Intracellular Energy-Transfer Networks and High-Resolution Respirometry: A Convenient Approach for Studying Their Function
Abstract
1. Introduction
2. Organization of Intracellular Environment, Influencing Bioenergetics
2.1. Diffusion Restrictions and Micro-Compartmentalization
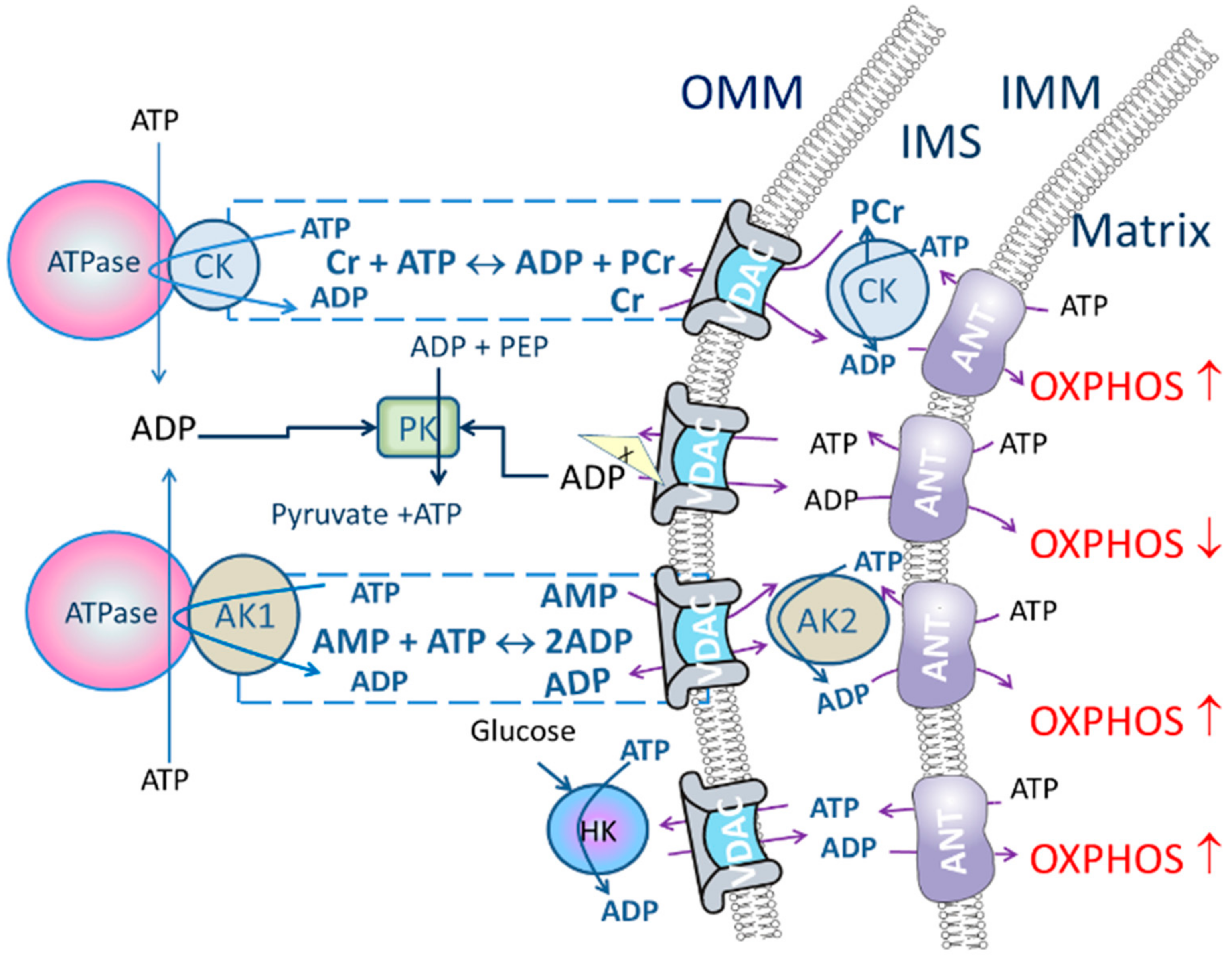
2.2. Energy Transport Systems as a Regulator of Oxidative Phosphorylation
3. Quantitative Assessment of Intracellular Diffusion Restrictions and Energy-Transfer Networks in Permeabilized Cells
3.1. Materials and Sample Preparation
3.2. Quantitative Assessment of Intracellular Diffusion Restrictions
Timing ~ 1 h
- Add cells/fiber into the oxygraphic chamber.
- Add respiratory substrates: malate (2 mM) and glutamate/pyruvate (5/10 mM).
- Register the basal respiration rate (V0).
- Start cumulative addition of ADP until the saturation of respiration rate. The ADP concentration range depends on the sample. For preparations with low Km(ADP) (e.g., isolated mitochondria and most cell cultures) the concentration range is 0–500 µM ADP. For permeabilized tissue samples the saturating ADP concentration may reach up to 5 mM ADP (usually 2 mM).
- Calculate the Km(ADP) and Vmax values from the [ADP] versus respiration rate (the basal rate of respiration, Km 0, subtracted) relationships on the basis of the Michaelis-Menten equation.
3.3. Quantitative Assessment of Energy-Transfer Pathways
3.3.1. Creatine Kinase Pathway
Timing ~ 1 h
- Intracellular diffusion of adenine nucleotides could be restricted (characterized by high Km(ADP) measured in permeabilized tissue/cells) but creatine/PCr transport through the VDAC could bypass the restrictions when CK pathway is functionally coupled to OXPHOS.
- Add creatine (10 mM) into the respiration media.
- Add cells/fiber into the oxygraphic chamber.
- Add respiratory substrates: malate (2 mM) and glutamate/pyruvate (5/10 mM).
- Register the basal respiration rate V0.
- Start cumulative addition of ADP until to respiration rate saturation.
- Calculate the Km(ADP) and Vmax values from the [ADP] versus respiration rate value (the basal rate of respiration, V0, subtracted) relationships on the basis of the Michaelis-Menten equation. When the calculated Km(ADP) value with creatine is significantly lower than the corresponding value without creatine, it confirms an effective functional coupling of OXPHOS to CK pathway.
Timing 0.5 h
- Add cells or permeabilized tissue sample into the oxygraph chamber.
- Add general respiratory substrates malate (2 mM) + glutamate/pyruvate (5/10 mM) in the oxygraphic chamber and register the basal respiration (V0).
- Add MgATP (2 mM) to induce maximal activity of ATPases (VATP). Slight oxygen consumption could be detected in these conditions in resting muscle cells.
- Add creatine to a final concentration of 20 mM (VCr). If there is a marked rise in respiratory rate after the addition of creatine then CK pathway is activated and concomitant increase in respiration rate reflects functional coupling between mitochondrial CK with OXPHOS as well as general ADP transport activity.
Optional:
- 5.
- Add ADP (2 mM) to register maximal ADP dependent oxygen consumption rate.
Timing 0.5–1 h
- Insert cells/fiber into the oxygraphic chamber in addition to the respiratory solution supplemented with substrates: malate (2 mM) and glutamate/pyruvate (5/10 mM) and PEP (5 mM)
- Add MgATP (2 mM) to activate ATPases. The increase in respiration rate is observable because ADP generated by ATPases is diffused to mitochondria.
- Add PK (10 U/mL) to activate PK/PEP system which is included to rephosphorylate ADP produced by cytosolic ATPases. While the CK pathway is not activated, energy transport between mitochondrion and ATPases is prevailing and taking place through direct ATP/ADP transfer. Therefore, addition of PK/PEP decreases oxygen consumption rate. In this situation ADP, formed by the ATPases, is regenerated by PK/PEP and backflow of the ADP to mitochondrion is smaller, and oxygen consumption rate, used for rephosphorylation inside mitochondrion, decreases (Figure 1).
- Start stepwise addition of creatine until saturation is reached (when no additional increase in the respiration is detected). If mitochondrial CK is coupled to OXPHOS, then the respiration in the presence of PK/PEP system is initiated only by ADP generated in mitochondrial intermembrane space by mitochondrial CK.
3.3.2. Adenylate Kinase Pathway
Timing 0.5 h
- Add cells/fiber into the oxygraphic chamber.
- Add respiratory substrates malate (2 mM) + glutamate/pyruvate (5/10 mM). Register the basal respiration rate.
- Add MgATP (2 mM or 0.1 mM) to activate ATPases and induce maximal endogenous (intra-systemic) ADP production which should increase the respiration rate.
- Add AMP (2 mM) to activate the AK reaction and register VAMP. Respiration should increase due to activation of cytosolic and mitochondrial AKs. The extent to which respiration is stimulated by AMP indicates the functional coupling of whole AK pathway.
- Inhibit AK with diadenosine pentaphosphate (AP5A, 0.2 mM, VAP5A) in order to measure AK-dependent part of AMP activated respiration. Consequently, in this setup the inhibitory effect of PK on the AMP-mediated O2 consumption correlates with intracellular AK1/AK2 ratio.
- Add carboxyatractyloside (CAT, 1 µM) to inhibit ATP/ADP transport through ANT. In intact mitochondria the respiration is controlled by ANT and if inner mitochondrial membrane is disrupted ANT does not control respiration.
- To express the strength of the AK functional coupling with OXPHOS calculate AK index (IAK) as IAK = (VAMP − VAP5A)/VAP5A.
Timing 45 min–1 h
- Insert cells/fiber into the oxygraphic chamber.
- Add respiratory substrates malate (2 mM) + glutamate/pyruvate (5/10 mM) to the respiratory solution supplemented with 5 mM PEP. Monitor the basal respiration rate.
- Add MgATP (2 mM) to induce maximal endogenous (intra-systemic) ADP production.
- Add AMP (2 mM) to activate the AK reaction coupled with OXPHOs and mediated by AK2 and AK1 and ANT. Register the maximal AMP stimulated respiration (VAMP).
- Injection of 10 IU/mL PK decreases the respiration to the level of AK2 coupled reaction. Because the PEP/PK system is formed and the present VPK demonstrates AK2-specific coupled reaction with ANT inside mitochondria.
- Add AP5A (0.2 mM) to inhibit AK. Respiration rate should fall significantly.
- Add CAT (1 μM) to check inner mitochondrial membrane (IMM) intactness. With intact IMM ANT controls the respiration and if control is lost the respiration rate with CAT significantly exceeds the basal respiration rate.
- The functional coupling with OXPHOS system with AK1 activity could be characterized by the corresponding AK index (IAK1). The IAK1 is calculated according to the following equation: IAK1 = ((VAMP − VPK))/(VAMP − VAP5A)) × 100%, where VAMP, VPK and VAP5A are the rates of O2 consumption that were measured in step 4, 5, 6 respectively. Calculate the index for AK2 functional coupling with OXPHOS s as IAK2 = 100% − IAK1.
Timing 1.5 h
- Add cells/fiber into the oxygraphic chamber.
- Add respiratory substrates glutamate/pyruvate (5/10 mM) and malate (2 mM).
- Add MgATP (50–100 µM) to produce a submaximal amount of endogenous ADP to stimulate mitochondria.
- Add AMP (2 mM) to activate the coupled reaction of mitochondrial AK (AK2) with ANT. In these conditions the rise in respiration rate (VAMP) is caused by coupling of AK to OXPHOS.
- Add AP5A (0.2 mM, VAP5A) to inhibit AK.
- Add creatine (20 mM) to activate coupled reaction between mitochondrial CK and ANT. In these conditions creatine stimulated respiration (VCr) is activated by local generation of ADP in the vicinity of ANT and associated rise in respiration rate indicates the strength of coupling of MtCK.
- Add ADP (2 mM) for maximum activation of respiration (VADP).
- Add cytochrome c (Cyt c, 10 µM) for quality control for intactness of outer mitochondrial membrane.
- Add CAT (1 µM) to check quality of inner mitochondrial membrane.
- To assess the strength of the functional coupling independently of mitochondrial content in individual preparations, activation of respiration by AMP can be normalized for the respiratory rate registered after addition of AP5A, thus producing the relative index (IAK = VAMP − VAP5A/VAP5A). The coupling of CK to OXPHOS is characterized by relative index ICK (ICK = VCr/VADP).
3.3.3. Coupling of Hexokinase to Oxidative Phosphorylation
Timing 40 min
- Add cells/fiber into the oxygraph chamber.
- Add respiratory substrates glutamate/pyruvate (5/10 mM) and malate (2 Mm).
- MgATP (0.1–2 mM) (VATP) is added to achieve maximal stimulation of mitochondria with endogenous ADP e.g., ADP produced by the ATPases.
- Add glucose (10 mM) to activate the HK reaction (VGluc).
- Add ADP (2 mM) to achieve maximal ADP-dependent respiration rate (VADP).
- Add Cyt c (10 µM) for outer mitochondrial membrane quality control.
- Add CAT (1 µM) for inner mitochondrial membrane quality control.
- The effect of glucose (glucose index) can be calculated as follows: (VGluc − VATP)/(VADP).
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | creatine kinase |
| AK | adenylate kinase |
| OXPHOS | oxidative phosphorylation |
| IMM | inner mitochondrial membrane |
| OMM | outer mitochondrial membrane |
| PCr | phosphocreatine |
| VDAC | voltage-dependent anion channel |
| IMS | mitochondrial intermembrane space |
| HK | hexokinase |
| ANT | adenine nucleotide translocase |
| MtCK | mitochondrial creatine kinase |
| uMtCK | ubiquitous MtCK |
| MI | Mitochondrial Interactosome |
| PK | pyruvate kinase |
| PEP | phosphoenol pyruvate |
| AP5A | diadenosine pentaphosphate |
| CAT | carboxyatractyloside |
References
- Chung, S.; Arrell, D.K.; Faustino, R.S.; Terzic, A.; Dzeja, P.P. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J. Mol. Cell Cardiol. 2010, 48, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Guzun, R.; Kaambre, T.; Bagur, R.; Grichine, A.; Usson, Y.; Varikmaa, M.; Anmann, T.; Tepp, K.; Timohhina, N.; Shevchuk, I.; et al. Modular organization of cardiac energy metabolism: Energy conversion, transfer and feedback regulation. Acta Physiol. 2015, 213, 84–106. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.; Dzeja, P.; Schlattner, U.; Vendelin, M.; Terzic, A.; Wallimann, T. Cardiac system bioenergetics: Metabolic basis of the frank-starling law. J. Physiol. 2006, 571, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Gruno, M.; Peet, N.; Seppet, E.; Kadaja, L.; Paju, K.; Eimre, M.; Orlova, E.; Peetsalu, M.; Tein, A.; Soplepmann, J.; et al. Oxidative phosphorylation and its coupling to mitochondrial creatine and adenylate kinases in human gastric mucosa. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R936–R946. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Terzic, A. Phosphotransfer networks and cellular energetics. J. Exp. Biol. 2003, 206, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Zeleznikar, R.J.; Goldberg, N.D. Suppression of creatine kinase-catalyzed phosphotransfer results in increased phosphoryl transfer by adenylate kinase in intact skeletal muscle. J. Biol. Chem. 1996, 271, 12847–12851. [Google Scholar] [CrossRef] [PubMed]
- Nemutlu, E.; Zhang, S.; Gupta, A.; Juranic, N.O.; Macura, S.I.; Terzic, A.; Jahangir, A.; Dzeja, P. Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted 31P NMR and mass spectrometry. Physiol. Genomics 2012, 44, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Pucar, D.; Dzeja, P.P.; Bast, P.; Juranic, N.; Macura, S.; Terzic, A. Cellular energetics in the preconditioned state: Protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J. Biol. Chem. 2001, 276, 44812–44819. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Hoyer, K.; Tian, R.; Zhang, S.; Nemutlu, E.; Spindler, M.; Ingwall, J.S. Rearrangement of energetic and substrate utilization networks compensate for chronic myocardial creatine kinase deficiency. J. Physiol. 2011, 589, 5193–5211. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Taivassalo, T.; Ritchie, D.; Wright, K.J.; Thomas, M.M.; Romestaing, C.; Hepple, R.T. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 2011, 6, e18317. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.A.; Veksler, V.I.; Kuznetsov, A.V.; Kay, L.; Sikk, P.; Tiivel, T.; Tranqui, L.; Olivares, J.; Winkler, K.; Wiedemann, F.; et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol. Cell. Biochem. 1998, 184, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Guzun, R.; Timohhina, N.; Tepp, K.; Gonzalez-Granillo, M.; Shevchuk, I.; Chekulayev, V.; Kuznetsov, A.V.; Kaambre, T.; Saks, V.A. Systems bioenergetics of creatine kinase networks: Physiological roles of creatine and phosphocreatine in regulation of cardiac cell function. Amino Acids 2011, 40, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Seppet, E.K.; Kaambre, T.; Sikk, P.; Tiivel, T.; Vija, H.; Tonkonogi, M.; Sahlin, K.; Kay, L.; Appaix, F.; Braun, U.; et al. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim. Biophys. Acta 2001, 1504, 379–395. [Google Scholar] [CrossRef]
- Saks, V.; Guzun, R.; Timohhina, N.; Tepp, K.; Varikmaa, M.; Monge, C.; Beraud, N.; Kaambre, T.; Kuznetsov, A.; Kadaja, L.; et al. Structure-function relationships in feedback regulation of energy fluxes in vivo in health and disease: Mitochondrial interactosome. Biochim. Biophys. Acta 2010, 1797, 678–697. [Google Scholar] [CrossRef] [PubMed]
- Vendelin, M.; Beraud, N.; Guerrero, K.; Andrienko, T.; Kuznetsov, A.V.; Olivares, J.; Kay, L.; Saks, V.A. Mitochondrial regular arrangement in muscle cells: A “crystal-like” pattern. Am J. Physiol. Cell Physiol. 2005, 288, C757–C767. [Google Scholar] [CrossRef] [PubMed]
- Anmann, T.; Guzun, R.; Beraud, N.; Pelloux, S.; Kuznetsov, A.V.; Kogerman, L.; Kaambre, T.; Sikk, P.; Paju, K.; Peet, N.; et al. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in hl-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim. Biophys. Acta 2006, 1757, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Tiivel, T.; Sikk, P.; Kaambre, T.; Kay, L.; Daneshrad, Z.; Rossi, A.; Kadaja, L.; Peet, N.; Seppet, E.; et al. Striking differences between the kinetics of regulation of respiration by adp in slow-twitch and fast-twitch muscles in vivo. Eur. J. Biochem. 1996, 241, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Burelle, Y.; Hochachka, P.W. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J. Appl. Physiol. 2002, 92, 2429–2438. [Google Scholar] [CrossRef] [PubMed]
- Tepp, K.; Timohhina, N.; Puurand, M.; Klepinin, A.; Chekulayev, V.; Shevchuk, I.; Kaambre, T. Bioenergetics of the aging heart and skeletal muscles: Modern concepts and controversies. Ageing Res. Rev. 2016, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.A.; Kaambre, T.; Sikk, P.; Eimre, M.; Orlova, E.; Paju, K.; Piirsoo, A.; Appaix, F.; Kay, L.; Regitz-Zagrosek, V.; et al. Intracellular energetic units in red muscle cells. Biochem. J. 2001, 356, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Rostovtseva, T.K.; Chamberlin, A.C.; Teijido, O.; Jiang, W.; Bezrukov, S.M. Current state of theoretical and experimental studies of the voltage-dependent anion channel (vdac). Biochim. Biophys. Acta 2016, 1858, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. The vdac channel: Molecular basis for selectivity. Biochim. Biophys. Acta 2016, 1863, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Beraud, N.; Kuznetsov, A.V.; Rostovtseva, T.; Sackett, D.; Schlattner, U.; Vendelin, M.; Saks, V.A. Regulation of respiration in brain mitochondria and synaptosomes: Restrictions of adp diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol. Cell. Biochem. 2008, 318, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Bezrukov, S.M. Vdac inhibition by tubulin and its physiological implications. Biochim. Biophys. Acta 2012, 1818, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Kaldma, A.; Klepinin, A.; Chekulayev, V.; Mado, K.; Shevchuk, I.; Timohhina, N.; Tepp, K.; Kandashvili, M.; Varikmaa, M.; Koit, A.; et al. An in situ study of bioenergetic properties of human colorectal cancer: The regulation of mitochondrial respiration and distribution of flux control among the components of atp synthasome. Int. J. Biochem. Cell Biol. 2014, 55, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Chekulayev, V.; Mado, K.; Shevchuk, I.; Koit, A.; Kaldma, A.; Klepinin, A.; Timohhina, N.; Tepp, K.; Kandashvili, M.; Ounpuu, L.; et al. Metabolic remodeling in human colorectal cancer and surrounding tissues: Alterations in regulation of mitochondrial respiration and metabolic fluxes. Biochem. Biophys. Rep. 2015, 4, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Vendelin, M.; Eimre, M.; Seppet, E.; Peet, N.; Andrienko, T.; Lemba, M.; Engelbrecht, J.; Seppet, E.K.; Saks, V.A. Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol. Cell. Biochem. 2004, 256, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.; Kuznetsov, A.; Andrienko, T.; Usson, Y.; Appaix, F.; Guerrero, K.; Kaambre, T.; Sikk, P.; Lemba, M.; Vendelin, M. Heterogeneity of adp diffusion and regulation of respiration in cardiac cells. Biophys. J. 2003, 84, 3436–3456. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Krause, S.; Roca, J.; Cascante, M. Modeling of spatial metabolite distributions in the cardiac sarcomere. Biophys. J. 2007, 92, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, A.E.; Reyes, S.; Selivanov, V.A.; Dzeja, P.P.; Terzic, A. Compartmentation of membrane processes and nucleotide dynamics in diffusion-restricted cardiac cell microenvironment. J. Mol. Cell Cardiol. 2012, 52, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kay, L.; Li, Z.; Mericskay, M.; Olivares, J.; Tranqui, L.; Fontaine, E.; Tiivel, T.; Sikk, P.; Kaambre, T.; Samuel, J.L.; et al. Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim. Biophys. Acta 1997, 1322, 41–59. [Google Scholar] [CrossRef]
- Timohhina, N.; Guzun, R.; Tepp, K.; Monge, C.; Varikmaa, M.; Vija, H.; Sikk, P.; Kaambre, T.; Sackett, D.; Saks, V. Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: Some evidence for mitochondrial interactosome. J. Bioenerg. Biomembr. 2009, 41, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Klaus, A.; Ramirez Rios, S.; Guzun, R.; Kay, L.; Tokarska-Schlattner, M. Cellular compartmentation of energy metabolism: Creatine kinase microcompartments and recruitment of b-type creatine kinase to specific subcellular sites. Amino Acids 2016, 48, 1751–1774. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Delannoy, M.; Hullihen, J.; Chiu, W.; Pedersen, P.L. Mitochondrial atp synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for pi and ADP/ATP. J. Biol. Chem. 2003, 278, 12305–12309. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ko, Y.; Delannoy, M.; Ludtke, S.J.; Chiu, W.; Pedersen, P.L. Mitochondrial ATP synthasome: Three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for pi and ADP/ATP. J. Biol. Chem. 2004, 279, 31761–31768. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.; Kuznetsov, A.V.; Gonzalez-Granillo, M.; Tepp, K.; Timohhina, N.; Karu-Varikmaa, M.; Kaambre, T.; Dos Santos, P.; Boucher, F.; Guzun, R. Intracellular energetic units regulate metabolism in cardiac cells. J. Mol. Cell. Cardiol. 2012, 52, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Guzun, R.; Saks, V. Application of the principles of systems biology and Wiener’s cybernetics for analysis of regulation of energy fluxes in muscle cells in vivo. Int. J. Mol. Sci. 2010, 11, 982–1019. [Google Scholar] [CrossRef] [PubMed]
- Tepp, K.; Shevchuk, I.; Chekulayev, V.; Timohhina, N.; Kuznetsov, A.V.; Guzun, R.; Saks, V.; Kaambre, T. High efficiency of energy flux controls within mitochondrial interactosome in cardiac intracellular energetic units. Biochim. Biophys. Acta 2011, 1807, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Khoo, J.C.; Russell, P.J. Isoenzymes of adenylate kinase in human tissue. Biochim. Biophys. Acta 1972, 268, 98–101. [Google Scholar] [CrossRef]
- Gellerich, F.N. The role of adenylate kinase in dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space. FEBS Lett. 1992, 297, 55–58. [Google Scholar] [CrossRef]
- Noma, T.; Song, S.; Yoon, Y.S.; Tanaka, S.; Nakazawa, A. Cdna cloning and tissue-specific expression of the gene encoding human adenylate kinase isozyme 2. Biochim. Biophys. Acta 1998, 1395, 34–39. [Google Scholar] [CrossRef]
- Noma, T. Dynamics of nucleotide metabolism as a supporter of life phenomena. J. Med. Investig. 2005, 52, 127–136. [Google Scholar] [CrossRef]
- Tanimura, A.; Horiguchi, T.; Miyoshi, K.; Hagita, H.; Noma, T. Differential expression of adenine nucleotide converting enzymes in mitochondrial intermembrane space: A potential role of adenylate kinase isozyme 2 in neutrophil differentiation. PLoS ONE 2014, 9, e89916. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Seo, M.; Yamada, Y.; Nakazawa, A. Increase of adenylate kinase isozyme 1 protein during neuronal differentiation in mouse embryonal carcinoma p19 cells and in rat brain primary cultured cells. J. Neurochem. 1998, 71, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.; Terzic, A. Adenylate kinase and amp signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 2009, 10, 1729–1772. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Chung, S.; Faustino, R.S.; Behfar, A.; Terzic, A. Developmental enhancement of adenylate kinase-ampk metabolic signaling axis supports stem cell cardiac differentiation. PLoS ONE 2011, 6, e19300. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Gahm, A.; Noma, T.; Nakazawa, A.; Orrenius, S.; Zhivotovsky, B. Release of adenylate kinase 2 from the mitochondrial intermembrane space during apoptosis. FEBS Lett. 1999, 447, 10–12. [Google Scholar] [CrossRef]
- Lee, H.J.; Pyo, J.O.; Oh, Y.; Kim, H.J.; Hong, S.H.; Jeon, Y.J.; Kim, H.; Cho, D.H.; Woo, H.N.; Song, S.; et al. Ak2 activates a novel apoptotic pathway through formation of a complex with fadd and caspase-10. Nat. Cell Biol. 2007, 9, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Dzeja, P.P.; Oerlemans, F.; Simonetti, A.W.; Heerschap, A.; de Haan, A.; Rush, P.S.; Terjung, R.R.; Wieringa, B.; Terzic, A. Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J. 2000, 19, 6371–6381. [Google Scholar] [CrossRef] [PubMed]
- Anmann, T.; Varikmaa, M.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Chekulayev, V.; Saks, V.; Kaambre, T. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim. Biophys. Acta 2014, 1837, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Tepp, K.; Puurand, M.; Timohhina, N.; Adamson, J.; Klepinin, A.; Truu, L.; Shevchuk, I.; Chekulayev, V.; Kaambre, T. Changes in the mitochondrial function and in the efficiency of energy transfer pathways during cardiomyocyte aging. Mol. Cell. Biochem. 2017, 432, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Nemutlu, E.; Gupta, A.; Zhang, S.; Viqar, M.; Holmuhamedov, E.; Terzic, A.; Jahangir, A.; Dzeja, P. Decline of phosphotransfer and substrate supply metabolic circuits hinders atp cycling in aging myocardium. PLoS ONE 2015, 10, e0136556. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.; Brdiczka, D.; Cusso, R.; Pette, D. Enhanced catalytic activity of hexokinase by work-induced mitochondrial binding in fast-twitch muscle of rat. FEBS Lett. 1997, 403, 279–282. [Google Scholar] [CrossRef]
- Nederlof, R.; Gurel-Gurevin, E.; Eerbeek, O.; Xie, C.; Deijs, G.S.; Konkel, M.; Hu, J.; Weber, N.C.; Schumacher, C.A.; Baartscheer, A.; et al. Reducing mitochondrial bound hexokinase II mediates transition from non-injurious into injurious ischemia/reperfusion of the intact heart. J. Physiol. Biochem. 2016, 73, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Smeele, K.M.; Wyatt, E.; Ichikawa, Y.; Eerbeek, O.; Sun, L.; Chawla, K.; Hollmann, M.W.; Nagpal, V.; Heikkinen, S.; et al. Reduction in hexokinase ii levels results in decreased cardiac function and altered remodeling after ischemia/reperfusion injury. Circ. Res. 2011, 108, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “warburg effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L.; Mathupala, S.; Rempel, A.; Geschwind, J.F.; Ko, Y.H. Mitochondrial bound type ii hexokinase: A key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim. Biophys. Acta 2002, 1555, 14–20. [Google Scholar] [CrossRef]
- Puurand, M.; Peet, N.; Piirsoo, A.; Peetsalu, M.; Soplepmann, J.; Sirotkina, M.; Peetsalu, A.; Hemminki, A.; Seppet, E. Deficiency of the complex I of the mitochondrial respiratory chain but improved adenylate control over succinate-dependent respiration are human gastric cancer-specific phenomena. Mol. Cell. Biochem. 2012, 370, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.; Kaambre, T.; Guzun, R.; Anmann, T.; Sikk, P.; Schlattner, U.; Wallimann, T.; Aliev, M.; Vendelin, M. The creatine kinase phosphotransfer network: Thermodynamic and kinetic considerations, the impact of the mitochondrial outer membrane and modelling approaches. Subcell. Biochem. 2007, 46, 27–65. [Google Scholar] [PubMed]
- Jacobus, W.E.; Saks, V.A. Creatine kinase of heart mitochondria: Changes in its kinetic properties induced by coupling to oxidative phosphorylation. Arch. Biochem. Biophys. 1982, 219, 167–178. [Google Scholar] [CrossRef]
- Saks, V.A.; Kuznetsov, A.V.; Kupriyanov, V.V.; Miceli, M.V.; Jacobus, W.E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J. Biol. Chem. 1985, 260, 7757–7764. [Google Scholar] [PubMed]
- Ventura-Clapier, R.; Kuznetsov, A.; Veksler, V.; Boehm, E.; Anflous, K. Functional coupling of creatine kinases in muscles: Species and tissue specificity. Mol. Cell. Biochem. 1998, 184, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Anflous, K.; Veksler, V.; Mateo, P.; Samson, F.; Saks, V.; Ventura-Clapier, R. Mitochondrial creatine kinase isoform expression does not correlate with its mode of action. Biochem. J. 1997, 322, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Seppet, E.K.; Eimre, M.; Anmann, T.; Seppet, E.; Peet, N.; Kaambre, T.; Paju, K.; Piirsoo, A.; Kuznetsov, A.V.; Vendelin, M.; et al. Intracellular energetic units in healthy and diseased hearts. Exp. Clin. Cardiol. 2005, 10, 173–183. [Google Scholar] [PubMed]
- Varikmaa, M.; Bagur, R.; Kaambre, T.; Grichine, A.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Chekulayev, V.; Metsis, M.; Boucher, F.; et al. Role of mitochondria-cytoskeleton interactions in respiration regulation and mitochondrial organization in striated muscles. Biochim. Biophys. Acta 2014, 1837, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Bera, S.; SinhaRoy, S.; Ghoshal, S.; Ray, S.; Basu, A.; Schlattner, U.; Wallimann, T.; Ray, M. Progressive decrease of phosphocreatine, creatine and creatine kinase in skeletal muscle upon transformation to sarcoma. FEBS J. 2008, 275, 3236–3247. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Ghosh, A.; Roy, S.S.; Bera, S.; Das, M.; Talukdar, D.; Ray, S.; Wallimann, T.; Ray, M. A short review on creatine-creatine kinase system in relation to cancer and some experimental results on creatine as adjuvant in cancer therapy. Amino Acids 2012, 42, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.J.; Hornung, T.V.; Garman, J.D.; Clayton, D.A.; O’Gorman, E.; Wallimann, T. Stimulation of mitochondrial gene expression and proliferation of mitochondria following impairment of cellular energy transfer by inhibition of the phosphocreatine circuit in rat hearts. J. Bioenerg. Biomembr. 1999, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Panayiotou, C.; Solaroli, N.; Karlsson, A. The many isoforms of human adenylate kinases. Int. J. Biochem. Cell Biol. 2014, 49, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Bortolon, R.; Perez-Terzic, C.; Holmuhamedov, E.L.; Terzic, A. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc. Natl. Acad. Sci. USA 2002, 99, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.W.; Yuan, Y.; Isaac, J.; Babu, C.V.; Meller, J.; Ho, S.M. Comprehensive identification and modified-site mapping of s-nitrosylated targets in prostate epithelial cells. PLoS ONE 2010, 5, e9075. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Go, V.L.; Hu, S. Membrane proteomic analysis of pancreatic cancer cells. J. Biomed. Sci. 2010, 17. [Google Scholar] [CrossRef] [PubMed]
- Klepinin, A.; Ounpuu, L.; Guzun, R.; Chekulayev, V.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Schlattner, U.; Kaambre, T. Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J. Bioenerg. Biomembr. 2016, 48, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Lagresle-Peyrou, C.; Six, E.M.; Picard, C.; Rieux-Laucat, F.; Michel, V.; Ditadi, A.; Demerens-de Chappedelaine, C.; Morillon, E.; Valensi, F.; Simon-Stoos, K.L.; et al. Human adenylate kinase 2 deficiency causes a profound hematopoietic defect associated with sensorineural deafness. Nat. Genet. 2009, 41, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Selivanov, V.A.; Alekseev, A.E.; Hodgson, D.M.; Dzeja, P.P.; Terzic, A. Nucleotide-gated katp channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol. Cell. Biochem. 2004, 256, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Eimre, M.; Paju, K.; Pelloux, S.; Beraud, N.; Roosimaa, M.; Kadaja, L.; Gruno, M.; Peet, N.; Orlova, E.; Remmelkoor, R.; et al. Distinct organization of energy metabolism in hl-1 cardiac cell line and cardiomyocytes. Biochim. Biophys. Acta 2008, 1777, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L. Voltage dependent anion channels (vdacs): A brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “warburg effect” in cancer. J. Bioenerg. Biomembr. 2008, 40, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Boil. 2003, 206, 2049–2057. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Mitochondrial hexokinases: Guardians of the mitochondria. Cell Cycle 2005, 4, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L. Warburg, me and hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “warburg effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr. 2007, 39, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Wilson, J.E. Functional organization of mammalian hexokinases: Both n- and c-terminal halves of the rat type ii isozyme possess catalytic sites. Arch. Biochem. Biophys. 1996, 329, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim. Biophys. Acta 2010, 1797, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Simson, P.; Jepihhina, N.; Laasmaa, M.; Peterson, P.; Birkedal, R.; Vendelin, M. Restricted adp movement in cardiomyocytes: Cytosolic diffusion obstacles are complemented with a small number of open mitochondrial voltage-dependent anion channels. J. Mol. Cell. Cardiol. 2016, 97, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Koit, A.; Shevchuk, I.; Ounpuu, L.; Klepinin, A.; Chekulayev, V.; Timohhina, N.; Tepp, K.; Puurand, M.; Truu, L.; Heck, K.; et al. Mitochondrial respiration in human colorectal and breast cancer clinical material is regulated differently. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
| Chemical | Stock Concentration (Solvent) | Notes | Storage (°C) |
|---|---|---|---|
| ADP | 0.2 M (water) | Adjust pH to 7.1 with KOH | −80, for a short time −20 |
| MgATP | 0.2 M (0.1 M HEPES buffer) | Add 0.2 M MgAc 4 H2O, adjust pH to 7.1 with NaOH | −80, for a short time −20 |
| AMP | 0.2 M (Mitomed, or 0.1 M HEPES) | −80, for a short time −20 | |
| Creatine | 0.2 M (water) | Keep the solution at +60 °C to avoid precipitation | Fresh |
| AP5A | 0.02 M (water) | −20 | |
| CAT | 0.2 mM (water) | −20 | |
| Cytochrome c | 2 mM (water) | −20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puurand, M.; Tepp, K.; Klepinin, A.; Klepinina, L.; Shevchuk, I.; Kaambre, T. Intracellular Energy-Transfer Networks and High-Resolution Respirometry: A Convenient Approach for Studying Their Function. Int. J. Mol. Sci. 2018, 19, 2933. https://doi.org/10.3390/ijms19102933
Puurand M, Tepp K, Klepinin A, Klepinina L, Shevchuk I, Kaambre T. Intracellular Energy-Transfer Networks and High-Resolution Respirometry: A Convenient Approach for Studying Their Function. International Journal of Molecular Sciences. 2018; 19(10):2933. https://doi.org/10.3390/ijms19102933
Chicago/Turabian StylePuurand, Marju, Kersti Tepp, Aleksandr Klepinin, Lyudmila Klepinina, Igor Shevchuk, and Tuuli Kaambre. 2018. "Intracellular Energy-Transfer Networks and High-Resolution Respirometry: A Convenient Approach for Studying Their Function" International Journal of Molecular Sciences 19, no. 10: 2933. https://doi.org/10.3390/ijms19102933
APA StylePuurand, M., Tepp, K., Klepinin, A., Klepinina, L., Shevchuk, I., & Kaambre, T. (2018). Intracellular Energy-Transfer Networks and High-Resolution Respirometry: A Convenient Approach for Studying Their Function. International Journal of Molecular Sciences, 19(10), 2933. https://doi.org/10.3390/ijms19102933





