MicroRNAs in Autoimmunity and Hematological Malignancies
Abstract
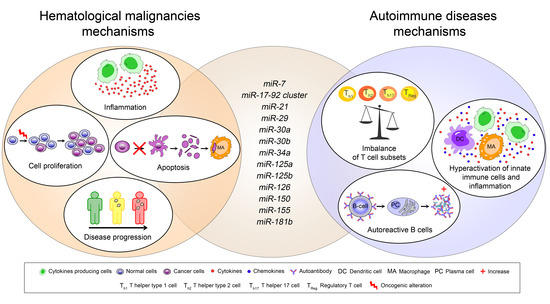
1. Introduction
2. MiRNAs in Autoimmune Diseases
2.1. Involvement of microRNAs in Innate Immune Cells Hyperactivation and Inflammation
2.2. Involvement of MicroRNAs in Autoreactive B Cells
2.3. Role of MicroRNAs in Effector and Regulatory T Cell Imbalance
3. MiRNAs in Hematological Malignancies
3.1. Chronic Lymphocytic Leukemia (CLL)
3.2. Acute Myeloid Leukemia (AML)
3.3. Chronic Myeloid Leukemia (CML)
3.4. Non-Hodgkin Lymphoma (NHL)
3.5. Hodgkin Lymphoma (HL)
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| SIR | Standardized incidence ratio |
| nd | not detected |
| SLE | Systemic Lupus Erythematous |
| RA | Rheumatic Arthritis |
| SSc | Systemic Sclerosis |
| Ps | Psoriasis |
| AIHA | Autoimmune Haemolytic Anemia |
| MS | Multiple Sclerosis |
| ITP | Idiopathic Thrombocytopenic Purpura |
| ALPS | Lymphoproliferative Syndrome |
| NHL | Non-Hodgkin Lymphoma |
| HL | Hodgkin Lymphoma |
| AML | Acute Myeloid Leukemia |
| CML | Chronic Myeloid Leukemia |
| CLL | Chronic Lymphoid Leukemia |
| DCs | Dendritic Cells |
| pDCs | plasmacytoid Dendritic Cells |
| SS | Sjögren’s syndrome |
References
- Ramos, P.S.; Shedlock, A.M.; Langefeld, C.D. Genetics of autoimmune diseases: Insights from population genetics. J. Hum. Genet. 2015, 60, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ngalamika, O.; Zhang, Y.; Yin, H.; Zhao, M.; Gershwin, M.E.; Lu, Q. Epigenetics, autoimmunity and hematologic malignancies: A comprehensive review. J. Autoimmun. 2012, 39, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xiong, K.; Szulwach, K.E.; Zhang, Y.; Wang, Z.; Peng, J.; Fu, M.; Jin, P.; Suzuki, H.I.; Liu, Q. Sjogren syndrome antigen B (SSB)/La promotes global microRNA expression by binding microRNA precursors through stem-loop recognition. J. Biol. Chem. 2013, 288, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.S.; Hertweck, A.; Smith, J.; O’Connor, E.; Graf, D.; Cook, T.; Smale, S.T.; Sakaguchi, S.; Livesey, F.J.; Fisher, A.G.; et al. A role for Dicer in immune regulation. J. Exp. Med. 2006, 203, 2519–2527. [Google Scholar] [CrossRef] [PubMed]
- Gelmez, M.Y.; Coskunpinar, E.; Saracoglu, B.; Deniz, G.; Aktan, M. Investigation of AID, Dicer, and Drosha Expressions in Patients with Chronic Lymphocytic Leukemia. Immunol. Investig. 2017, 46, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.-X.; Fan, L.; Lu, R.-N.; Fang, C.; Shen, W.-Y.; Zou, Z.-J.; Wang, Y.-H.; Zhu, H.-Y.; Miao, K.-R.; Liu, P.; et al. Downregulated Dicer expression predicts poor prognosis in chronic lymphocytic leukemia. Cancer Sci. 2012, 103, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Qiu, G.-R.; Zhou, F.; Gong, L.-Y.; Gao, F.; Sun, K.-L. Overexpression of DICER1 induced by the upregulation of GATA1 contributes to the proliferation and apoptosis of leukemia cells. Int. J. Oncol. 2013, 42, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Lam, I.K.Y.; Chow, J.X.; Lau, C.S.; Chan, V.S.F. MicroRNA-mediated immune regulation in rheumatic diseases. Cancer Lett. 2018, 431, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Nguyen, G.H.; Fujita, M.; Florell, S.R.; Callis Duffin, K.; Krueger, G.G.; O’Connell, R.M. microRNAs in Psoriasis. J. Investig. Dermatol. 2016, 136, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kapsogeorgou, E.K.; Gourzi, V.C.; Manoussakis, M.N.; Moutsopoulos, H.M.; Tzioufas, A.G. Cellular microRNAs (miRNAs) and Sjögren’s syndrome: Candidate regulators of autoimmune response and autoantigen expression. J. Autoimmun. 2011, 37, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Yu, S.; He, Y.; Sun, T.; Liang, W.; Yu, L.; Xu, D.; Li, Q.; Zhang, R. MicroRNA profiling of platelets from immune thrombocytopenia and target gene prediction. Mol. Med. Rep. 2017, 16, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zhai, J.; You, L.; Zhao, Y.; Yang, J.; Weng, Z.; Dai, L.; Wu, Q.; Ruan, C.; He, Y. Plasma microRNAs characterising patients with immune thrombocytopenic purpura. Thromb. Haemost. 2017, 117, 1420–1431. [Google Scholar] [PubMed]
- Ferrer, G.; Navarro, A.; Hodgson, K.; Aymerich, M.; Pereira, A.; Baumann, T.; Monzo, M.; Moreno, C.; Montserrat, E. MicroRNA expression in chronic lymphocytic leukemia developing autoimmune hemolytic anemia. Leuk. Lymphoma 2013, 54, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Iqbal, J.; Teruya-Feldstein, J.; Shen, Y.; Dabrowska, M.J.; Dybkaer, K.; Lim, M.S.; Piva, R.; Barreca, A.; Pellegrino, E.; et al. MicroRNA expression profiling identifies molecular signatures associated with anaplastic large cell lymphoma. Blood 2013, 122, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Rosato, P.; Anastasiadou, E.; Garg, N.; Lenze, D.; Boccellato, F.; Vincenti, S.; Severa, M.; Coccia, E.M.; Bigi, R.; Cirone, M.; et al. Differential regulation of miR-21 and miR-146a by Epstein-Barr virus-encoded EBNA2. Leukemia 2012, 26, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Croce, C.M. MicroRNAs in normal and malignant hematopoiesis. Curr. Opin. Hematol. 2008, 15, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Bonelli, M.; Niederreiter, B.; Puchner, A.; Mayr, G.; Hayer, S.; Koenders, M.I.; van den Berg, W.B.; Smolen, J.; Redlich, K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheumatol. 2011, 63, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, L.P.; Dijkstra, M.K.; van Lom, K.; Robertus, J.-L.; Harms, G.; Blokzijl, T.; Kooistra, K.; van T’veer, M.B.; Rosati, S.; et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: Proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J. Pathol. 2008, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Shimizu, M.; Barbarotto, E.; Nicoloso, M.S.; Dimitri, F.; Sampath, D.; Fabbri, M.; Lerner, S.; Barron, L.L.; Rassenti, L.Z.; et al. microRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood 2010, 116, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Vasilatou, D.; Papageorgiou, S.; Pappa, V.; Papageorgiou, E.; Dervenoulas, J. The role of microRNAs in normal and malignant hematopoiesis. Eur. J. Haematol. 2010, 84, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.L.; Slansky, J.E. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012, 32, 1119–1136. [Google Scholar] [PubMed]
- Fallah, M.; Liu, X.; Ji, J.; Försti, A.; Sundquist, K.; Hemminki, K. Autoimmune diseases associated with non-Hodgkin lymphoma: A nationwide cohort study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, M.; Olsson, A.M.; Steel, K.J.A.; Georgouli, M.; Ranasinghe, U.; Brender Read, C.; Frederiksen, K.S.; Taams, L.S. MicroRNA-155 contributes to enhanced resistance to apoptosis in monocytes from patients with rheumatoid arthritis. J. Autoimmun. 2017, 79, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Elmesmari, A.; Fraser, A.R.; Wood, C.; Gilchrist, D.; Vaughan, D.; Stewart, L.; McSharry, C.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155 regulates monocyte chemokine and chemokine receptor expression in Rheumatoid Arthritis. Rheumatology 2016, 55, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.F.; Millar, D.G.; Dissanayake, D.; Savage, J.C.; Grimshaw, N.K.; Kerr, W.G.; Ohashi, P.S. miR-155 Upregulation in Dendritic Cells Is Sufficient To Break Tolerance In Vivo by Negatively Regulating SHIP1. J. Immunol. 2015, 195, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yim, L.Y.; Tam, R.C.Y.; Chan, A.; Lu, L.; Lau, C.S.; Chan, V.S.-F. MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. Int. J. Mol. Sci. 2016, 17, 1282. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C.M.; Sassi-Gaha, S.; Hope, J.L.; Feghali-Bostwick, C.A.; Katsikis, P.D. Mir-155 is overexpressed in systemic sclerosis fibroblasts and is required for NLRP3 inflammasome-mediated collagen synthesis during fibrosis. Arthritis Res. Ther. 2017, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Gianello, V.; Busatto, S.; Bergese, P.; Andreoli, L.; D’Oro, U.; Zingoni, A.; Tincani, A.; Sozzani, S.; Bosisio, D. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 2018, 3, 98204. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Y.; Zhang, X.; Qin, Y.; Qu, B.; Wu, L.; Ma, J.; Zhou, Z.; Qian, J.; Dai, M.; et al. MicroRNA-130b Ameliorates Murine Lupus Nephritis Through Targeting the Type I Interferon Pathway on Renal Mesangial Cells. Arthritis Rheumatol. 2016, 68, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Fernando, T.; Wu, P.W.; Seo, J.; Ní Gabhann, J.; Piskareva, O.; McCarthy, E.; Howard, D.; O’Connell, P.; Conway, R.; et al. MicroRNA-302d targets IRF9 to regulate the IFN-induced gene expression in SLE. J. Autoimmun. 2017, 79, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhu, H.; Zhou, B.; Xiao, X.; Zuo, X. MicroRNA-130b regulates scleroderma fibrosis by targeting peroxisome proliferator-activated receptor γ. Mod. Rheumatol. 2015, 25, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Affandi, A.J.; Thordardottir, S.; Wichers, C.G.K.; Cossu, M.; Broen, J.C.A.; Moret, F.M.; Bossini-Castillo, L.; Chouri, E.; van Bon, L.; et al. Association of MicroRNA-618 Expression With Altered Frequency and Activation of Plasmacytoid Dendritic Cells in Patients With Systemic Sclerosis. Arthritis Rheumatol. 2017, 69, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Cao, J.; Zhang, F.; Cui, H.; Teng, J.; Li, J.; Liu, Z.; Morehouse, C.; Jallal, B.; Tang, Y.; et al. Type I Interferon Inhibition of MicroRNA-146a Maturation Through Up-Regulation of Monocyte Chemotactic Protein-Induced Protein 1 in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. MicroRNA-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheumatol. 2009, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Gauna, A.E.; Park, Y.-J.; Nayar, G.; Onate, M.; Jin, J.; Stewart, C.M.; Yu, Q.; Cha, S. Dysregulated co-stimulatory molecule expression in a Sjögren’s syndrome mouse model with potential implications by microRNA-146a. Mol. Immunol. 2015, 68, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Lv, A.-K.; Yao, H. Relationship of miRNA-146a to primary Sjögren’s syndrome and to systemic lupus erythematosus: A meta-analysis. Rheumatol. Int. 2017, 37, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Melchor, E.G.; Elmesmari, A.; Tolusso, B.; Tange, C.; Petricca, L.; Gilchrist, D.S.; Di Sante, G.; Keijzer, C.; et al. MicroRNA-34a dependent regulation of AXL controls the activation of dendritic cells in inflammatory arthritis. Nat. Commun. 2017, 8, 15877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, J.; Qin, H.; Ge, Y.; Du, J.; Lin, J.; Zhu, X.; Wang, J.; Xu, J. Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE. Arthritis Res. Ther. 2016, 18, 263. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Gregersen, P.K.; Diamond, B. Regulation of dendritic cell activation by microRNA let-7c and BLIMP1. J. Clin. Investig. 2013, 123, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yuan, L.; Wang, Y.; Hua, C. Enhanced expression of TREM-1 in splenic cDCs in lupus prone mice and it was modulated by miRNA-150. Mol. Immunol. 2017, 81, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hasni, S.A.; Perez, P.; Tandon, M.; Jang, S.-I.; Zheng, C.; Kopp, J.B.; Austin, H.; Balow, J.E.; Alevizos, I.; et al. miR-150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J. Am. Soc. Nephrol. 2013, 24, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Heindryckx, F.; Binet, F.; Ponticos, M.; Rombouts, K.; Lau, J.; Kreuger, J.; Gerwins, P. Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol. Med. 2016, 8, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Jinnin, M.; Kira-Etoh, T.; Makino, K.; Kajihara, I.; Makino, T.; Fukushima, S.; Inoue, Y.; Okamoto, Y.; Hasegawa, M.; et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am. J. Pathol. 2013, 182, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Chouri, E.; Servaas, N.H.; Bekker, C.P.J.; Affandi, A.J.; Cossu, M.; Hillen, M.R.; Angiolilli, C.; Mertens, J.S.; van den Hoogen, L.L.; Silva-Cardoso, S.; et al. Serum microRNA screening and functional studies reveal miR-483-5p as a potential driver of fibrosis in systemic sclerosis. J. Autoimmun. 2018, 89, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhu, H.; Luo, H.; Gao, S.; Dai, X.; Li, Y.; Zuo, X. MicroRNA-202-3p regulates scleroderma fibrosis by targeting matrix metalloproteinase 1. Biomed. Pharmacother. 2017, 87, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad-Farsangi, S.; Farazmand, A.; Mahmoudi, M.; Gharibdoost, F.; Karimizadeh, E.; Noorbakhsh, F.; Faridani, H.; Jamshidi, A.R. MicroRNA-29a induces apoptosis via increasing the Bax:Bcl-2 ratio in dermal fibroblasts of patients with systemic sclerosis. Autoimmunity 2015, 48, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, M.; O’Reilly, S.; Suwara, M.; Bogunia-Kubik, K.; van Laar, J.M. MiR-29a reduces TIMP-1 production by dermal fibroblasts via targeting TGF-β activated kinase 1 binding protein 1, implications for systemic sclerosis. PLoS ONE 2014, 9, e115596. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Suto, A.; Ikeda, K.; Sanayama, Y.; Nakagomi, D.; Iwamoto, T.; Suzuki, K.; Kambe, N.; Matsue, H.; Matsumura, R.; et al. Alteration of circulating miRNAs in SSc: MiR-30b regulates the expression of PDGF receptor β. Rheumatology 2013, 52, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Li, Y.; Zhou, Y.; Jiang, Y.; Chai, J.; Xiao, X.; You, Y.; Zuo, X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-β-regulated fibrosis-related genes expression. J. Clin. Immunol. 2013, 33, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Etoh, M.; Jinnin, M.; Makino, K.; Yamane, K.; Nakayama, W.; Aoi, J.; Honda, N.; Kajihara, I.; Makino, T.; Fukushima, S.; et al. microRNA-7 down-regulation mediates excessive collagen expression in localized scleroderma. Arch. Dermatol. Res. 2013, 305, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Jinnin, M.; Aoi, J.; Hirano, A.; Kajihara, I.; Makino, T.; Sakai, K.; Fukushima, S.; Inoue, Y.; Ihn, H. Discoidin domain receptor 2-microRNA 196a-mediated negative feedback against excess type I collagen expression is impaired in scleroderma dermal fibroblasts. J. Investig. Dermatol. 2013, 133, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-β-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Meisgen, F.; Butler, L.M.; Han, G.; Wang, X.-J.; Söderberg-Nauclér, C.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J. Immunol. 2013, 190, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, Z.; Lou, F.; Zhang, L.; Ke, F.; Bai, J.; Liu, Z.; Liu, J.; Wang, H.; Zhu, H.; et al. NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat. Commun. 2015, 6, 7652. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Gu, J.; Huang, T.; Zhang, C.; Shu, Z.; Li, M.; Hao, Q.; Li, W.; Zhang, W.; Zhao, J.; et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci. Rep. 2016, 6, 20059. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Zhu, W.; Jiang, C.; Xu, J.; Wu, X.; Geng, M.; Hussain, S.; Cai, Y.; Xu, K.; Xu, P.; et al. Down-regulation of miR-10a-5p in synoviocytes contributes to TBX5-controlled joint inflammation. J. Cell. Mol. Med. 2018, 22, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Singh, A.K.; Ahmed, S. MicroRNA-17 Suppresses TNF-α Signaling by Interfering with TRAF2 and cIAP2 Association in Rheumatoid Arthritis Synovial Fibroblasts. J. Immunol. 2016, 197, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, X.; An, Y.; Guo, S.; Li, S.; Song, P.; Chang, Y.; Zhang, S.; Gao, T.; Wang, G.; et al. MicroRNA-17-92 cluster promotes the proliferation and the chemokine production of keratinocytes: Implication for the pathogenesis of psoriasis. Cell Death Dis. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Kurowska-Stolarska, M.; Schütte, F.; Burden, A.D.; McKimmie, C.S.; Graham, G.J. MicroRNA-146 and cell trauma down-regulate expression of the psoriasis-associated atypical chemokine receptor ACKR2. J. Biol. Chem. 2018, 293, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sun, Z.; Dang, E.; Li, B.; Fang, H.; Li, J.; Gao, L.; Zhang, K.; Wang, G. TGFβ/SMAD/microRNA-486-3p Signaling Axis Mediates Keratin 17 Expression and Keratinocyte Hyperproliferation in Psoriasis. J. Investig. Dermatol. 2017, 137, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Leng, H.; Shi, X.; Ji, J.; Fu, J.; Leng, H. MiR-155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed. Pharmacother. 2017, 90, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-J.; Chu, R.-Q.; Ma, J.; Wang, Z.-X.; Zhang, G.-J.; Yang, X.-F.; Song, Z.; Ma, Y.-Y. MicroRNA138 regulates keratin 17 protein expression to affect HaCaT cell proliferation and apoptosis by targeting hTERT in psoriasis vulgaris. Biomed. Pharmacother. 2017, 85, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Bai, M.; Yu, N.-Z.; Wang, X.-J.; Liu, Z. MicroRNA-181b negatively regulates the proliferation of human epidermal keratinocytes in psoriasis through targeting TLR4. J. Cell. Mol. Med. 2017, 21, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Brodin, P.; Wei, T.; Meisgen, F.; Eidsmo, L.; Nagy, N.; Kemeny, L.; Ståhle, M.; Sonkoly, E.; Pivarcsi, A. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J. Investig. Dermatol. 2011, 131, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.-H.; Patterson, H.C.; Pham, D.-H.; Kis-Toth, K.; Kaminski, D.A.; Tsokos, G.C. Deletion of microRNA-155 reduces autoantibody responses and alleviates lupus-like disease in the Fas(lpr) mouse. Proc. Natl. Acad. Sci. USA 2013, 110, 20194–20199. [Google Scholar] [CrossRef] [PubMed]
- Vigorito, E.; Perks, K.L.; Abreu-Goodger, C.; Bunting, S.; Xiang, Z.; Kohlhaas, S.; Das, P.P.; Miska, E.A.; Rodriguez, A.; Bradley, A.; et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 2007, 27, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Kurowska-Stolarska, M.; Tolusso, B.; Benvenuto, R.; Elmesmari, A.; Canestri, S.; Petricca, L.; Mangoni, A.; Fedele, A.L.; Di Mario, C.; et al. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nat. Commun. 2016, 7, 12970. [Google Scholar] [CrossRef] [PubMed]
- Steri, M.; Orrù, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faà, V.; Floris, M.; Deiana, M.; et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Liang, G.; Zhao, M.; Wu, H.; Liang, Y.; Qiu, X.; Tan, Y.; Dai, Y.; Yung, S.; et al. The role of microRNA-1246 in the regulation of B cell activation and the pathogenesis of systemic lupus erythematosus. Clin. Epigenet. 2015, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liang, Y.; Zhao, M.; Liang, G.; Long, H.; Zhao, S.; Wang, Y.; Yin, H.; Zhang, P.; Zhang, Q.; et al. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheumatol. 2012, 64, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, J.; Mu, R.; Gao, Y.; Tan, X.; Li, Y.; Li, Z.; Yang, G. MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn. Arthritis Rheumatol. 2013, 65, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, A.; Adams, B.D.; Lai, M.; Shepherd, J.; Salvador-Bernaldez, M.; Salvador, J.M.; Lu, J.; Nemazee, D.; Xiao, C. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat. Immunol. 2016, 17, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tao, J.-H.; Fang, X.; Xiang, N.; Dai, X.-J.; Jin, L.; Li, X.-M.; Wang, Y.-P.; Li, X.-P. MicroRNA-326 Upregulates B Cell Activity and Autoantibody Production in Lupus Disease of MRL/lpr Mice. Mol. Ther. Nucleic Acids 2018, 11, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, J.; Li, J.; Zou, L.; Zhang, J.; Xie, Z.; Fu, X.; Jiang, S.; Chen, G.; Jia, Q.; et al. Forced miR-146a expression causes autoimmune lymphoproliferative syndrome in mice via downregulation of Fas in germinal center B cells. Blood 2013, 121, 4875–4883. [Google Scholar] [CrossRef] [PubMed]
- Stagakis, E.; Bertsias, G.; Verginis, P.; Nakou, M.; Hatziapostolou, M.; Kritikos, H.; Iliopoulos, D.; Boumpas, D.T. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: MiR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann. Rheum. Dis. 2011, 70, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Garchow, B.; Kiriakidou, M. MicroRNA-21 deficiency protects from lupus-like autoimmunity in the chronic graft-versus-host disease model of systemic lupus erythematosus. Clin. Immunol. 2016, 162, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Schutt, S.; Paz, K.; Zhang, M.; Flynn, R.P.; Bastian, D.; Sofi, M.H.; Nguyen, H.; Dai, M.; Liu, C.; et al. MicroRNA-17-92 is required for T-cell and B-cell pathogenicity in chronic graft-versus-host disease in mice. Blood 2018, 131, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Xu, W.; Qu, Y.; Zhao, M.; Zhu, H.; Liu, H.; Wang, H.; Su, X.; Shao, Z. miR-150 regulates B lymphocyte in autoimmune hemolytic anemia/Evans syndrome by c-Myb. Int. J. Hematol. 2018, 107, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Díaz, R.; Blanco-Dominguez, R.; Lasarte, S.; Tsilingiri, K.; Martín-Gayo, E.; Linillos-Pradillo, B.; de la Fuente, H.; Sánchez-Madrid, F.; Nakagawa, R.; Toribio, M.L.; et al. Thymus-Derived Regulatory T Cell Development Is Regulated by C-Type Lectin-Mediated BIC/MicroRNA 155 Expression. Mol. Cell. Biol. 2017, 37, e00341-16. [Google Scholar] [CrossRef] [PubMed]
- Divekar, A.A.; Dubey, S.; Gangalum, P.R.; Singh, R.R. Dicer insufficiency and microRNA-155 overexpression in lupus regulatory T cells: An apparent paradox in the setting of an inflammatory milieu. J. Immunol. 2011, 186, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Lashine, Y.A.; Salah, S.; Aboelenein, H.R.; Abdelaziz, A.I. Correcting the expression of miRNA-155 represses PP2Ac and enhances the release of IL-2 in PBMCs of juvenile SLE patients. Lupus 2015, 24, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liang, D.; Tang, Y.; Qu, B.; Cui, H.; Luo, X.; Huang, X.; Chen, S.; Higgs, B.W.; Jallal, B.; et al. Identification of microRNA-31 as a novel regulator contributing to impaired interleukin-2 production in T cells from patients with systemic lupus erythematosus. Arthritis Rheumatol. 2012, 64, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Haupt, S.; Kreuzer, J.T.; Hammitzsch, A.; Proft, F.; Neumann, C.; Leipe, J.; Witt, M.; Schulze-Koops, H.; Skapenko, A. Decreased expression of miR-146a and miR-155 contributes to an abnormal Treg phenotype in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Meisgen, F.; Xu, N.; Wei, T.; Janson, P.C.; Obad, S.; Broom, O.; Nagy, N.; Kauppinen, S.; Kemény, L.; Ståhle, M.; et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp. Dermatol. 2012, 21, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, X.; Tan, J.; Li, H.; Qian, W.; Chen, J.; Chen, Q.; Wang, J.; Xu, W.; Tao, C.; et al. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J. Cell. Mol. Med. 2014, 18, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhu, S.; Yuan, M.; Cui, H.; Wang, L.; Luo, X.; Li, J.; Zhou, H.; Tang, Y.; Shen, N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010, 184, 6773–6781. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, X.; Liang, J.; Wu, J.; Yang, Y.; Wang, S.; Shi, W.; Xu, J. MicroRNA-29b contributes to DNA hypomethylation of CD4+ T cells in systemic lupus erythematosus by indirectly targeting DNA methyltransferase 1. J. Dermatol. Sci. 2013, 69, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Liang, Y.; Zhao, M.; Long, H.; Ding, S.; Yin, H.; Lu, Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheumatol. 2011, 63, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Liang, G.; Zhang, P.; Deng, X.; Tang, Q.; Zhai, H.; Chang, C.C.; Su, Y.; Lu, Q. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin. Immunol. 2014, 150, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zeng, J.; Yuan, J.; Deng, X.; Huang, Y.; Chen, L.; Zhang, P.; Feng, H.; Liu, Z.; Wang, Z.; et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J. Clin. Investig. 2018, 128, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Choi, I.Y.; Wang, Y.-C.; Liu, S.; Pham, A.T.; Moon, H.; Smith, D.J.; Rao, D.S.; Boldin, M.P.; et al. miR-146a modulates autoreactive Th17 cell differentiation and regulates organ-specific autoimmunity. J. Clin. Investig. 2017, 127, 3702–3716. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hua, M.; Liu, C.; He, N.; Li, Z.; Ma, D. The aberrant expression of microRNAs and correlations with T cell subsets in patients with immune thrombocytopenia. Oncotarget 2016, 7, 76453–76463. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Hu, S.-Y.; Wang, Z.-Y.; Lin, J.; Jian, S.; Dong, Y.-C.; Wu, X.-F.; Cao, L.-J. Long non-coding RNA MEG3 inhibits microRNA-125a-5p expression and induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura. Biomed. Pharmacother. 2016, 83, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Q.; Hu, S.-Y.; Wang, Z.-Y.; Lin, J.; Jian, S.; Dong, Y.-C.; Wu, X.-F.; Lan, D.; Cao, L.-J. MicroRNA-125-5p targeted CXCL13: A potential biomarker associated with immune thrombocytopenia. Am. J. Transl. Res. 2015, 7, 772–780. [Google Scholar] [PubMed]
- Ichiyama, K.; Gonzalez-Martin, A.; Kim, B.-S.; Jin, H.Y.; Jin, W.; Xu, W.; Sabouri-Ghomi, M.; Xu, S.; Zheng, P.; Xiao, C.; et al. The MicroRNA-183-96-182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression. Immunity 2016, 44, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-G.; Tao, J.-H.; Xiang, N.; Li, X.-M.; Wang, G.-S.; Fang, X.; Dai, C.; Zhang, M.; Chen, Z.; Li, X.-P. Negative Correlation Between miR-326 and Ets-1 in Regulatory T Cells from new-Onset SLE Patients. Inflammation 2016, 39, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pan, W.; Song, X.; Liu, Y.; Shao, X.; Tang, Y.; Liang, D.; He, D.; Wang, H.; Liu, W.; et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat. Med. 2012, 18, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Yu, W.; Li, M.; Wang, H.; Liu, D.; Song, X.; Li, Z.; Tian, Z. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol. Lett. 2015, 166, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Andreeff, M.; Kantarjian, H.M.; Garcia-Manero, G.; Calin, G. a MicroRNAs and noncoding RNAs in hematological malignancies: Molecular, clinical and therapeutic implications. Leukemia 2008, 22, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H. MicroRNAs in hematological malignancies. Blood Rev. 2013, 27, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Frediani, J.N.; Fabbri, M. Essential role of miRNAs in orchestrating the biology of the tumor microenvironment. Mol. Cancer 2016, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Shahi, P.; Werb, Z. microRNA-mediated regulation of the tumor microenvironment. Cell Cycle 2013, 12, 3262–3271. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Paggetti, J.; Haderk, F.; Seiffert, M.; Janji, B.; Distler, U.; Ammerlaan, W.; Kim, Y.J.; Adam, J.; Lichter, P.; Solary, E.; et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015, 126, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Garcia, M.; Murakami, J.L.; Chen, C.-C. Exosome-mediated microenvironment dysregulation in leukemia. Biochim. Biophys. Acta 2016, 1863, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Underbayev, C.; Kasar, S.; Ruezinsky, W.; Degheidy, H.; Schneider, J.S.; Marti, G.; Bauer, S.R.; Fraidenraich, D.; Lightfoote, M.M.; Parashar, V.; et al. Role of mir-15a/16-1 in early B cell development in a mouse model of chronic lymphocytic leukemia. Oncotarget 2016, 7, 60986–60999. [Google Scholar] [CrossRef] [PubMed]
- Klein, U.; Lia, M.; Crespo, M.; Siegel, R.; Shen, Q.; Mo, T.; Ambesi-Impiombato, A.; Califano, A.; Migliazza, A.; Bhagat, G.; et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 2010, 17, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Farahani, M.; Yang, Y.; Johnson, G.G.; Oates, M.; Atherton, M.; Douglas, A.; Kalakonda, N.; Pettitt, A.R. Loss of MIR15A and MIR16-1 at 13q14 is associated with increased TP53 mRNA, de-repression of BCL2 and adverse outcome in chronic lymphocytic leukaemia. Br. J. Haematol. 2014, 167, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Cutrona, G.; Matis, S.; Colombo, M.; Massucco, C.; Baio, G.; Valdora, F.; Emionite, L.; Fabris, S.; Recchia, A.G.; Gentile, M.; et al. Effects of miRNA-15 and miRNA-16 expression replacement in chronic lymphocytic leukemia: Implication for therapy. Leukemia 2017, 31, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Veronese, A.; Pepe, F.; Chiacchia, J.; Pagotto, S.; Lanuti, P.; Veschi, S.; Di Marco, M.; D’Argenio, A.; Innocenti, I.; Vannata, B.; et al. Allele-specific loss and transcription of the miR-15a/16-1 cluster in chronic lymphocytic leukemia. Leukemia 2015, 29, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kasar, S.; Underbayev, C.; Hassan, M.; Ilev, I.; Degheidy, H.; Bauer, S.; Marti, G.; Lutz, C.; Raveche, E.; Batish, M. Alterations in the mir-15a/16-1 Loci Impairs Its Processing and Augments B-1 Expansion in De Novo Mouse Model of Chronic Lymphocytic Leukemia (CLL). PLoS ONE 2016, 11, e0149331. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Bottoni, A.; Shimizu, M.; Spizzo, R.; Nicoloso, M.S.; Rossi, S.; Barbarotto, E.; Cimmino, A.; Adair, B.; Wojcik, S.E.; et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA 2011, 305, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Chen, L.; Rassenti, L.Z.; Ghia, E.M.; Li, H.; Jepsen, K.; Smith, E.N.; Messer, K.; Frazer, K.A.; Kipps, T.J. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood 2014, 124, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Visone, R.; Veronese, A.; Rassenti, L.Z.; Balatti, V.; Pearl, D.K.; Acunzo, M.; Volinia, S.; Taccioli, C.; Kipps, T.J.; Croce, C.M. miR-181b is a biomarker of disease progression in chronic lymphocytic leukemia. Blood 2011, 118, 3072–3079. [Google Scholar] [CrossRef] [PubMed]
- Bresin, A.; Callegari, E.; D’Abundo, L.; Cattani, C.; Bassi, C.; Zagatti, B.; Narducci, M.G.; Caprini, E.; Pekarsky, Y.; Croce, C.M.; et al. miR-181b as a therapeutic agent for chronic lymphocytic leukemia in the Eµ-TCL1 mouse model. Oncotarget 2015, 6, 19807–19818. [Google Scholar] [CrossRef] [PubMed]
- Fulci, V.; Chiaretti, S.; Goldoni, M.; Azzalin, G.; Carucci, N.; Tavolaro, S.; Castellano, L.; Magrelli, A.; Citarella, F.; Messina, M.; et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007, 109, 4944–4951. [Google Scholar] [CrossRef] [PubMed]
- Ferracin, M.; Zagatti, B.; Rizzotto, L.; Cavazzini, F.; Veronese, A.; Ciccone, M.; Saccenti, E.; Lupini, L.; Grilli, A.; De Angeli, C.; et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol. Cancer 2010, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Wiestner, A.; Rosenwald, A.; Barry, T.S.; Wright, G.; Davis, R.E.; Henrickson, S.E.; Zhao, H.; Ibbotson, R.E.; Orchard, J.A.; Davis, Z.; et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood 2003, 101, 4944–4951. [Google Scholar] [CrossRef] [PubMed]
- Carabia, J.; Carpio, C.; Abrisqueta, P.; Jiménez, I.; Purroy, N.; Calpe, E.; Palacio, C.; Bosch, F.; Crespo, M. Microenvironment regulates the expression of MIR-21 and tumor suppressor genes PTEN, PIAS3 and PDCD4 through ZAP-70 in chronic lymphocytic leukemia. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lafuente, N.; Alcaraz-García, M.-J.; Sebastián-Ruiz, S.; García-Serna, A.-M.; Gómez-Espuch, J.; Moraleda, J.-M.; Minguela, A.; García-Alonso, A.-M.; Parrado, A. IL-4 Up-Regulates MiR-21 and the MiRNAs Hosted in the CLCN5 Gene in Chronic Lymphocytic Leukemia. PLoS ONE 2015, 10, e0124936. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Ferrajoli, A.; Shanafelt, T.D.; Ivan, C.; Shimizu, M.; Rabe, K.G.; Nouraee, N.; Ikuo, M.; Ghosh, A.K.; Lerner, S.; Rassenti, L.Z.; et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood 2013, 122, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Grgurevic, S.; Liu, Z.; Harris, D.; Rozovski, U.; Calin, G.A.; Keating, M.J.; Estrov, Z. Signal transducer and activator of transcription-3 induces microRNA-155 expression in chronic lymphocytic leukemia. PLoS ONE 2013, 8, e64678. [Google Scholar] [CrossRef] [PubMed]
- Costinean, S.; Zanesi, N.; Pekarsky, Y.; Tili, E.; Volinia, S.; Heerema, N.; Croce, C.M. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Kipps, T.J. MicroRNAs and B cell receptor signaling in chronic lymphocytic leukemia. Leuk. Lymphoma 2013, 54, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Chen, L.; Zhang, S.; Mraz, M.; Fecteau, J.-F.; Yu, J.; Ghia, E.M.; Zhang, L.; Bao, L.; Rassenti, L.Z.; et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood 2014, 124, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.K.; Krysov, S.; Davies, A.J.; Steele, A.J.; Packham, G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood 2011, 118, 4313–4320. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, S.; Veronese, A.; Soranno, A.; Lanuti, P.; Di Marco, M.; Russo, M.V.; Ramassone, A.; Marchisio, M.; Simeone, P.; Guanciali-Franchi, P.E.; et al. Hsa-miR-155-5p drives aneuploidy at early stages of cellular transformation. Oncotarget 2018, 9, 13036–13047. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Garofalo, M.; Martelli, M.P.; Briesewitz, R.; Wang, L.; Fernandez-Cymering, C.; Volinia, S.; Liu, C.-G.; Schnittger, S.; Haferlach, T.; et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc. Natl. Acad. Sci. USA 2008, 105, 3945–3950. [Google Scholar] [CrossRef] [PubMed]
- Jongen-Lavrencic, M.; Sun, S.M.; Dijkstra, M.K.; Valk, P.J.M.; Löwenberg, B. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood 2008, 111, 5078–5085. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, G.; Augugliaro, L.; Salemi, D.; Agueli, C.; La Rosa, M.; Dagnino, L.; Civiletto, G.; Messana, F.; Marfia, A.; Bica, M.G.; et al. Differential expression of specific microRNA and their targets in acute myeloid leukemia. Am. J. Hematol. 2010, 85, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Ludwig, M.; Leibundgut, E.O.; Mueller, B.U.; Ratschiller, D.; Solenthaler, M.; Fey, M.F.; Pabst, T. Risk assessment in patients with acute myeloid leukemia and a normal karyotype. Clin. Cancer Res. 2005, 11, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, D.; Grundler, R.; Wurm, A.A.; Bräuer-Hartmann, D.; Katzerke, C.; Hartmann, J.-U.; Madan, V.; Müller-Tidow, C.; Duyster, J.; Tenen, D.G.; et al. NF-κB/STAT5/miR-155 network targets PU.1 in FLT3-ITD-driven acute myeloid leukemia. Leukemia 2015, 29, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.A.; Kagele, D.A.; Eiring, A.M.; Kim, C.N.; Hu, R.; Runtsch, M.C.; Alexander, M.; Huffaker, T.B.; Lee, S.-H.; Patel, A.B.; et al. miR-155 promotes FLT3-ITD-induced myeloproliferative disease through inhibition of the interferon response. Blood 2017, 129, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Narayan, N.; Morenos, L.; Phipson, B.; Willis, S.N.; Brumatti, G.; Eggers, S.; Lalaoui, N.; Brown, L.M.; Kosasih, H.J.; Bartolo, R.C.; et al. Functionally distinct roles for different miR-155 expression levels through contrasting effects on gene expression, in acute myeloid leukaemia. Leukemia 2017, 31, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Hornick, N.I.; Doron, B.; Abdelhamed, S.; Huan, J.; Harrington, C.A.; Shen, R.; Cambronne, X.A.; Chakkaramakkil Verghese, S.; Kurre, P. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci. Signal. 2016, 9, ra88. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Heaphy, C.E.A.; Havelange, V.; Fabbri, M.; Volinia, S.; Tsao, T.; Zanesi, N.; Kornblau, S.M.; Marcucci, G.; Calin, G.A.; et al. MicroRNA 29b functions in acute myeloid leukemia. Blood 2009, 114, 5331–5341. [Google Scholar] [CrossRef] [PubMed]
- Ngankeu, A.; Ranganathan, P.; Havelange, V.; Nicolet, D.; Volinia, S.; Powell, B.L.; Kolitz, J.E.; Uy, G.L.; Stone, R.M.; Kornblau, S.M.; et al. Discovery and functional implications of a miR-29b-1/miR-29a cluster polymorphism in acute myeloid leukemia. Oncotarget 2018, 9, 4354–4365. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.K.; Perez, A.W.; White, E.S.; Lian, J.B.; Stein, J.L.; Stein, G.S. An AML1-ETO/miR-29b-1 regulatory circuit modulates phenotypic properties of acute myeloid leukemia cells. Oncotarget 2017, 8, 39994–40005. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Radmacher, M.D.; Maharry, K.; Mrózek, K.; Ruppert, A.S.; Paschka, P.; Vukosavljevic, T.; Whitman, S.P.; Baldus, C.D.; Langer, C.; et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schwind, S.; Santhanam, R.; Eisfeld, A.-K.; Chiang, C.-L.; Lankenau, M.; Yu, B.; Hoellerbauer, P.; Jin, Y.; Tarighat, S.S.; et al. Targeting the RAS/MAPK pathway with miR-181a in acute myeloid leukemia. Oncotarget 2016, 7, 59273–59286. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Quelen, C.; Rosati, R.; Mansat-De Mas, V.; La Starza, R.; Bastard, C.; Lippert, E.; Talmant, P.; Lafage-Pochitaloff, M.; Leroux, D.; et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J. Exp. Med. 2008, 205, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Nguyen, D.; Chen, C.; Shields, L.; Lodish, H.F. MicroRNA-125b transforms myeloid cell lines by repressing multiple mRNA. Haematologica 2012, 97, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, M.; Haga, C.L.; Das, S.; Leu, C.-M.; Hodson, D.; Josson, S.; Turner, M.; Cooper, M.D. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int. Immunol. 2010, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-M.; Lin, K.-Y.; Chen, Y.-Q. Diverse functions of miR-125 family in different cell contexts. J. Hematol. Oncol. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.H.; Wang, S.L.; Zhao, J.T.; Lin, Z.J.; Chen, L.Y.; Su, R.; Xie, S.T.; Carter, B.Z.; Xu, B. miR-150 exerts antileukemia activity in vitro and in vivo through regulating genes in multiple pathways. Cell Death Dis. 2016, 7, e2371. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Li, R.-C.; Lu, J.; Meng, F.-K.; Feng, Y.-K.; Fang, M.-H. MicroRNA-192 regulates cell proliferation and cell cycle transition in acute myeloid leukemia via interaction with CCNT2. Int. J. Hematol. 2017, 106, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bhayadia, R.; Krowiorz, K.; Haetscher, N.; Jammal, R.; Emmrich, S.; Obulkasim, A.; Fiedler, J.; Schwarzer, A.; Rouhi, A.; Heuser, M.; et al. Endogenous Tumor Suppressor microRNA-193b: Therapeutic and Prognostic Value in Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversana, C.; Giorgio, C.; D’Amato, L.; Lania, G.; Matarese, F.; Saeed, S.; Di Costanzo, A.; Belsito Petrizzi, V.; Ingenito, C.; Martens, J.H.A.; et al. miR-194-5p/BCLAF1 deregulation in AML tumorigenesis. Leukemia 2018, 32, 573. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Zhou, B.; Li, H.; He, L.; Wang, C.; Wang, Z.; Zhu, L.; Chen, M.; Gao, S. A novel miR-375-HOXB3-CDCA3/DNMT3B regulatory circuitry contributes to leukemogenesis in acute myeloid leukemia. BMC Cancer 2018, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-J.; Lin, J.; Zhou, J.-D.; Li, X.-X.; Zhang, W.; Guo, H.; Xu, Z.-J.; Yan, Y.; Ma, J.-C.; Qian, J. High bone marrow miR-19b level predicts poor prognosis and disease recurrence in de novo acute myeloid leukemia. Gene 2018, 640, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zhang, X.; Hao, X.; Li, Y.; Chen, Z.; Ding, Y.; Shi, H.; Bai, J.; Gao, Y.; Cheng, T.; et al. Upregulation of miR-99a is associated with poor prognosis of acute myeloid leukemia and promotes myeloid leukemia cell expansion. Oncotarget 2016, 7, 78095–78109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Wang, J.-H.; Yang, M.; Wang, H.-P.; Jin, J. MiR-362-5p as a novel prognostic predictor of cytogenetically normal acute myeloid leukemia. J. Transl. Med. 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, Q.; Ren, Y.; Zhu, H.Q.; Huang, Z. MicroRNA-126 attenuates cell apoptosis by targeting TRAF7 in acute myeloid leukemia cells. Biochem. Cell Biol. 2018, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Xu, X. Knockdown of LncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J. Cell. Biochem. 2018, 119, 6296–6308. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, X.; Liu, Q.; Xiao, Y.; Pan, S.; Jia, L. Aberrant mannosylation profile and FTX/miR-342/ALG3-axis contribute to development of drug resistance in acute myeloid leukemia. Cell Death Dis. 2018, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Wang, B.; Li, X.; Jiang, G. miRNAs in acute myeloid leukemia. Oncotarget 2017, 8, 3666–3682. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.A.; O’Connell, R.M. MicroRNAs and acute myeloid leukemia: Therapeutic implications and emerging concepts. Blood 2017, 130, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Gabra, M.M.; Salmena, L. microRNAs and Acute Myeloid Leukemia Chemoresistance: A Mechanistic Overview. Front. Oncol. 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Faderl, S.; Talpaz, M.; Estrov, Z.; O’Brien, S.; Kurzrock, R.; Kantarjian, H.M. The biology of chronic myeloid leukemia. N. Engl. J. Med. 1999, 341, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dai, J.; Gu, D.; Huang, Q.; Tian, L. MicroRNA-7 inhibits cell proliferation of chronic myeloid leukemia and sensitizes it to imatinib in vitro. Biochem. Biophys. Res. Commun. 2017, 494, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.G.; Antman, K.H. Imatinib mesylate—A new oral targeted therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Song, Y.; Zheng, W.; Ma, W. miRNA143 Induces K562 Cell Apoptosis Through Downregulating BCR-ABL. Med. Sci. Monit. 2016, 22, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Xishan, Z.; Ziying, L.; Jing, D.; Gang, L. MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL oncogene in chronic myeloid leukemia. Sci. Rep. 2015, 5, 12460. [Google Scholar] [CrossRef] [PubMed]
- Espadinha, A.-S.; Prouzet-Mauléon, V.; Claverol, S.; Lagarde, V.; Bonneu, M.; Mahon, F.-X.; Cardinaud, B. A tyrosine kinase-STAT5-miR21-PDCD4 regulatory axis in chronic and acute myeloid leukemia cells. Oncotarget 2017, 8, 76174–76188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Liu, X.; Nie, Z.; Luo, J. Long noncoding RNA MEG3 inhibits proliferation of chronic myeloid leukemia cells by sponging microRNA21. Biomed. Pharmacother. 2018, 104, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Z.; Pu, Q.-H.; Lin, X.-H.; Liu, M.-Y.; Wu, L.-R.; Wu, Q.-Q.; Chen, Y.-H.; Liao, F.-F.; Zhu, J.-Y.; Jin, X.-B. Silencing of miR-21 sensitizes CML CD34+ stem/progenitor cells to imatinib-induced apoptosis by blocking PI3K/AKT pathway. Leuk. Res. 2015, 39, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Eiring, A.M.; Harb, J.G.; Neviani, P.; Garton, C.; Oaks, J.J.; Spizzo, R.; Liu, S.; Schwind, S.; Santhanam, R.; Hickey, C.J.; et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 2010, 140, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Yokoyama, A. BCR/ABL increases EZH2 levels which regulates XIAP expression via miRNA-219 in chronic myeloid leukemia cells. Leuk. Res. 2016, 45, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, D.; Shao, K.; Ye, B.; Huang, J.; Gao, Y. MiR-15a-5p negatively regulates cell survival and metastasis by targeting CXCL10 in chronic myeloid leukemia. Am. J. Transl. Res. 2017, 9, 4308–4316. [Google Scholar] [PubMed]
- Zhou, H.; Li, Y.; Liu, B.; Shan, Y.; Li, Y.; Zhao, L.; Su, Z.; Jia, L. Downregulation of miR-224 and let-7i contribute to cell survival and chemoresistance in chronic myeloid leukemia cells by regulating ST3GAL IV expression. Gene 2017, 626, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, L.; Guo, Z.-K.; Zheng, X.-L.; Wang, L.-S.; Sun, H.-Y.; Jin, Z.-G.; Wang, H.-X. The epigenetically-regulated miR-34a targeting c-SRC suppresses RAF/MEK/ERK signaling pathway in K-562 cells. Leuk. Res. 2017, 55, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Eis, P.S.; Tam, W.; Sun, L.; Chadburn, A.; Li, Z.; Gomez, M.F.; Lund, E.; Dahlberg, J.E. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 2005, 102, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Kluiver, J.; Poppema, S.; de Jong, D.; Blokzijl, T.; Harms, G.; Jacobs, S.; Kroesen, B.-J.; van den Berg, A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J. Pathol. 2005, 207, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Kim, S.-W.; McKeller, M.R.; Dahia, P.L.M.; Aguiar, R.C.T. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Liu, Z.B.; Wang, W.G.; Sun, C.B.; Wei, P.; Yang, Y.L.; You, M.J.; Yu, B.H.; Li, X.Q.; Zhou, X.Y. HDAC6 regulates microRNA-27b that suppresses proliferation, promotes apoptosis and target MET in diffuse large B-cell lymphoma. Leukemia 2018, 32, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.M.; Inguscio, A.; Kunderfranco, P.; Cortesi, A.; Elia, L.; Quintavalle, M. MicroRNA-26a/cyclin-dependent kinase 5 axis controls proliferation, apoptosis and in vivo tumor growth of diffuse large B-cell lymphoma cell lines. Cell Death Dis. 2017, 8, e2890. [Google Scholar] [CrossRef] [PubMed]
- Kozloski, G.A.; Jiang, X.; Bhatt, S.; Ruiz, J.; Vega, F.; Shaknovich, R.; Melnick, A.; Lossos, I.S. miR-181a negatively regulates NF-κB signaling and affects activated B-cell-like diffuse large B-cell lymphoma pathogenesis. Blood 2016, 127, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Cogliatti, S.B.; Imig, J.; Renner, C.; Neuenschwander, S.; Rehrauer, H.; Schlapbach, R.; Dirnhofer, S.; Tzankov, A.; Müller, A. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood 2011, 117, 6227–6236. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Tzankov, A.; Flori, M.; Schmid, C.A.; Bader, A.G.; Müller, A. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 2012, 26, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, D.M.; Banham, A.H. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk. Lymphoma 2017, 58, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Gorniak, P.; Szydlowski, M.; Sewastianik, T.; Bialopiotrowicz, E.; Polak, A.; Warzocha, K.; Juszczynski, P. MiR-17-92 represses PTPROt and PP2A phosphatases and amplifies tonic BCR signaling in DLBCL cells. Exp. Hematol. 2017, 46, 56.e1–61.e1. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé-Izquierdo, N.; de Yébenes, V.G.; Álvarez-Prado, A.F.; Mur, S.M.; Lopez Del Olmo, J.A.; Roa, S.; Vazquez, J.; Ramiro, A.R. miR-28 regulates the germinal center reaction and blocks tumor growth in preclinical models of non-Hodgkin lymphoma. Blood 2017, 129, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lwin, T.; Zhao, J.-J.; Tam, W.; Choi, Y.S.; Moscinski, L.C.; Dalton, W.S.; Sotomayor, E.M.; Wright, K.L.; Tao, J. Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin’s B-cell lymphomas. Leukemia 2011, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lenze, D.; Leoncini, L.; Hummel, M.; Volinia, S.; Liu, C.G.; Amato, T.; De Falco, G.; Githanga, J.; Horn, H.; Nyagol, J.; et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia 2011, 25, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, Y.; McBride, K.M.; Jankovic, M.; Gazumyan, A.; Thai, T.-H.; Robbiani, D.F.; Di Virgilio, M.; Reina San-Martin, B.; Heidkamp, G.; Schwickert, T.A.; et al. MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 2008, 28, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef]
- Mazzoccoli, L.; Robaina, M.C.; Apa, A.G.; Bonamino, M.; Pinto, L.W.; Queiroga, E.; Bacchi, C.E.; Klumb, C.E. MiR-29 silencing modulates the expression of target genes related to proliferation, apoptosis and methylation in Burkitt lymphoma cells. J. Cancer Res. Clin. Oncol. 2018, 144, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Sander, S.; Bullinger, L.; Klapproth, K.; Fiedler, K.; Kestler, H.A.; Barth, T.F.E.; Möller, P.; Stilgenbauer, S.; Pollack, J.R.; Wirth, T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood 2008, 112, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Ralfkiaer, U.; Hagedorn, P.H.; Bangsgaard, N.; Løvendorf, M.B.; Ahler, C.B.; Svensson, L.; Kopp, K.L.; Vennegaard, M.T.; Lauenborg, B.; Zibert, J.R.; et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL). Blood 2011, 118, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mercado, M.; Manterola, L.; Lawrie, C.H. MicroRNAs in Lymphoma: Regulatory Role and Biomarker Potential. Curr. Genom. 2015, 16, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Laginestra, M.A.; Piccaluga, P.P.; Fuligni, F.; Rossi, M.; Agostinelli, C.; Righi, S.; Sapienza, M.R.; Motta, G.; Gazzola, A.; Mannu, C.; et al. Pathogenetic and diagnostic significance of microRNA deregulation in peripheral T-cell lymphoma not otherwise specified. Blood Cancer J. 2014, 4, 259. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, B.; Li, K.; Wang, L.; Zhao, X.; Xue, F.; Shi, R.; Zheng, J. MicroRNA Signatures in Diagnosis and Prognosis of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, W.; Huang, R.; Li, L.; Wang, X.; Li, L.; Fu, X.; Sun, Z.; Li, Z.; Chen, Q.; et al. MicroRNA-155 is a potential molecular marker of natural killer/T-cell lymphoma. Oncotarget 2016, 7, 53808–53819. [Google Scholar] [CrossRef] [PubMed]
- Foss, F.M.; Querfeld, C.; Porcu, P.; Kim, Y.H.; Pacheco, T.; Halwani, A.S.; DeSimone, J.; William, B.M.; Seto, A.G.; Ruckman, J.; et al. Phase 1 trial evaluating MRG-106, a synthetic inhibitor of microRNA-155, in patients with cutaneous t-cell lymphoma (CTCL). J. Clin. Oncol. 2017, 35, 7564. [Google Scholar] [CrossRef]
- Querfeld, C.; Pacheco, T.; Foss, F.M.; Halwani, A.S.; Porcu, P.; Seto, A.G.; Ruckman, J.; Landry, M.L.; Jackson, A.L.; Pestano, L.A.; et al. Preliminary Results of a Phase 1 Trial Evaluating MRG-106, a Synthetic microRNA Antagonist (LNA antimiR) of microRNA-155, in Patients with CTCL. Blood 2016, 128, 1829. [Google Scholar]
- Kuppers, D.A.; Schmitt, T.M.; Hwang, H.C.; Samraj, L.; Clurman, B.E.; Fero, M.L. The miR-106a~363Xpcl1 miRNA cluster induces murine T cell lymphoma despite transcriptional activation of the p27Kip1 cell cycle inhibitor. Oncotarget 2017, 8, 50680–50691. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Chen, J.; Wu, B.; Wang, Y.J.; Guo, K.Y. MicroRNA-150 enhances radiosensitivity by inhibiting the AKT pathway in NK/T cell lymphoma. J. Exp. Clin. Cancer Res. 2018, 37, 18. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Saunders, N.J.; Soneji, S.; Palazzo, S.; Dunlop, H.M.; Cooper, C.D.O.; Brown, P.J.; Troussard, X.; Mossafa, H.; Enver, T.; et al. MicroRNA expression in lymphocyte development and malignancy. Leukemia 2008, 22, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Leucci, E.; Zriwil, A.; Gregersen, L.H.; Jensen, K.T.; Obad, S.; Bellan, C.; Leoncini, L.; Kauppinen, S.; Lund, A.H. Inhibition of miR-9 de-represses HuR and DICER1 and impairs Hodgkin lymphoma tumour outgrowth in vivo. Oncogene 2012, 31, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kluiver, J.; Koerts, J.; de Jong, D.; Rutgers, B.; Abdul Razak, F.R.; Terpstra, M.; Plaat, B.E.; Nolte, I.M.; Diepstra, A.; et al. miR-24-3p Is Overexpressed in Hodgkin Lymphoma and Protects Hodgkin and Reed-Sternberg Cells from Apoptosis. Am. J. Pathol. 2017, 187, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Ben Dhiab, M.; Ziadi, S.; Ksiaa, F.; Louhichi, T.; Ben Gacem, R.; Ben Zineb, A.; Amara, K.; Hachana, M.; Trimeche, M. Methylation of miR124a-1, miR124a-2, and miR124a-3 in Hodgkin lymphoma. Tumour Biol. 2015, 36, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; So, C.C.; Loong, F.; Chung, L.P.; Lam, W.W.L.; Liang, R.; Li, G.K.H.; Jin, D.-Y.; Chim, C.S. Epigenetic inactivation of the miR-124-1 in haematological malignancies. PLoS ONE 2011, 6, e19027. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Díaz, T.; Cordeiro, A.; Beyá, M.D.; Ferrer, G.; Fuster, D.; Martinez, A.; Monzó, M. Epigenetic regulation of microRNA expression in Hodgkin lymphoma. Leuk. Lymphoma 2015, 56, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ushmorov, A.; Leithäuser, F.; Guan, H.; Steidl, C.; Färbinger, J.; Pelzer, C.; Vogel, M.J.; Maier, H.J.; Gascoyne, R.D.; et al. FOXO1 is a tumor suppressor in classical Hodgkin lymphoma. Blood 2012, 119, 3503–3511. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Liu, X.; Försti, A.; Ji, J.; Sundquist, J.; Sundquist, K. Subsequent leukaemia in autoimmune disease patients. Br. J. Haematol. 2013, 161, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Brewster, D.H.; Black, R.J.; Macfarlane, G.J. Risk of malignancy among patients with rheumatic conditions. Int. J. Cancer 2000, 88, 497–502. [Google Scholar] [CrossRef]
- Ekström, K.; Hjalgrim, H.; Brandt, L.; Baecklund, E.; Klareskog, L.; Ekbom, A.; Askling, J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheumatol. 2003, 48, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Abásolo, L.; Júdez, E.; Descalzo, M.A.; González-Alvaro, I.; Jover, J.A.; Carmona, L.; EMECAR Study Group. Cancer in rheumatoid arthritis: Occurrence, mortality, and associated factors in a South European population. Semin. Arthritis Rheumatol. 2008, 37, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X.; Sundquist, K.; Sundquist, J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatology 2008, 47, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Parikh-Patel, A.; White, R.H.; Allen, M.; Cress, R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control 2009, 20, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chang, Y.-T.; Wang, C.-B.; Wu, C.-Y. Malignancy in systemic lupus erythematosus: A nationwide cohort study in Taiwan. Am. J. Med. 2010, 123, 1150.e1-6. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, L.; Faurschou, M.; Mogensen, M.; Jacobsen, S. High incidence of potentially virus-induced malignancies in systemic lupus erythematosus: A long-term followup study in a Danish cohort. Arthritis Rheumatol. 2011, 63, 3032–3037. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Park, J.K.; Lee, Y.J.; Yang, J.A.; Lee, E.Y.; Song, Y.W.; Lee, E.B. Comparison of cancer incidence among patients with rheumatic disease: A retrospective cohort study. Arthritis Res. Ther. 2014, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Chiou, M.-J.; Kuo, C.-F.; Lin, Y.-C.; Yu, K.-H.; See, L.-C. No overall increased risk of cancer in patients with rheumatoid arthritis: A nationwide dynamic cohort study in Taiwan. Rheumatol. Int. 2014, 34, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, S.; Efthimiou, P. Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk? BioMed Res. Int. 2016, 2016, 8631061. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela-Svahn, A.; Pukkala, E.; Läärä, E.; Poikolainen, K.; Karvonen, J. Psoriasis, its treatment, and cancer in a cohort of Finnish patients. J. Investig. Dermatol. 2000, 114, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Dombi, G.W.; Severson, R.K.; Mayes, M.D. Risk of malignancy in scleroderma: A population-based cohort study. Arthritis Rheumatol. 2005, 52, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.K.; McLaughlin, J.K.; Gridley, G.; Nyrén, O. Incidence of cancer among patients with systemic sclerosis. Cancer 1995, 76, 910–914. [Google Scholar] [CrossRef]
- Olesen, A.B.; Svaerke, C.; Farkas, D.K.; Sørensen, H.T. Systemic sclerosis and the risk of cancer: A nationwide population-based cohort study. Br. J. Dermatol. 2010, 163, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Fored, C.M.; Baecklund, E.; Brandt, L.; Backlin, C.; Ekbom, A.; Sundström, C.; Bertilsson, L.; Cöster, L.; Geborek, P.; et al. Haematopoietic malignancies in rheumatoid arthritis: Lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann. Rheum. Dis. 2005, 64, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Cibere, J.; Sibley, J.; Haga, M. Systemic lupus erythematosus and the risk of malignancy. Lupus 2001, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Björnådal, L.; Löfström, B.; Yin, L.; Lundberg, I.E.; Ekbom, A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand. J. Rheumatol. 2002, 31, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Tarr, T.; Gyorfy, B.; Szekanecz, E.; Bhattoa, H.P.; Zeher, M.; Szegedi, G.; Kiss, E. Occurrence of malignancies in Hungarian patients with systemic lupus erythematosus: Results from a single center. Ann. N. Y. Acad. Sci. 2007, 1108, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Parikh-Patel, A.; White, R.H.; Allen, M.; Cress, R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control 2008, 19, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Kim, H.O.; Yoon, H.S.; Lee, J.; Lee, W.C.; Ko, H.-J.; Ju, J.H.; Cho, C.-S.; Kim, H.-Y.; Park, S.-H. Incidence of cancer among female patients with systemic lupus erythematosus in Korea. Clin. Rheumatol. 2010, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Ramsey-Goldman, R.; Labrecque, J.; Joseph, L.; Boivin, J.-F.; Petri, M.; Zoma, A.; Manzi, S.; Urowitz, M.B.; Gladman, D.; et al. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J. Autoimmun. 2013, 42, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Apor, E.; O’Brien, J.; Stephen, M.; Castillo, J.J. Systemic lupus erythematosus is associated with increased incidence of hematologic malignancies: A meta-analysis of prospective cohort studies. Leuk. Res. 2014, 38, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Tallbacka, K.R.; Pettersson, T.; Pukkala, E. Increased incidence of cancer in systemic lupus erythematosus: A Finnish cohort study with more than 25 years of follow-up. Scand. J. Rheumatol. 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mercer, L.K.; Davies, R.; Galloway, J.B.; Low, A.; Lunt, M.; Dixon, W.G.; Watson, K.D.; Symmons, D.P.M.; Hyrich, K.L. British Society for Rheumatology Biologics Register (BSRBR) Control Centre Consortium. Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatology 2013, 52, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.M.; Fouad, T.M.; Summa, V.; Hasan, S.K.H.; Lo-Coco, F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica 2012, 97, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.B.; Chen, X.; Pujato, M.; Miller, D.; Maddox, A.; Forney, C.; Magnusen, A.F.; Lynch, A.; Chetal, K.; Yukawa, M.; et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat. Genet. 2018, 50, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Mizukawa, Y.; Shiohara, T. Virus-induced immune dysregulation as a triggering factor for the development of drug rashes and autoimmune diseases: With emphasis on EB virus, human herpesvirus 6 and hepatitis C virus. J. Dermatol. Sci. 2000, 22, 169–180. [Google Scholar] [CrossRef]
- Zignego, A.L.; Gragnani, L.; Piluso, A.; Sebastiani, M.; Giuggioli, D.; Fallahi, P.; Antonelli, A.; Ferri, C. Virus-driven autoimmunity and lymphoproliferation: The example of HCV infection. Expert Rev. Clin. Immunol. 2015, 11, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Lossius, A.; Johansen, J.N.; Torkildsen, Ø.; Vartdal, F.; Holmøy, T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis—Association and causation. Viruses 2012, 4, 3701–3730. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Cohen, J.I.; Fischer, S.; Li, L.; Sneller, M.; Goldbach-Mansky, R.; Raab-Traub, N.; Delecluse, H.-J.; Kenney, S.C. Reactivation of latent Epstein-Barr virus by methotrexate: A potential contributor to methotrexate-associated lymphomas. J. Natl. Cancer Inst. 2004, 96, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Polliack, A.; Gafter-Gvili, A. Rheumatoid arthritis and lymphoma: Incidence, pathogenesis, biology, and outcome. Hematol. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Yang, Y.; Chang, C.; Lu, Q. The epigenetic mechanism for discordance of autoimmunity in monozygotic twins. J. Autoimmun. 2017, 83, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R. Epigenetics in autoimmune diseases: Pathogenesis and prospects for therapy. Autoimmun. Rev. 2015, 14, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Yin, H.; Wang, L.; Gershwin, M.E.; Lu, Q. The critical role of epigenetics in systemic lupus erythematosus and autoimmunity. J. Autoimmun. 2016, 74, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wu, H.; Chan, V.; Lau, C.-S.; Lu, Q. Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity. Autoimmunity 2017, 50, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Stroopinsky, D.; Alimperti, S.; Jiao, A.L.; Pyzer, A.R.; Cippitelli, C.; Pepe, G.; Severa, M.; Rosenblatt, J.; Etna, M.P.; et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia 2018. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Boccellato, F.; Vincenti, S.; Rosato, P.; Bozzoni, I.; Frati, L.; Faggioni, A.; Presutti, C.; Trivedi, P. Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene 2010, 29, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F.; Wu, W.; Wang, Y.; Ding, H.; Qian, L. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int. J. Biol. Sci. 2018, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Ferrajoli, A.; Ivan, C.; Ciccone, M.; Shimizu, M.; Kita, Y.; Ohtsuka, M.; D’Abundo, L.; Qiang, J.; Lerner, S.; Nouraee, N.; et al. Epstein-Barr Virus MicroRNAs are Expressed in Patients with Chronic Lymphocytic Leukemia and Correlate with Overall Survival. EBioMedicine 2015, 2, 572–582. [Google Scholar] [CrossRef] [PubMed]
| miRNAs | Innate Immune Cells Hyperactivation and Inflammation | Autoreactive B Cell | T Cell Imbalance | Hematologic Disease | Standardized Incidence Ratio (SIR) |
|---|---|---|---|---|---|
| Let-7a | Cell proliferation in NHL [191] | ||||
| Let-7b | pDC activation and cytokine (IFNa) production in SLE [30]; | ||||
| Let-7c | DCs activation in SLE-like mouse models; pro-inflammatory cytokines production [41] | ||||
| Let-7i | Cell proliferation in CML [176] | ||||
| miR-7 | Fibrosis in SSc [52] | Apoptosis and cell proliferation in CML [166] | SSc-CML: 1.23 [213] | ||
| miR-9 | Cell proliferation and inflammation in HL [206,207]; cell proliferation in NHL [191] | ||||
| miR-10a | Pro-inflammatory cytokines production in RA [57,58] | ||||
| miR-10b | Chemokines receptor production in Ps keratinocytes [61] | ||||
| miR-15a/miR-16-1 cluster | Apoptosis and cell proliferation in CLL [109,110,111,112,113,114,115,116,117] | ||||
| miR-15a | Apoptosis and cell proliferation in CML [175] | ||||
| miR-17-92 cluster | Pro-inflammatory cytokines production in RA [59]; Chemokines production in Ps keratinocytes [60] | IgG autoantibodies production in SSc-like mouse model [79] | Th17 differentiation in SSc-like mouse model [79] | Apoptosis and cell proliferation in NHL [189] | RA-NHL: Male 2.39, Female 2.04 [214]; 1.89 [215]; 5.4 [216]; 2.34 [217]; Male 2.07, Female 1.37 [218]; 3.54 [219]; 2.27 [220]; 2.0 [23]; 3.38 [221]; 3.31 [222]; [22,223]; Ps-NHL: 2.2 [224]; 1.4 [23]. SSc-NHL: 1.18 [225]; 2.9 [226]; 2.5 [227]; 2.1 [23]; 4.14 [221] |
| miR-19b | Disease progression in AML [156] | ||||
| miR-21 | pDCs activation and cytokine (IFNa) production in SLE [30]; Fibrosis in SSc [51] | B cells differentiation and IgG autoantibodies production in SLE [77]; IgG autoantibodies and co-stimulatory molecules production in SLE-like mouse model [78] | Pro-inflammatory cytokines production in SLE-like mouse model [78]; T cells apoptosis in Ps [86]; Th17 differentiation in RA [87]; methylation of autoimmune-associated-genes in SLE and SLE-like mouse model [88] | Disease progression and apoptosis in CLL [121,122,124,125]; apoptosis and cell proliferation in CML [170,171,172] | SLE-CLL: 1.17 [213]; SSc-CLL: nd; Ps-CLL: 1.10 [213]; RA-CLL: 1.09 [213]; SLE-CML: 1.90 [213]; SSc-CML: 1.23 [213]; Ps-CML: nd; RA-CML: 2.4 [228]; 1.43 [213] |
| miR-23b | Pro-inflammatory cytokine production in SLE or RA and SLE and RA-like mouse models [99] | ||||
| miR-24 | Apoptosis in HL [208] | ||||
| miR-26a | Apoptosis and cell proliferation in NHL [183,196] | ||||
| miR-27b | Cell proliferation in NHL [182] | ||||
| miR-28 | Cell proliferation in NHL [190] | ||||
| miR-29 | Fibrosis in SSc [48,49] | Apoptosis and cell proliferation in AML [142,143,144]; apoptosis and cell proliferation in NHL [195] | SSc-AML: 1.01 [213]; SSc-NHL: 1.18 [225]; 2.9 [226]; 2.5 [227]; 2.1 [23]; 4.14 [221]; SLE-AML: nd; SLE-NHL: 7.01 [229]; 2.86 [230]; 3.50 [231]; 2.74 [232]; 15.37 [233]; 7.27 [219]; 5.0 [220]; 4.39 [234]; 5.70 [235]; 7.40 [221] 4.40 [23]; 12.10 [236]; [22,223] | ||
| miR-30a | B cells proliferation and IgG autoantibodies production in SLE [73] | Apoptosis in NHL [191] | SLE-NHL: 7.01 [229]; 2.86 [230]; 3.50 [231]; 2.74 [232]; 15.37 [233]; 7.27 [219]; 5.0 [220]; 4.39 [234]; 5.70 [235]; 7.40 [221] 4.40 [23]; 12.10 [236]; [22,223] | ||
| miR-30b | Fibrosis in SSc [50] | Apoptosis in NHL [191] | SSc-NHL: 1.18 [225]; 2.9 [226]; 2.5 [227]; 2.1 [23]; 4.14 [221] | ||
| miR-31 | Pro-inflammatory cytokines and chemokines production in Ps [55]; keratinocyte proliferation in Ps [56] | ||||
| miR-34a | DCs activation in RA [39] | Cell proliferation in CML [177]; apoptosis and cell proliferation in NHL [185,186]; cell proliferation in HL [211] | RA-CML: 2.4 [228], 1.43 [213]; RA-NHL: Male 2.39, Female 2.04 [214]; 1.89 [215]; 5.4 [216]; 2.34 [217]; Male 2.07, Female 1.37 [218]; 3.54 [219]; 2.27 [220]; 2.0 [23]; 3.38 [221]; 3.31 [222]; [22,223]; RA-HL: 3.06 [215]; 4.05 [217]; 1.76 [219]; 12.82 [237]; 3.31 [222] | ||
| miR-34b/c | Disease progression in CLL [117] | ||||
| miR-99a | Disease progression in AML [157] | ||||
| miR-106a~363Xpcl1 cluster | Cell proliferation in NHL [204] | ||||
| miR-124a | Disease progression in HL [209,210] | ||||
| miR-125a | Treg differentiation in ITP [95] | AML [160] | ITP-AML: 3.46 [213] | ||
| miR-125b | Keratinocytes proliferation and differentiation in Ps [66] | Disease progression, apoptosis and cell proliferation in AML [147,148,149,150] | Ps-AML: 1.26 [213] | ||
| miR-126 | Methylation of autoimmune-associated-genes in SLE [90] | Apoptosis in AML [159] | SLE-AML: nd | ||
| miR-130b | Cytokines production (regulator type I IFN pathway) in renal cells (SLE) [31]; fibrosis in SSc [33]; | ||||
| miR-138 | Proliferation in Ps [64] | Th1 differentiation in Ps [100] | |||
| miR-142 | pro-inflammatory cytokines production in SLE [40] | T cell activation and B cell stimulation in SLE [72] | |||
| miR-143 | Apoptosis and cell proliferation in CML [168] | ||||
| miR-146a | type I IFN pathway in SLE [36]; co-stimulatory molecules production in SS-prone mice [37]; | IgG autoantibodies production in ALPS-like mouse model [76] | |||
| miR-146b | chemokines receptor production in Ps keratinocyte [61] | Co-stimulatory molecules production in patients with CLL associated with AIHA [14] | Treg differentiation in ITP [94] | ||
| miR-148a | Survival of immature B cells in SLE-like mouse model [74] | Methylation of autoimmune-associated-genes in SLE and SLE-like mouse model [88] | |||
| miR-150 | DCs activation and cytokines production in SLE-like mouse model [42]; fibrosis in lupus nephritis [43]; fibrosis in SSc [44,45] | Disease progression and cell proliferation in CLL [118]; apoptosis and cell proliferation in AML [151]; apoptosis in NHL [205] | SLE-CLL: 1.17 [213]; SSc-CLL: nd; SLE-AML: nd; SSc-AML: 1.01 [213]; SLE-NHL: 7.01 [229]; 2.86 [230]; 3.50 [231]; 2.74 [232]; 15.37 [233]; 7.27 [219]; 5.0 [220]; 4.39 [234]; 5.70 [235]; 7.40 [221] 4.40 [23]; 12.10 [236]; [22,223]; SSc-NHL: 1.18 [225]; 2.9 [226]; 2.5 [227]; 2.1 [23]; 4.14 [221] | ||
| miR-155 | Chemokine production and pro-inflammatory chemokine receptor expression in RA monocytes [25]; pro-inflammatory cytokines production in RA synovial CD14(+) [26]; resistance to apoptosis in CD14+ RA [24]; DCs hyperactivation [27]; co-stimulatory molecules production in pDCs from SLE-like mice model [28]; fibrosis in SSc [29]; proliferation in Ps [63] | IgG autoantibodies production in SLE-like mouse model [67]; in RA [69] and in RA-like mouse model [26]. | Treg cells development in SLE-like mouse model [82]; pro-inflammatory cytokines production in RA [85] and in RA-like mouse model [18] | Disease progression and cell proliferation in CLL [126,127,128,129,130,131,133]; disease progression and cell proliferation in AML [134,135,136,138,139,140,141]; cell proliferation in NHL [178,179,180,181,192,193,194] | RA-CLL: 1.09 [213]; SLE-CLL: 1.17 [213]; RA-AML: 2.4 [213,228,238]; SLE-AML: nd; RA-NHL: Male 2.39, Female 2.04 [214]; 1.89 [215]; 5.4 [216]; 2.34 [217]; Male 2.07, Female 1.37 [218]; 3.54 [219]; 2.27 [220]; 2.0 [23]; 3.38 [221]; 3.31 [222]; [22,223]; SLE-NHL: 7.01 [229]; 2.86 [230]; 3.50 [231]; 2.74 [232]; 15.37 [233]; 7.27 [219]; 5.0 [220]; 4.39 [234]; 5.70 [235]; 7.40 [221] 4.40 [23]; 12.10 [236]; [22,223] |
| miR-181a | Disease progression and cell proliferation in AML [145,146]; cell proliferation in NHL [184] | ||||
| miR-181b | Keratinocytes proliferation in Ps [65] | Disease progression and apoptosis in CLL [119,120] | Ps-CLL: 1.10 [213] | ||
| miR-192 | Cell proliferation in AML [152] | ||||
| miR-193b | Disease progression and cell proliferation in AML [153] | ||||
| miR-194 | Apoptosis in AML [154] | ||||
| miR-196a | Fibrosis in SSc [53,54] | ||||
| miR-202 | Fibrosis in SSc [47] | ||||
| miR-203 | Cell proliferation in HL [211] | ||||
| miR-210 | Th1 and Th17 cell differentiation [92] and pro-inflammatory cytokines production in Ps [91] | ||||
| miR-219 | Apoptosis in CML [174] | ||||
| miR-224 | Cell proliferation in CML [176] | ||||
| miR-302d | Cytokines production (regulator type I IFN pathway) in SLE [32] | ||||
| mir-320a | Apoptosis and cell proliferation in CML [169] | ||||
| miR-326 | B cells hyper activation of and IgG autoantibodies production in SLE-like mouse model [75] | ||||
| miR-328 | Cell proliferation in CML [173] | ||||
| miR-342 | Cell proliferation and apoptosis in AML [161] | ||||
| miR-362 | Disease progression in AML [158] | ||||
| miR-375 | Disease progression in AML [155] | ||||
| miR-483 | Fibrosis in SSc [46] | ||||
| miR-486 | Keratinocytes proliferation in Ps [62] | ||||
| miR-574 | pDCs activation and cytokine (IFNa) production in SLE [30] | ||||
| miR-618 | Citokines production (IFNA1) from pDCs in SSc [34] | ||||
| miR-1246 | B cells hyper activation and co-stimulatory molecules production in SLE [71] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco, M.D.; Ramassone, A.; Pagotto, S.; Anastasiadou, E.; Veronese, A.; Visone, R. MicroRNAs in Autoimmunity and Hematological Malignancies. Int. J. Mol. Sci. 2018, 19, 3139. https://doi.org/10.3390/ijms19103139
Marco MD, Ramassone A, Pagotto S, Anastasiadou E, Veronese A, Visone R. MicroRNAs in Autoimmunity and Hematological Malignancies. International Journal of Molecular Sciences. 2018; 19(10):3139. https://doi.org/10.3390/ijms19103139
Chicago/Turabian StyleMarco, Mirco Di, Alice Ramassone, Sara Pagotto, Eleni Anastasiadou, Angelo Veronese, and Rosa Visone. 2018. "MicroRNAs in Autoimmunity and Hematological Malignancies" International Journal of Molecular Sciences 19, no. 10: 3139. https://doi.org/10.3390/ijms19103139
APA StyleMarco, M. D., Ramassone, A., Pagotto, S., Anastasiadou, E., Veronese, A., & Visone, R. (2018). MicroRNAs in Autoimmunity and Hematological Malignancies. International Journal of Molecular Sciences, 19(10), 3139. https://doi.org/10.3390/ijms19103139