra, A.; Kanthaje, S.; Ghosh, S.; Chakraborti, A. Crosstalk between microRNA-122 and FOX family genes in HepG2 cells. Exp. Biol. Med. 2017, 242, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Lulla, A.R.; Slifker, M.J.; Zhou, Y.; Lev, A.; Einarson, M.B.; Dicker, D.T.; El-Deiry, W.S. miR-6883 family miRNAs target CDK4/6 to induce G1 phase cell-cycle arrest in colon cancer cells. Cancer Res. 2017, 77, 6902–6913. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Du, J.; Xie, K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim. Biophys. Acta 2014, 1845, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Haeusler, R.A.; Lindén, D.; Ericson, E.; Norris, T.; Johansson, A.; Cook, J.R.; Aizawa, K.; Wang, L.; Buettner, C.; Accili, D. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell 2017, 171, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Cautain, B.; Castillo, F.; Musso, L.; Ferreira, B.I.; de Pedro, N.; Rodriguez Quesada, L.; Machado, S.; Vicente, F.; Dallavalle, S.; Link, W. Discovery of a novel, isothiazolonaphthoquinone-based small molecule activator of FOXO nuclear-cytoplasmic shuttling. PLoS ONE 2016, 11, e0167491. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.M.; Ackerson, T.; Ramakrishna, S.; Tretiakova, M.; Wang, I.C.; Kalin, T.V.; Major, M.L.; Gusarova, G.A.; Yoder, H.M.; Costa, R.H.; et al. The forkhead box M1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006, 66, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Millour, J.; Constantinidou, D.; Stavropoulou, A.V.; Wilson, M.S.; Myatt, S.S.; Kwok, J.M.; Sivanandan, K.; Coombes, R.C.; Medema, R.H.; Hartman, J.; et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene 2010, 29, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Gartel, A.L. Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA-damage. PLoS ONE 2012, 7, e31761. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, C.; Toi, M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Bhat, U.G.; Halasi, M.; Gartel, A.L. FOXM1 is a general target for proteasome inhibitors. PLoS ONE 2009, 4, e6593. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L. A new target for proteasome inhibitors: FOXM1. Expert. Opin. Investig Drugs 2010, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jagani, Z.; Song, K.; Kutok, J.L.; Dewar, M.R.; Melet, A.; Santos, T.; Grassian, A.; Ghaffari, S.; Wu, C.; Yeckes-Rodin, H.; et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009, 69, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Vizcaíno, J.A. A golden age for working with public proteomics data. Trends Biochem. Sci. 2017, 42, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Long, N.P.; Jung, K.H.; Yoon, S.J.; Anh, N.H.; Nghi, T.D.; Kang, Y.P.; Yan, H.H.; Min, J.E.; Hong, S.S.; Kwon, S.W. Systematic assessment of cervical cancer initiation and progression uncovers genetic panels for deep learning-based early diagnosis and proposes novel diagnostic and prognostic biomarkers. Oncotarget 2017, 8, 109436–109456. [Google Scholar] [CrossRef] [PubMed]
- Long, N.P.; Yoon, S.J.; Anh, N.H.; Nghi, T.D.; Lim, D.K.; Hong, Y.J.; Hong, S.S.; Kwon, S.W. A systematic review on metabolomics-based diagnostic biomarker discovery and validation in pancreatic cancer. Metabolomics 2018, 14, 109. [Google Scholar] [CrossRef]
- Lu, Z.R.; Qiao, P. Drug delivery in cancer therapy, quo vadis? Mol. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rationalizing combination therapies. Nat. Med. 2017, 23, 1113. [CrossRef] [PubMed]
- Bost, F.; Decoux-Poullot, A.G.; Tanti, J.F.; Clavel, S. Energy disruptors: Rising stars in anticancer therapy? Oncogenesis 2016, 5, e188. [Google Scholar] [CrossRef] [PubMed]
| FOX Members | Model/Cell Type | Corresponding Drug | Function | Ref |
|---|---|---|---|---|
| FOXM1 | Non-small cell lung cancer (NSCLC) patients | Tyrosine kinase inhibitor (TKI) | Contributes to TKI-resistant NSCLC cells Associated with unfavorable prognosis in NSCLC patients | [123] |
| Ovarian cancer patients | Platinum | Overexpressed in ovarian cancer cell lines and cancer cells in patients’ ascites Targeting FOXM1 improves the cytotoxicity of paclitaxel and cisplatinum in platinum-resistant ovarian cancer | [124] | |
| Lung adenocarcinoma | Gefitinib | FOXM1 stimulates acquired resistance to gefitinib in lung adenocarcinoma cells through a MET/Akt-dependent positive feedback loop | [125] | |
| Leukemia patient samples | Chemoresistance | Nuclear FOXM1 contributes to chemoresistance in acute myeloid leukemia (AML) FOXM1 inactivation causes a favorable prognosis and provides fertile ground for strategies to suppress this oncogenic transcription factor in AML | [126] | |
| Colorectal cancer | 5-Fluorouracil | FOXM1 can evoke 5-fluorouracil resistance depending on ATP binding cassette subfamily C member 10 (ABCC10) | [127] | |
| Glioma cells | Temozolomide | FOXM1-mediated repair gene replication factor 5 promotes temozolomide resistance in glioma cells independent of methylguanine-DNA-methyltransferase activation | [128] | |
| Nasopharyngeal carcinoma cells | Paclitaxel | FOXM1 can contribute to drug efflux and paclitaxel resistance by regulating the gene transcription of ABCC5, one of the ABC transporters | [129] | |
| Ovarian cancer patients | Chemo-resistance | The expression of FOXM1 is highly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients | [130] | |
| Bladder cancer | Chemo-resistance | FOXM1 is proposed to directly active ABC G member 2 to enhance drug resistance and drug efflux activation | [131] | |
| Breast cancer patients | Epirubicin | FOXM1 can target nijmegen breakage syndrome gene to modulate DNA damage-stimulated senescence and epirubicin resistance | [132] | |
| Gastric cancer | Docetaxel | FOXM1 might be a new therapeutic target in docetaxel-resistant gastric cancer and can be used as a marker for predicting patient prognosis and monitoring the response to docetaxel | [133] | |
| Cervical cancer | Chemoresistance | The prolyl isomerase Pin1 can modulate chemoresistance by up-regulating FOXM1 and involvement in the Wnt/β-catenin pathway | [134] | |
| Breast cancer patients | Chemoresistance | Targeting X-linked inhibitor of apoptosis gene (XIAP) and Survivin by FOXM1 may contribute to chemoresistance in breast cancer survivors | [135] | |
| Leukemia | Chemoresistance | FOXM1 is overexpressed in B-acute lymphoblastic leukemia (B-ALL) Inhibition of FOXM1 may sensitize B-ALL cells to chemotherapeutic drugs | [136] | |
| Breast cancer | Epirubicin | The suppression of ubiquitination and degradation of FOXM1 by ubiquitin thioesterase OTUB1 has been suggested to play a key role in genotoxic agent resistance | [137] | |
| Breast cancer | Paclitaxel | Paclitaxel resistance can be modulated by deregulating FOXM1 expression to regulate kinesin family member 20A in mitotic catastrophe | [138] | |
| Ovarian cancer | Chemoresistance | Overexpression of FOXM1 can enhance the expression and activity of β-catenin in chemoresistant cells, whereas downregulation of FOXM1 may suppress these events | [139] | |
| Gastric cancer | Oxaliplatin | FOXM1-stimulated resistance to oxaliplatin is partially mediated through its target gene Mcl-1 | [140] | |
| Ovarian cancer | Paclitaxel | Upregulation of FOXM1 contributes to paclitaxel resistance by suppressing mitotic catastrophe | [141] | |
| Ovarian cancer | Cisplatin | FOXM1 can contribute to cisplatin sensitivity by modulating exonuclease 1 | [142] | |
| FOXC1 | Breast cancer patients | Endocrine | FOXC1 expression is related to decreased or undetectable estrogen receptor (ER) expression in recurrent tumors FOXC1 is involved in ERα silencing through counteracting GATA binding protein 3 binding and has been implicated in endocrine resistance | [143] |
| FOXQ1 | Breast cancer | Chemoresistance | Platelet-derived growth factor receptors have been suggested as critical mediators of breast cancer chemoresistance driven by FOXQ1 and have potential implications for investigating novel therapeutic combinations to treat breast cancer | [106] |
| NSCLC | Chemoresistance | Overexpression of FOXQ1 elicits opposing effects on these phenotypes in vivo by regulating epithelial-mesenchymal transition (EMT) and modulating chemosensitivity in NSCLC | [144] | |
| FOXC2 | Ovarian cancer | Cisplatin | FOXC2 stimulates EMT and metastasis in cisplatin-resistant human ovarian cancer cells | [145] |
| FOXC2 promotes the resistance of human ovarian cancer cells to cisplatin by activating the Amkt and MAPK-signaling pathways | [146] | |||
| Nasopharyngeal carcinomas | Chemoresistance | FOXC2 may stimulate chemoresistance through activation of EMT | [147] | |
| FOXD1 | Breast cancer | Chemoresistance | FOXD1 can stimulate breast cancer growth and chemoresistance by modulating p27 | [148] |
| FOXO3a | Lung cancer | Gefitinib | NF-ĸB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance | [149] |
| Colorectal cancer | Cetuximab | FOXO3a contributes to cetuximab resistance in RAS wild-type metastasis through c-Myc | [150] | |
| Multi drug resistance cells | Docetaxel and Paclitaxel | Paclitaxel-resistant cancer cell-derived secretomes escape from apoptosis through FOXO3a-driven glycolytic modulation in association with ABCB1 | [151] | |
| HeLa cells | Cisplatin | Butein may sensitize HeLa cells to cisplatin through the ERK/p38 MAPK and Akt pathways by targeting FOXO3a | [152] | |
| Ovarian cancer | Cisplatin | -8-Bromo-7-methoxychrysin-induced apoptosis in cisplatin-sensitive and -resistant cells can occur through modulation of Akt/FOXO3a | [153] | |
| FOXO1 | Hepatocellular carcinoma | Doxorubicin | Expression of Bim is mediated by FOXO1 and indirectly downregulated by thyroid hormone/hormone receptor, causing chemotherapy resistance and doxorubicin-stimulated metastasis of hepatoma cells | [154] |
| Esophageal squamous cell carcinoma | Chemoresistance | Cancer-associated fibroblasts mediate chemoresistance through a FOXO1/TGFβ signaling loop | [155] | |
| Gastric cancer | Lapatinib | Inactivation of FOXO1 is suggested as a determinant of acquired lapatinib-resistance in HER2-positive breast cancer through upregulation of MET | [156] | |
| Gastric cancer | Cisplatin | FOXO1 may contribute to cisplatin resistance by stimulating the phosphoinositide 3-kinase/Akt pathway | [157] | |
| Leukemia | TKI | Overexpressed FOXO1 can contribute to BCR-ABL1 kinase-independent resistance in chronic myeloid leukemia patients | [158] | |
| NSCLC | TKI | FOXO1 acetylation suppresses cell growth and stimulates apoptosis of NSCLC Posttranslational modifications of FOXO1 modulate EGFR-TKI resistance in NSCLC cells | [159] | |
| FOXJ2 | Prostate cancer | Castration | The phosphorylation of FOXJ2 is associated with increased expression of NEK6 that can mediate castration resistance in prostate cancer | [160] |
| FOXL2 | Gastric cancer | Chemoresistance | The HMGA2-FOXL2 axis can modulate EMT and metastasis of chemoresistant gastric cancer | [161] |
| FOXP3∆3 | Bladder cancer | Cisplatin | Biased expression of the FOXP3∆3 isoform in aggressive bladder cancer contributes to differentiation and cisplatin chemotherapy resistance | [162] |
| FOXP3 | Lung adenocarcinoma | Cisplatin | Downregulation of FOXP3 can enhance chemosensitivity to cisplatin and suppress cell proliferation in human lung adenocarcinoma | [163] |
| FOXP1 | Gastric cancer | Chemoresistance | FOXP1 may interact with nuclear aurora kinase A, which regulates survivin stability by modulating F-box and leucine rich repeat protein 7 in gastric cancer drug resistance and affects prognosis | [164] |
| Ovarian cancer | Chemoresistance | The expression of nuclear FOXP1 is an independent risk factor related to chemotherapy resistance and the prognosis of patients with ovarian cancer | [165] | |
| FOXA1 | Breast cancer | Tamoxifen | Down-regulation of FOXA1 causes cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through stimulation of interleukin-6 | [166] |
| Prostate cancer | Castrate | FOXA1 modulates androgen receptor variant activity in models of castrate-resistant prostate cancer | [167] | |
| FOXF2 | Breast cancer patients | Multidrug resistance | FOXF2 may contribute to multidrug resistance of basal-like breast cancer by suppressing FOXC2-mediated EMT | [168] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach, D.-H.; Long, N.P.; Luu, T.-T.-T.; Anh, N.H.; Kwon, S.W.; Lee, S.K. The Dominant Role of Forkhead Box Proteins in Cancer. Int. J. Mol. Sci. 2018, 19, 3279. https://doi.org/10.3390/ijms19103279
Bach D-H, Long NP, Luu T-T-T, Anh NH, Kwon SW, Lee SK. The Dominant Role of Forkhead Box Proteins in Cancer. International Journal of Molecular Sciences. 2018; 19(10):3279. https://doi.org/10.3390/ijms19103279
Chicago/Turabian StyleBach, Duc-Hiep, Nguyen Phuoc Long, Thi-Thu-Trang Luu, Nguyen Hoang Anh, Sung Won Kwon, and Sang Kook Lee. 2018. "The Dominant Role of Forkhead Box Proteins in Cancer" International Journal of Molecular Sciences 19, no. 10: 3279. https://doi.org/10.3390/ijms19103279
APA StyleBach, D.-H., Long, N. P., Luu, T.-T.-T., Anh, N. H., Kwon, S. W., & Lee, S. K. (2018). The Dominant Role of Forkhead Box Proteins in Cancer. International Journal of Molecular Sciences, 19(10), 3279. https://doi.org/10.3390/ijms19103279