Resveratrol Attenuates Staphylococcus Aureus-Induced Monocyte Adhesion through Downregulating PDGFR/AP-1 Activation in Human Lung Epithelial Cells
Abstract
1. Introduction
2. Results
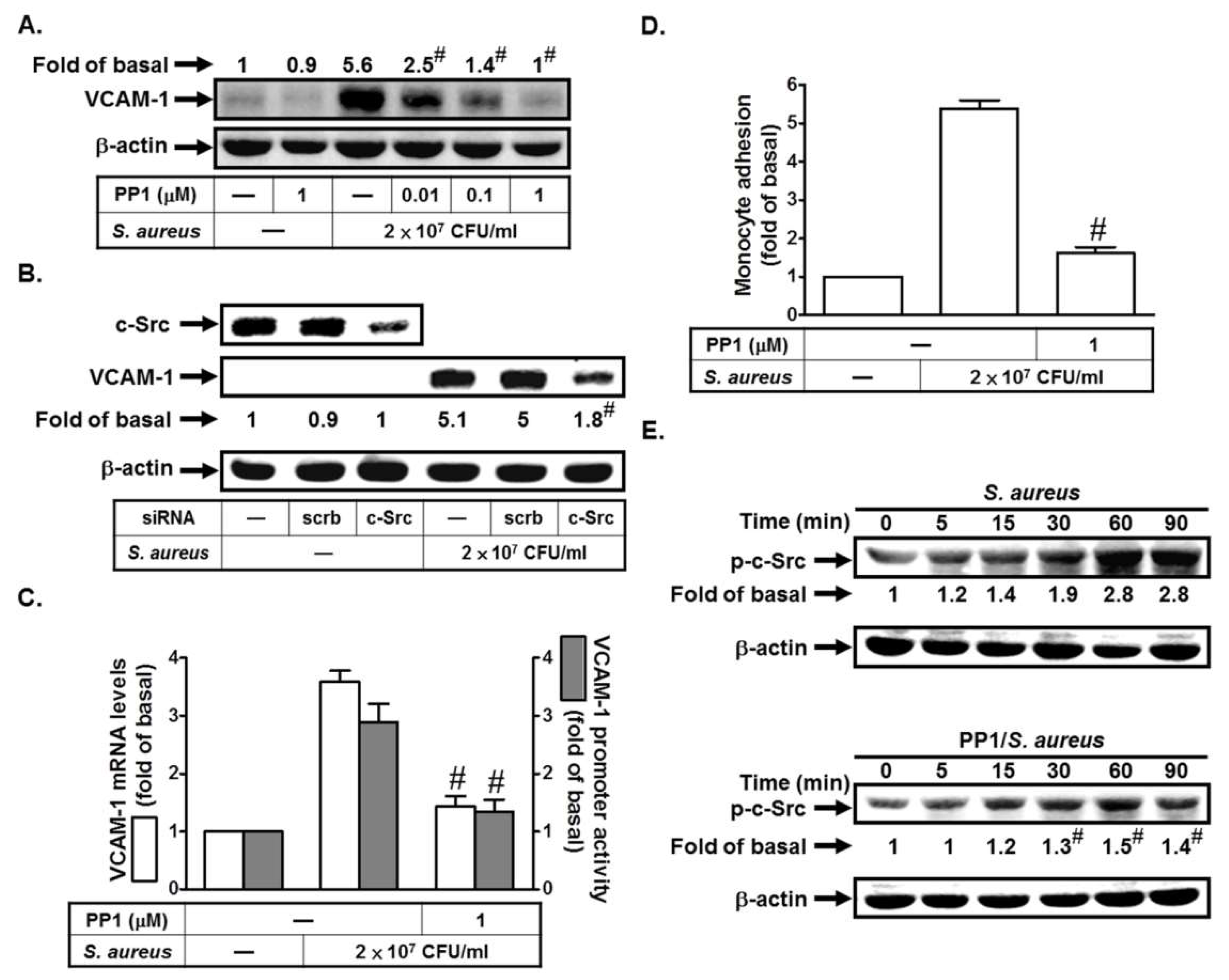
2.1. S. aureus Induces VCAM-1 Expression in HPAEpiCs via c-Src
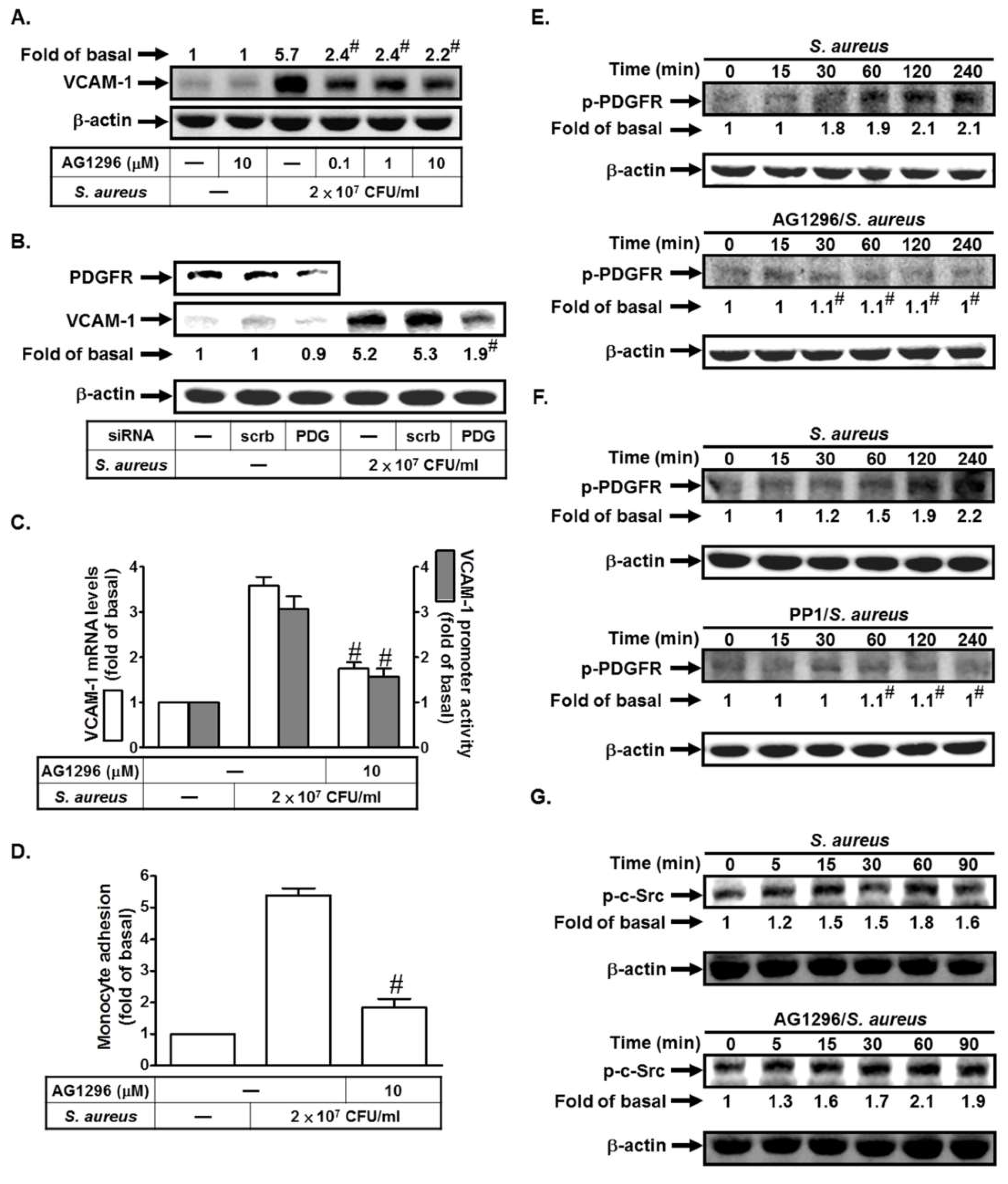
2.2. S. aureus Induces VCAM-1 Expression in HPAEpiCs via PDGFR
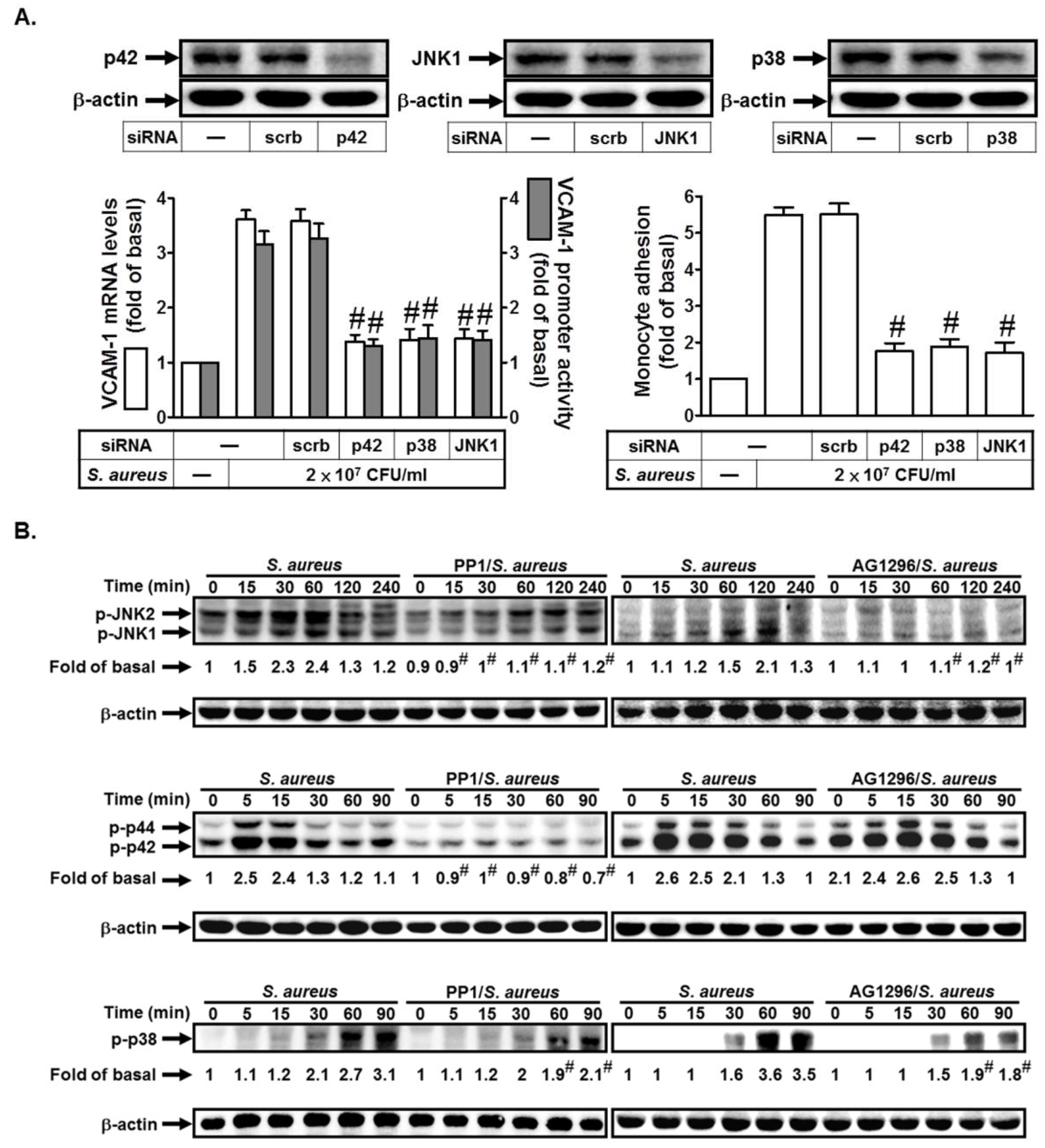
2.3. S. aureus Induces VCAM-1 Expression in HPAEpiCs via MAPKs
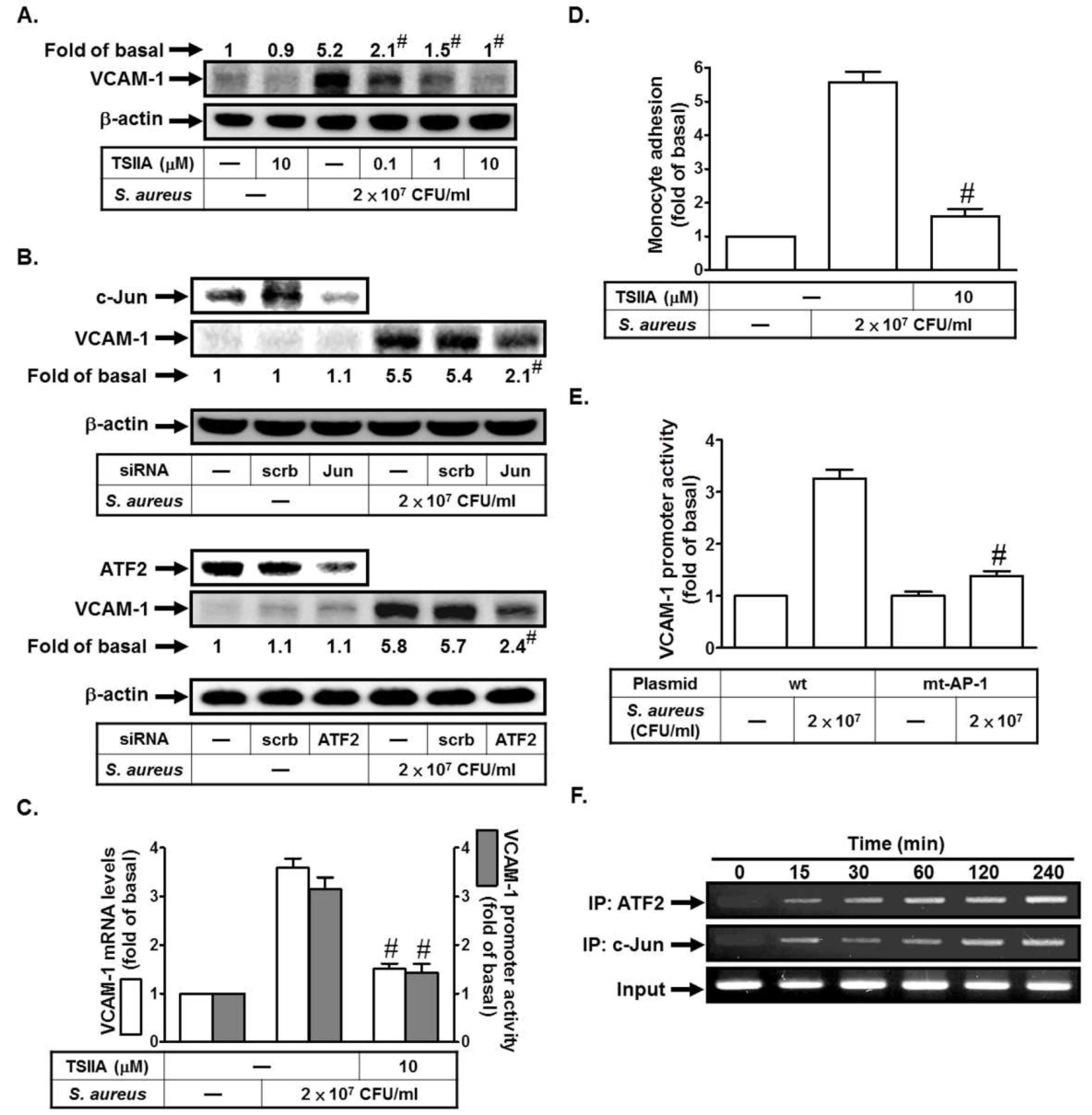
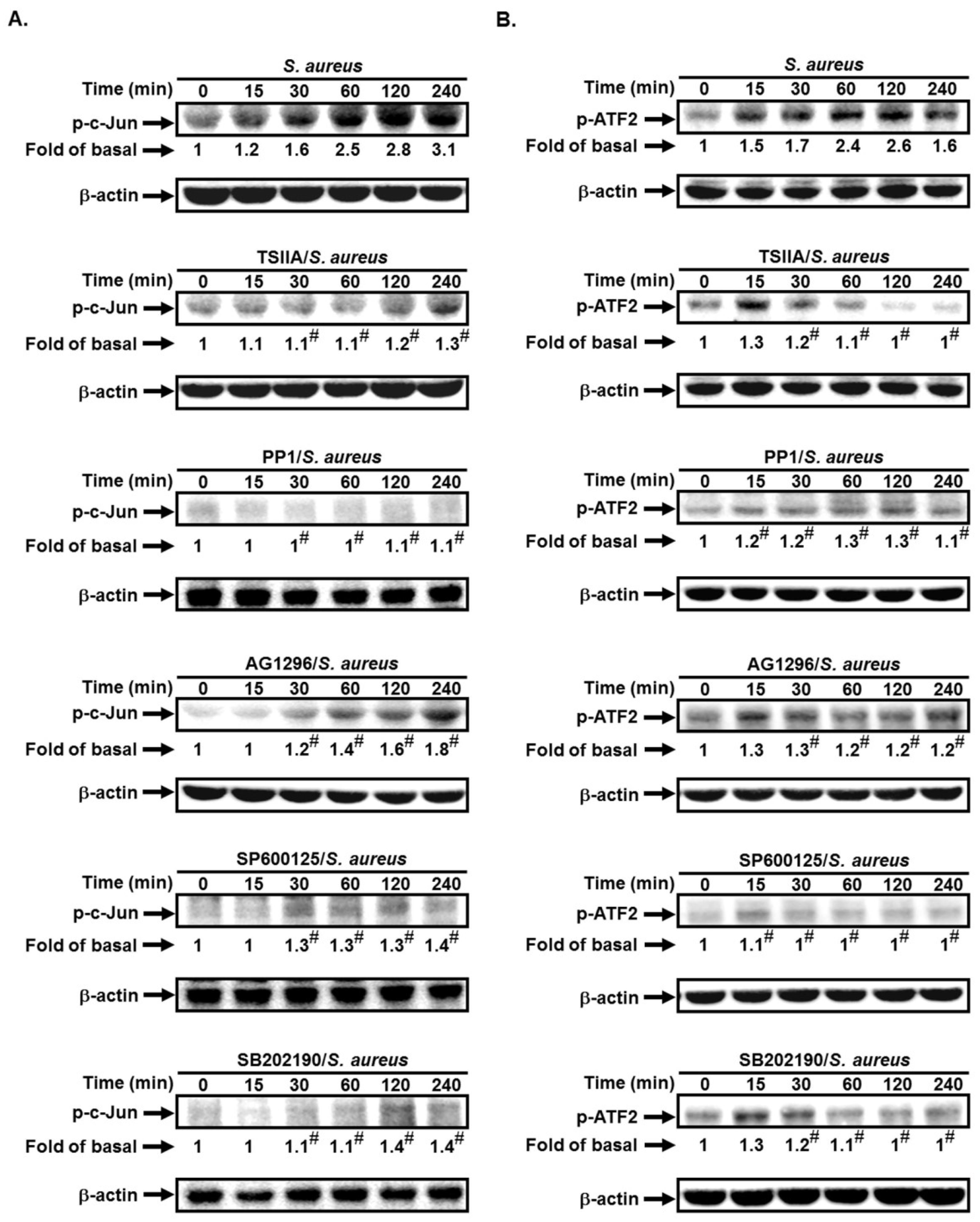
2.4. S. aureus Induces VCAM-1 Expression in HPAEpiCs via AP-1
2.5. S. aureus Induces AP-1 Activation via c-Src/PDGFR/JNK1/2 and p38 MAPK Pathways
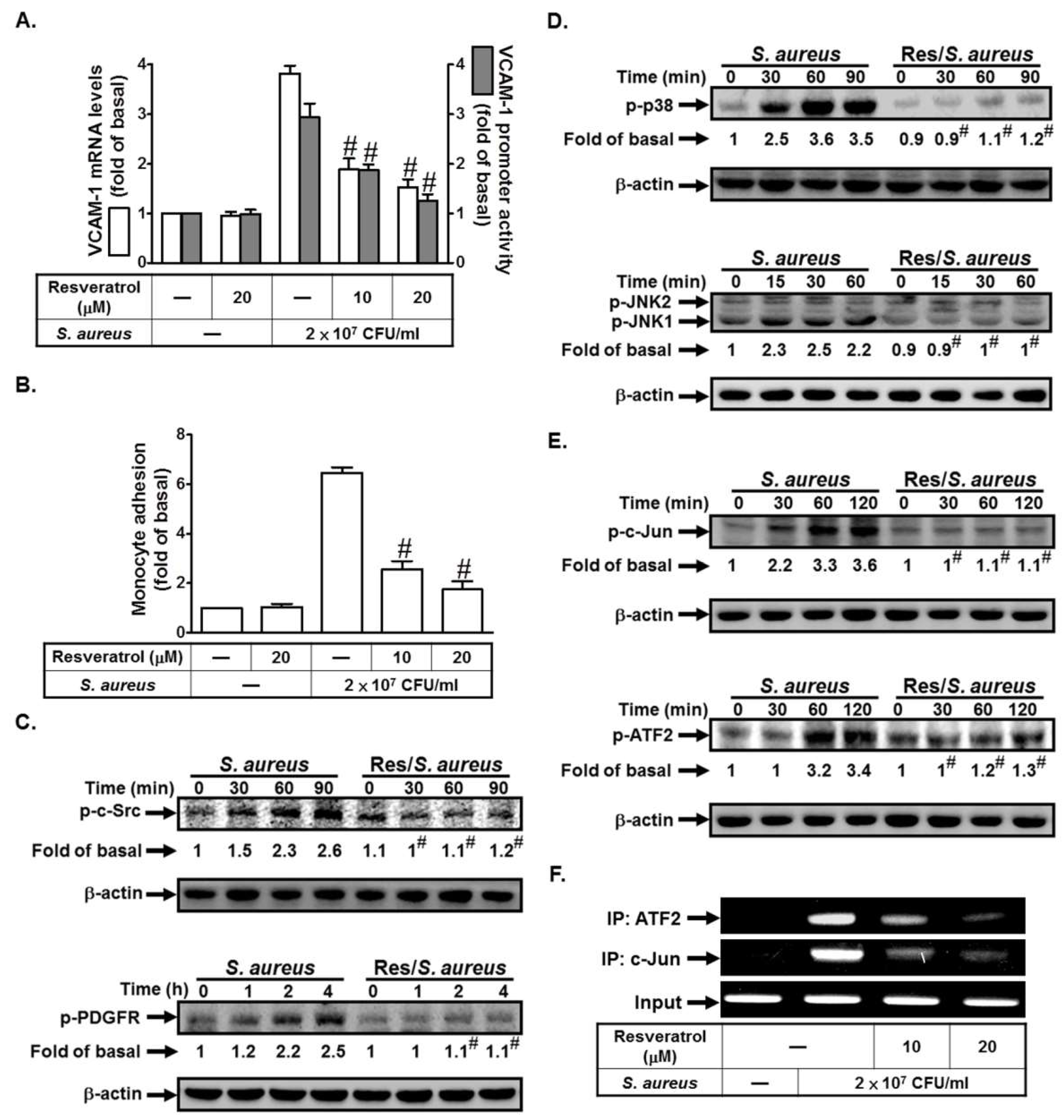
2.6. Resveratrol Inhibits S. aureus-Induced VCAM-1 Expression and Inflammatory Signaling Pathways
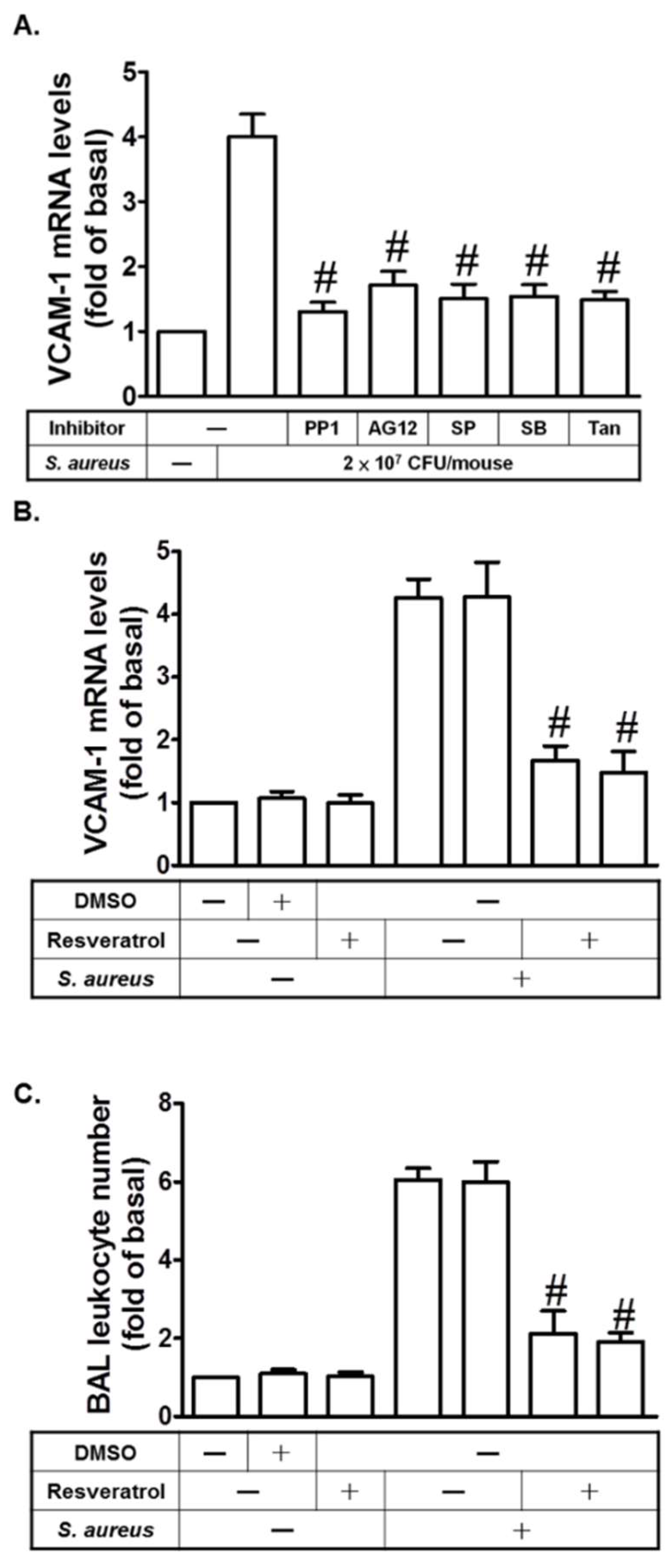
2.7. Resveratrol Inhibits S. aureus-Induced VCAM-1 Expression in the Lungs of Mice
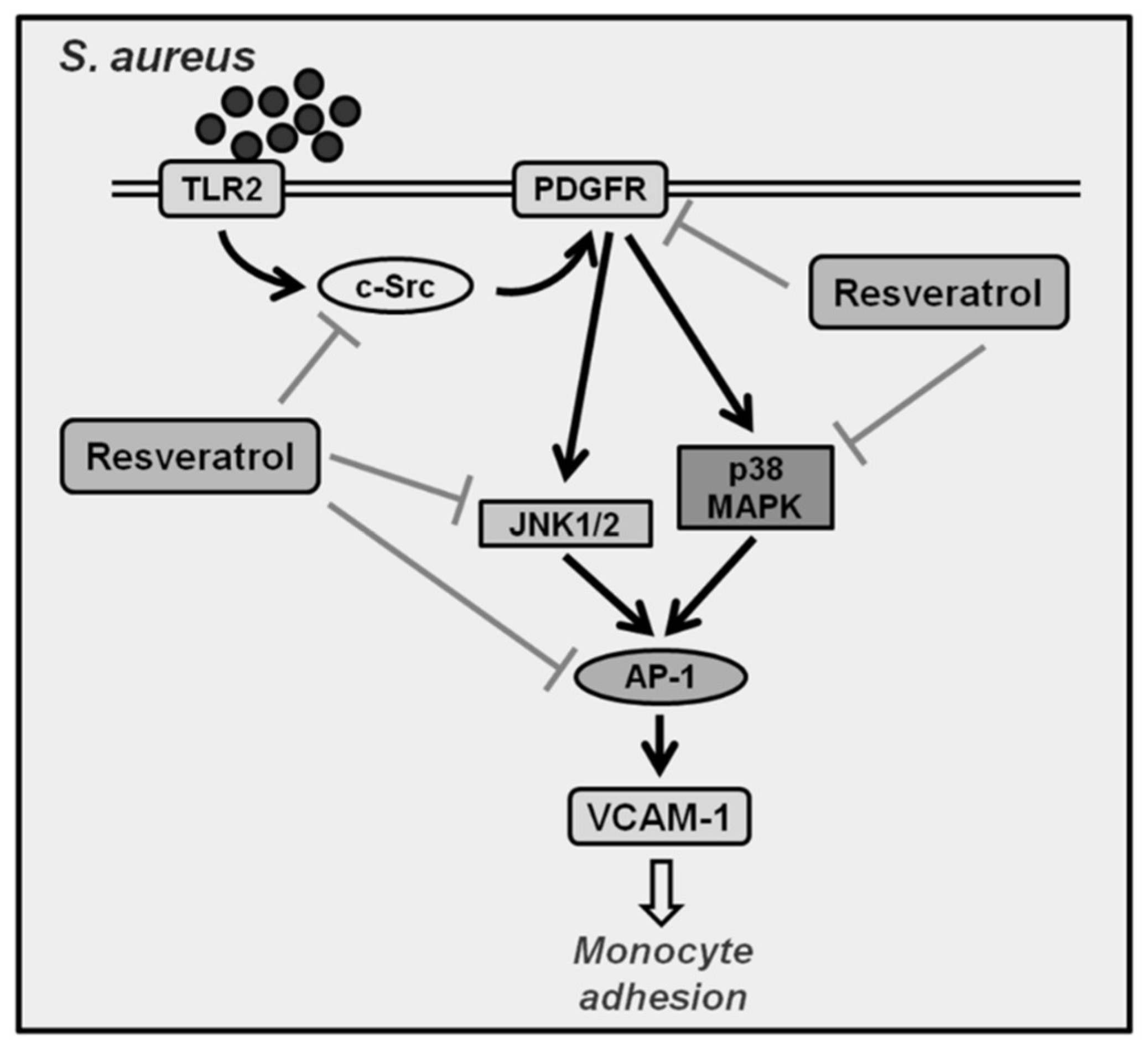
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Preparation of S. aureus
4.4. Animal Care and Experimental Procedures
4.5. Isolation of BAL Fluid
4.6. Transient Transfection with siRNAs
4.7.Western Blot
4.8. Real-Time RT-PCR
4.9. Measurement of VCAM-1 Luciferase Activity
4.10. Adhesion Assay
4.11. Chromatin Immunoprecipitation Assay
4.12. Statistical Software and Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| S. aureus | Staphylococcus aureus |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| SFKs | Src family kinases |
| HPAEpiCs | Human lung epithelial cells |
| MMP-9 | Matrix metalloproteinase-9 |
| EV71 | Enterovirus 71 |
| TLRs | Toll-like receptors |
| LPS | Lipopolysaccharide |
References
- Dai, X.H.; Li, H.E.; Lu, C.J.; Wang, J.F.; Dong, J.; Wei, J.Y.; Zhang, Y.; Wang, X.; Tan, W.; Deng, X.M.; et al. Liquiritigenin prevents Staphylococcus aureus-mediated lung cell injury via inhibiting the production of α-hemolysin. J. Asian Nat. Prod. Res. 2013, 15, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, IT.; Lee, C.W.; Tung, W.H.; Wang, S.W.; Lin, C.C.; Shu, J.C.; Yang, C.M. Cooperation of TLR2 with MyD88, PI3K, and Rac1 in lipoteichoic acid-induced cPLA2/COX-2-dependent airway inflammatory responses. Am. J. Pathol. 2010, 176, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Lee, IT.; Wang, S.W.; Lee, C.W.; Chang, C.C.; Lin, C.C.; Luo, S.F.; Yang, C.M. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J. Immunol. 2008, 181, 5098–5110. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, I.C.; Shaw, N. Atypical lipoteichoic acids of gram-positive bacteria. J. Bacteriol. 1991, 173, 7065–7069. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Yang, C.M. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013, 2013, 791231. [Google Scholar] [CrossRef] [PubMed]
- Lazaar, A.L.; Panettieri, R.A., Jr. Airway smooth muscle: a modulator of airway remodeling in asthma. J. Allergy Clin. Immunol. 2005, 116, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, IT.; Lin, C.C.; Lee, C.Y.; Hsieh, P.W.; Yang, C.M. Protective effects of (-)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 2013, 24, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, IT.; Yang, C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, IT.; Shih, R.H.; Lin, C.C.; Chen, J.T.; Yang, C.M. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell. Commun. Signal. 2012, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, S.M.; Tsai, C.H.; Huang, C.Y.; Hsu, C.J.; Tang, C.H. CCN4 induces vascular cell adhesion molecule-1 expression in human synovial fibroblasts and promotes monocyte adhesion. Biochim. Biophys. Acta 2013, 1833, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.H.; Sun, C.C.; Hsieh, H.L.; Wang, S.W.; Horng, J.T.; Yang, C.M. EV71 induces VCAM-1 expression via PDGF receptor, PI3-K/Akt, p38 MAPK, JNK and NF-κB in vascular smooth muscle cells. Cell. Signal. 2007, 19, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Wark, P.A.; Garg, M.L. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid. Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Biol. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tosaki, A.; Bagchi, D.; Maulik, N.; Das, D.K. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J. Pharmacol. Exp. Ther. 2006, 317, 980–988. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, A.M.; White, R.E. Resveratrol inhibits MAPK activity and nuclear translocation in coronary artery smooth muscle: reversal of endothelin-1 stimulatory effects. FEBS Lett. 1999, 451, 63–67. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Chen, W.C.; Lee, I.T.; Chi, P.L.; Cheng, S.E.; Yang, C.M. c-Src-dependent transactivation of PDGFR contributes to TNF-α-induced MMP-9 expression and functional impairment in osteoblasts. Bone 2014, 60, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lin, C.C.; Lee, I.T.; Lin, Y.H.; Yang, C.M.; Chen, W.J.; Jou, M.J.; Hsiao, L.D. Japanese encephalitis virus induces matrix metalloproteinase-9 expression via a ROS/c-Src/PDGFR/PI3K/Akt/MAPKs-dependent AP-1 pathway in rat brain astrocytes. J. Neuroinflammation 2012, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lee, I.T.; Lin, C.C.; Wang, C.H.; Cherng, W.J.; Hsiao, L.D. c-Src-dependent MAPKs/AP-1 activation is involved in TNF-α-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Biochem. Pharmacol. 2013, 85, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Ferraz da Costa, D.C.; Casanova, F.A.; Quarti, J.; Malheiros, M.S.; Sanches, D.; Dos Santos, P.S.; Fialho, E.; Silva, J.L. Transient transfection of a wild-type p53 gene triggers resveratrol-induced apoptosis in cancer cells. PLoS ONE 2012, 7, e48746. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Peschel, A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008, 3, 437–451. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.; Ainis, T.; Bottari, C.; Fimiani, V. Effect of resveratrol on some activities of isolated and in whole blood human neutrophils. Physiol. Res. 2003, 52, 555–562. [Google Scholar] [PubMed]
- Ferrero, M.E.; Bertelli, A.A.; Pellegatta, F.; Fulgenzi, A.; Corsi, M.M.; Bertelli, A. Phytoalexin resveratrol (3–4′-5-trihydroxystilbene) modulates granulocyte and monocyte endothelial adhesion. Transplant. Proc. 1998, 30, 4191–4193. [Google Scholar] [CrossRef]
- Tang, C.H.; Hsu, C.J.; Yang, W.H.; Fong, Y.C. Lipoteichoic acid enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, PKCδ and c-Src dependent pathways. Biochem. Pharmacol. 2010, 79, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Pang, L.; Inoue, H.; Knox, A.J. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1β in human airway smooth muscle cells: involvement of different promoter elements, transcription factors, and histone h4 acetylation. Mol. Cell. Biol. 2003, 23, 9233–9244. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-T.; Lin, C.-C.; Yang, C.-C.; Hsiao, L.-D.; Wu, M.-Y.; Yang, C.-M. Resveratrol Attenuates Staphylococcus Aureus-Induced Monocyte Adhesion through Downregulating PDGFR/AP-1 Activation in Human Lung Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 3058. https://doi.org/10.3390/ijms19103058
Lee I-T, Lin C-C, Yang C-C, Hsiao L-D, Wu M-Y, Yang C-M. Resveratrol Attenuates Staphylococcus Aureus-Induced Monocyte Adhesion through Downregulating PDGFR/AP-1 Activation in Human Lung Epithelial Cells. International Journal of Molecular Sciences. 2018; 19(10):3058. https://doi.org/10.3390/ijms19103058
Chicago/Turabian StyleLee, I-Ta, Chih-Chung Lin, Chien-Chung Yang, Li-Der Hsiao, Ming-Yen Wu, and Chuen-Mao Yang. 2018. "Resveratrol Attenuates Staphylococcus Aureus-Induced Monocyte Adhesion through Downregulating PDGFR/AP-1 Activation in Human Lung Epithelial Cells" International Journal of Molecular Sciences 19, no. 10: 3058. https://doi.org/10.3390/ijms19103058
APA StyleLee, I.-T., Lin, C.-C., Yang, C.-C., Hsiao, L.-D., Wu, M.-Y., & Yang, C.-M. (2018). Resveratrol Attenuates Staphylococcus Aureus-Induced Monocyte Adhesion through Downregulating PDGFR/AP-1 Activation in Human Lung Epithelial Cells. International Journal of Molecular Sciences, 19(10), 3058. https://doi.org/10.3390/ijms19103058




