Microparticles Produced by Activated Platelets Carry a Potent and Functionally Active Angiogenic Signal in Subjects with Crohn’s Disease
Abstract
1. Introduction
2. Results
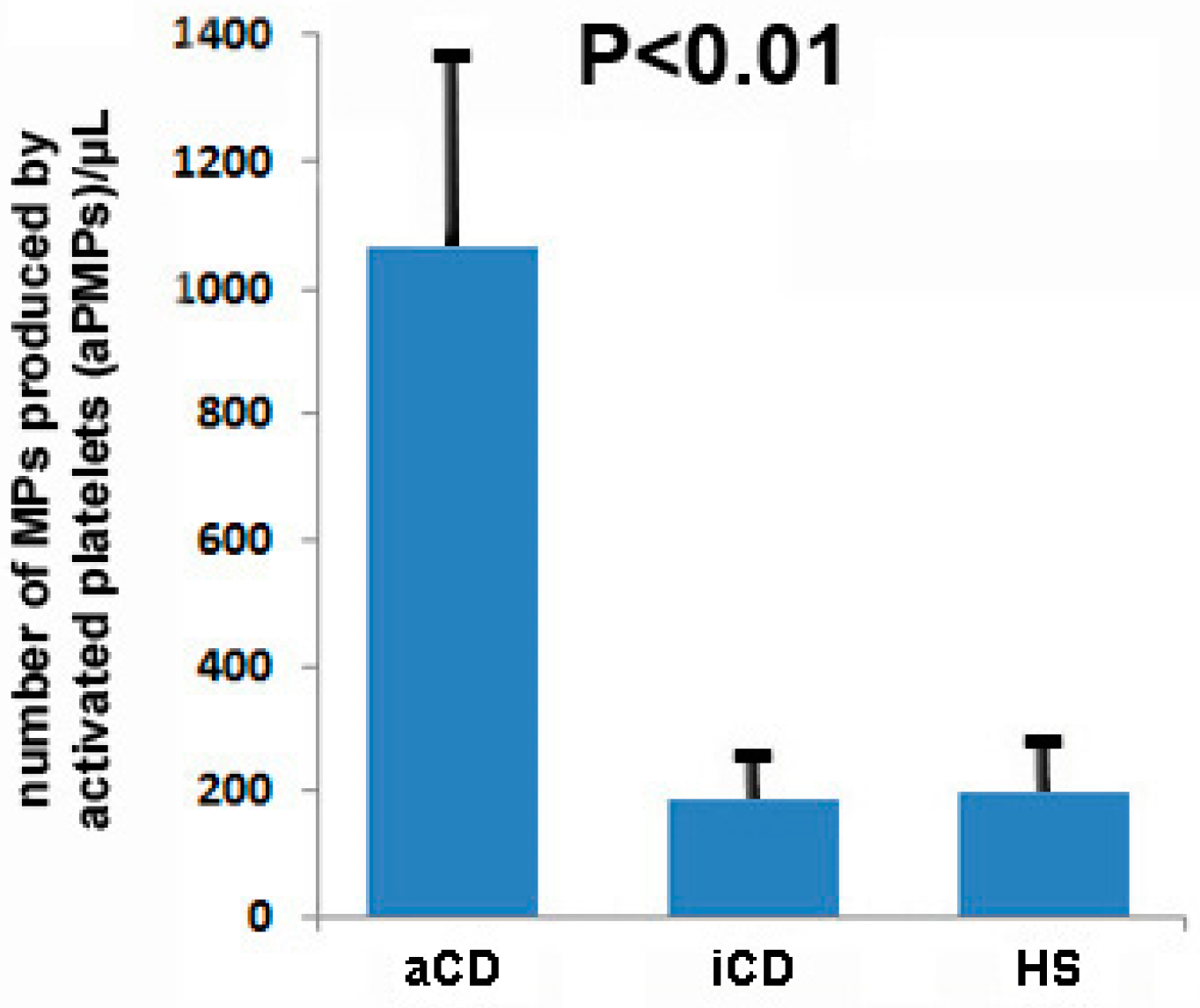
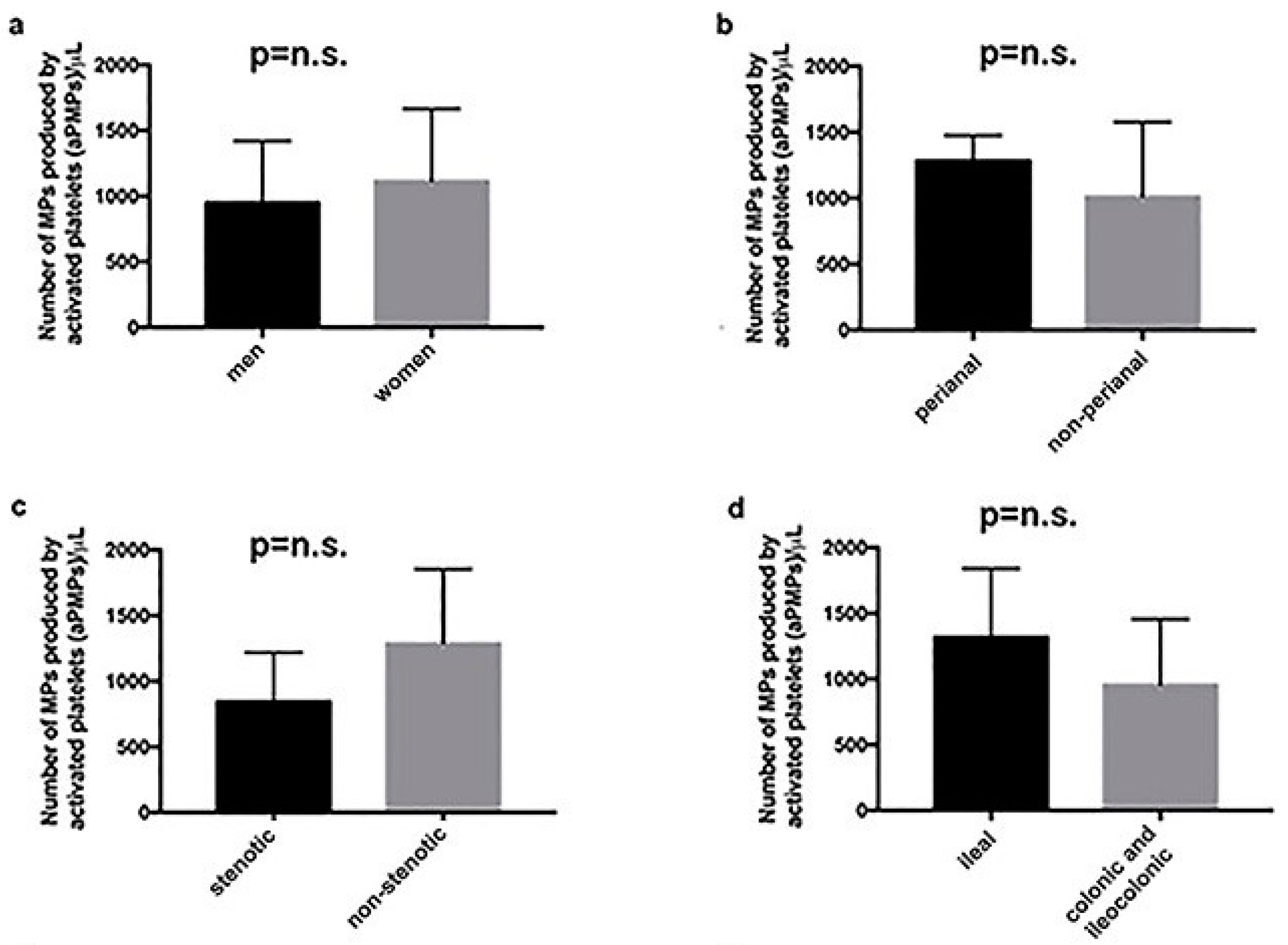
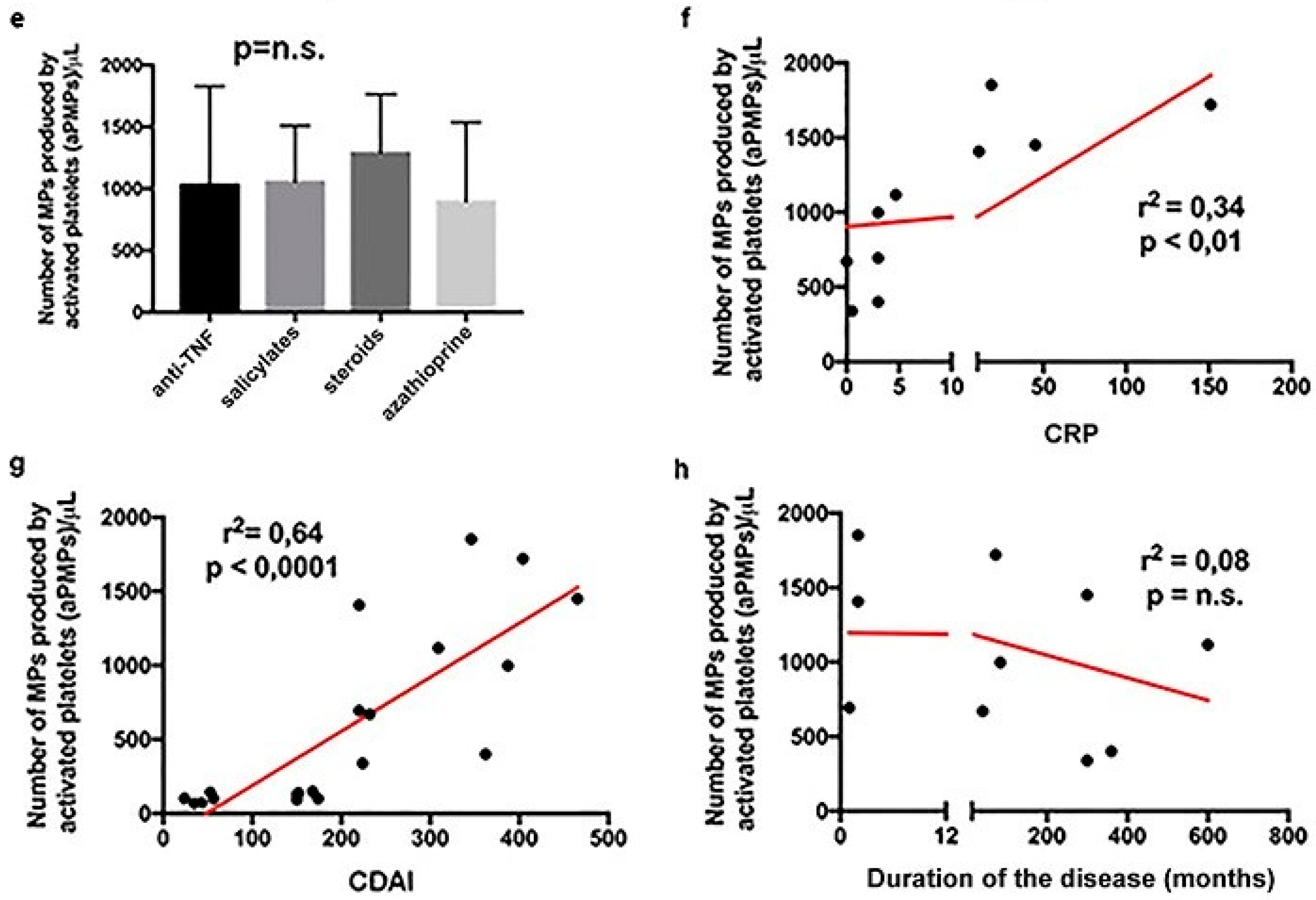
2.1. Subjects with aCD Have an Increased Number of Circulating aPMPs
2.2. Circulating aPMPs Are Rich in Angiogenic mRNAs and Proteins in Subjects with aCD
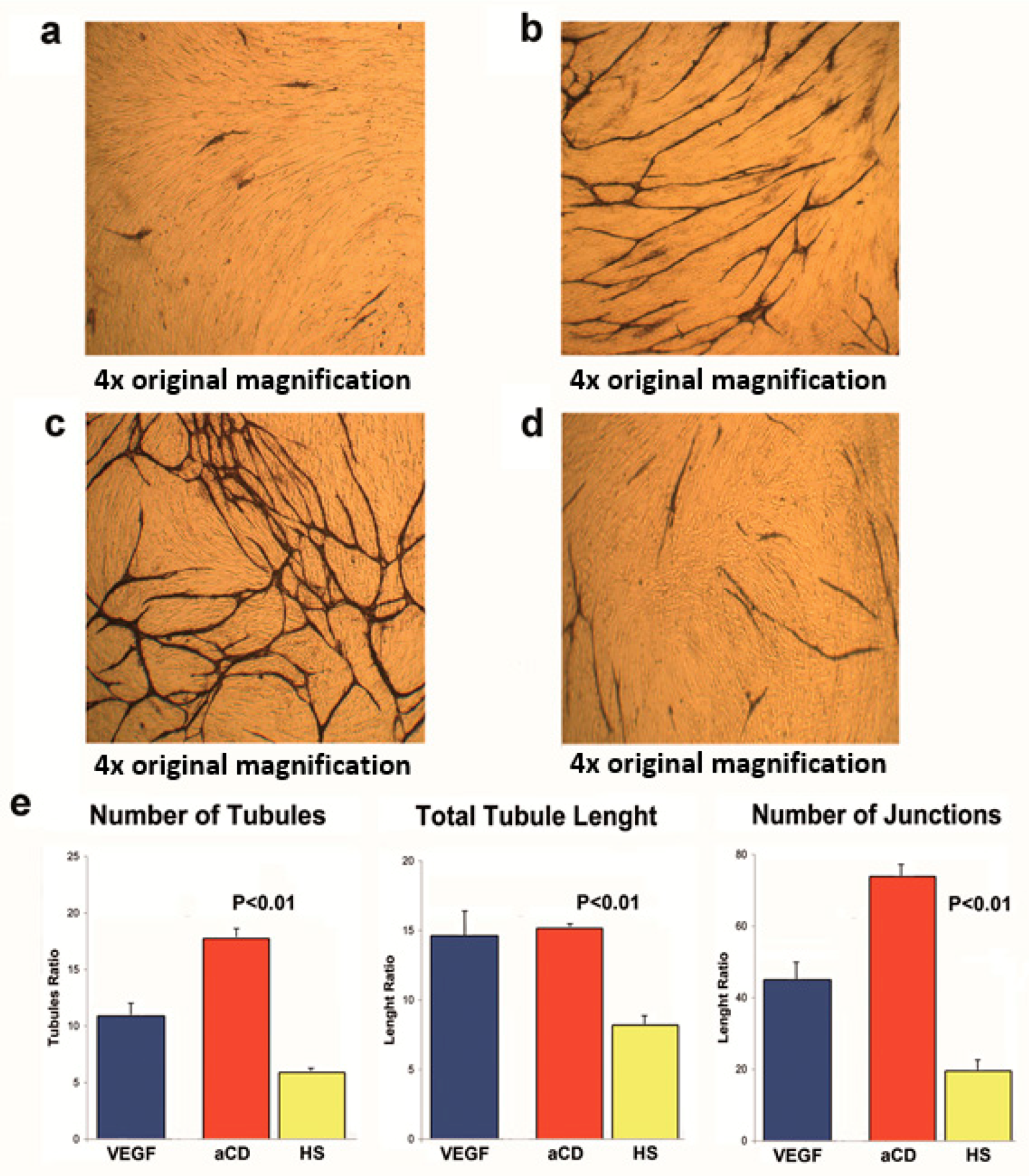
2.3. Proteins Extracted from aPMPs of Subjects with aCD Have the Ability to Promote Angiogenesis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation and Characterization of aPMPs
4.3. Angiogenic mRNA Signature of Circulating aPMPs
4.4. Analysis of Angiogenic Proteins Contained in Circulating aPMPs
4.5. Endothelial Cell/Interstitial Cell Co-Culture Assay
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Rautou, P.E.; Vion, A.C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef] [PubMed]
- McGinn, C.M.; MacDonnell, B.F.; Shan, C.X.; Wallace, R.; Cummins, P.M.; Murphy, R.P. Microparticles: A Pivotal Nexus in Vascular Homeostasis and Disease. Curr. Clin. Pharmacol. 2016, 11, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Chamouard, P.; Desprez, D.; Hugel, B.; Kunzelmann, C.; Gidon-Jeangirard, C.; Lessard, M.; Baumann, R.; Freyssinet, J.M.; Grunebaum, L. Circulating cell-derived microparticles in Crohn’s disease. Dig. Dis. Sci. 2005, 50, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Tsujikawa, T.; Hata, K.; Araki, Y.; Kitoh, K.; Sasaki, M.; Yoshida, T.; Fujiyama, Y. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am. J. Gastroenterol. 2005, 100, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, D.; Reimund, J.M.; Tesse, A.; Viennot, S.; Martinez, M.C.; Bretagne, A.L.; Andriantsitohaina, R. Circulating microparticles from Crohn’s disease patients cause endothelial and vascular dysfunctions. PLoS ONE 2013, 8, e73088. [Google Scholar] [CrossRef] [PubMed]
- Voudoukis, E.; Karmiris, K.; Koutroubakis, I.E. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. World J. Gastroenterol. 2014, 20, 3180–3190. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; de la Motte, C.; Graziani, C.; West, G.; Phillips, M.H.; Pola, R.; Rutella, S.; Willis, J.; Gasbarrini, A.; et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006, 130, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Vetrano, S.; Sans, M.; Arena, V.; Straface, G.; Stigliano, E.; Repici, A.; Sturm, A.; Malesci, A.; Panes, J.; et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 2009, 136, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Knod, L.; Donovan, E.C.; Chernoguz, A.; Crawford, K.M.; Dusing, M.R.; Frischer, J.S. Vascular endothelial growth factor receptor-2 inhibition in experimental murine colitis. J. Surg. Res. 2013, 184, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelet microparticles and miRNA transfer in cancer progression: Many targets, modes of action, and effects across cancer stages. Front. Cardiovasc. Med. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Anene, C.; Graham, A.M.; Boyne, J.; Roberts, W. Platelet microparticle delivered microRNA-Let-7a promotes the angiogenic switch. Biochim. Biophys. Acta 2018, 1864, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Polymeros, D.; Spathis, A.; Triantafyllou, M.; Gkolfakis, P.; Karakitsos, P.; Dimitriadis, G.; Triantafyllou, K. Increased levels of circulating platelet derived microparticles in Crohn’s disease patients. Scand. J. Gastroenterol. 2016, 51, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Muraro, D.; Simmons, A. An integrative analysis of gene expression and molecular interaction data to identify dys-regulated sub-networks in inflammatory bowel disease. BMC Bioinform. 2016, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Ohtani, H.; Nagai, T.; Funa, K.; Hiwatashi, N.O.; Shimosegawa Nagura, H. Platelet-derived growth factor and its receptors are expressed in areas of both active inflammation and active fibrosis in inflammatory bowel disease. Tohoku J. Exp. Med. 2001, 195, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Ciccocioppo, R.; Armellini, E.; Morera, R.; Ricevuti, L.; Cazzola, P.; Fulle, I.; Corazza, G.R. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn’s disease. Inflamm. Bowel Dis. 2004, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Algaba, A.; Linares, P.M.; Encarnación Fernández-Contreras, M.; Figuerola, A.; Calvet, X.; Guerra, I.; de Pousa, I.; Chaparro, M.; Gisbert, J.P.; Bermejo, F. The effects of infliximab or adalimumab on vascular endothelial growth factor and angiopoietin 1 angiogenic factor levels in inflammatory bowel disease: Serial observations in 37 patients. Inflamm. Bowel Dis. 2014, 20, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, K.A.; Kapsoritakis, A.N.; Kapsoritaki, A.I.; Manolakis, A.C.; Tiaka, E.K.; Tsiopoulos, F.D.; Tsiompanidis, I.A.; Potamianos, S.P. Angiogenin, angiopoietin-1, angiopoietin-2, and endostatin serum levels in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.H.; Braegger, C.P.; Sessa, W.C.; MacDonald, T.T. High endothelin-1 immunoreactivity in Crohn’s disease and ulcerative colitis. Lancet 1992, 339, 381–385. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Xia, L.; Xi, X.; Liu, B.; Yang, M. Serum pentraxin 3 is a novel marker in Crohn’s disease. Mol. Med. Rep. 2015, 12, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Hayon, Y.; Dashevsky, O.; Shai, E.; Brill, A.; Varon, D.; Leker, R.R. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr. Neurovasc. Res. 2012, 9, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Sasaki, K.; Ueno, T.; Seki, R.; Nakayoshi, T.; Koiwaya, H.; Toyama, Y.; Yokoyama, S.; Mitsutake, Y.; Chibana, H.; et al. Platelet-derived microparticles augment the adhesion and neovascularization capacities of circulating angiogenic cells obtained from atherosclerotic patients. Atherosclerosis 2013, 227, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 1989, 170, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F., Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [PubMed]
- Biscetti, F.; Gaetani, E.; Flex, A.; Aprahamian, T.; Hopkins, T.; Straface, G.; Pecorini, G.; Stigliano, E.; Smith, R.C.; Angelini, F.; et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)α and PPAR γ induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes 2008, 57, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
| Demographical and Clinical Characteristics | aCD | iCD | HS | p |
|---|---|---|---|---|
| Gender (men/women) | 16/24 | 13/17 | 18/22 | n.s. |
| Age (mean ± SD) | 44.1 ± 8.4 | 43.8 ± 7.9 | 45.1 ± 5.3 | n.s. |
| CDAI (mean ± SD) | 318.9 ± 95.3 | 105.5 ± 64.1 | – | <0.01 |
| Disease duration in months (mean ± SD) | 185.1 ± 102.5 | 151.6 ± 193.5 | – | n.s. |
| Type of disease (inflammatory/stenotic) | 26/14 | 17/13 | – | n.s |
| Extension of disease (only ileal/colonic or ileocolonic) | 10/30 | 7/23 | – | n.s. |
| Perianal disease (yes/no) | 9/31 | 5/25 | – | n.s. |
| Treatment (steroids/salicylates/azathioprine/anti-TNF) | 24/4/8/8 | 3/3/3/30 | – | n.s. |
| C-reactive protein (CRP) (mg/L) | 31.8 ± 56.7 | 1.6 ± 1.8 | 0.9 ± 0.8 | <0.01 |
| Total leukocytes (×1000/mcl) | 7.3 ± 3.2 | 6.5 ± 2.1 | 6.8 ± 1.5 | n.s. |
| Polymorphonuclear leukocytes (×1000/mcl) | 5.4 ± 3.4 | 3.7 ± 1.8 | 4.5 ± 1.9 | n.s. |
| Lymphocytes (×1000/mcl) | 1.5 ± 0.9 | 1.9 ± 0.7 | 1.7 ± 0.5 | n.s. |
| Platelets (×1000/mcl) | 335.7 ± 152.2 | 256.8 ± 51.8 | 318.1 ± 88.5 | n.s. |
| Hemoglobin (g/dL) | 10.8 ± 2.3 | 12.8 ± 1.7 | 13.8 ± 1.6 | <0.05 |
| mRNA | Fold-Change | p |
|---|---|---|
| EGF | +2.4 | 0.01 |
| FGF-2 | +4.9 | 0.006 |
| IGF-1 | +4.0 | 0.02 |
| PDGFα | +2.0 | 0.03 |
| ANGPT1 | +2.9 | 0.03 |
| MMP2 | +14.2 | 0.02 |
| MMP9 | +6.12 | 0.04 |
| FN1 | +5.6 | 0.04 |
| ITGAV | +6.5 | 0.04 |
| ITGB3 | +3.5 | 0.02 |
| SERPINF1 | −4.3 | 0.04 |
| VEGF-B | −5.5 | 0.01 |
| mRNA | Fold-Change | p |
|---|---|---|
| EGF | +3.2 | 0.01 |
| FGF-2 | +5.4 | 0.004 |
| PDGFα | +3.2 | 0.01 |
| ANGPT1 | +2.8 | 0.01 |
| Protein | Fold-Change | p |
|---|---|---|
| Angiogenin | +4.2 | 0.005 |
| Platelet factor-4 | +4.4 | 0.005 |
| SERPINF1 | +3.8 | 0.01 |
| Plasminogen activator inhibitor-1 | +3.5 | 0.01 |
| Metallopeptidase inhibitor 1 | +3.1 | 0.01 |
| Thrombospondin 1 | +4.2 | 0.01 |
| Protein | aCD Subjects | HS |
|---|---|---|
| Angiopoietin 1 | ++++/−−− | −−−−−−− |
| EGF | ++++/−−− | −−−−−−− |
| Endoglin | ++++/−−− | −−−−−−− |
| Endothelin-1 | +++++/−− | −−−−−−− |
| IGFBP-1 | ++++/−−− | −−−−−−− |
| IGFBP-2 | ++++/−−− | −−−−−−− |
| IGFBP-3 | ++++/−−− | −−−−−−− |
| TGFB1 | ++++/−−− | −−−−−−− |
| MMP8 | ++++/−−− | −−−−−−− |
| MMP9 | ++++/−−− | −−−−−−− |
| Pentraxin 3 | +++++/−− | −−−−−−− |
| VEGF-A | +++++/−− | −−−−−−− |
| PDGF-AA | ++++/−−− | −−−−−−− |
| PDGF-AB | ++++/−−− | −−−−−−− |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaetani, E.; Del Zompo, F.; Marcantoni, M.; Gatto, I.; Giarretta, I.; Porfidia, A.; Scaldaferri, F.; Laterza, L.; Lopetuso, L.; Gasbarrini, A.; et al. Microparticles Produced by Activated Platelets Carry a Potent and Functionally Active Angiogenic Signal in Subjects with Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2921. https://doi.org/10.3390/ijms19102921
Gaetani E, Del Zompo F, Marcantoni M, Gatto I, Giarretta I, Porfidia A, Scaldaferri F, Laterza L, Lopetuso L, Gasbarrini A, et al. Microparticles Produced by Activated Platelets Carry a Potent and Functionally Active Angiogenic Signal in Subjects with Crohn’s Disease. International Journal of Molecular Sciences. 2018; 19(10):2921. https://doi.org/10.3390/ijms19102921
Chicago/Turabian StyleGaetani, Eleonora, Fabio Del Zompo, Margherita Marcantoni, Ilaria Gatto, Igor Giarretta, Angelo Porfidia, Franco Scaldaferri, Lucrezia Laterza, Loris Lopetuso, Antonio Gasbarrini, and et al. 2018. "Microparticles Produced by Activated Platelets Carry a Potent and Functionally Active Angiogenic Signal in Subjects with Crohn’s Disease" International Journal of Molecular Sciences 19, no. 10: 2921. https://doi.org/10.3390/ijms19102921
APA StyleGaetani, E., Del Zompo, F., Marcantoni, M., Gatto, I., Giarretta, I., Porfidia, A., Scaldaferri, F., Laterza, L., Lopetuso, L., Gasbarrini, A., & Pola, R. (2018). Microparticles Produced by Activated Platelets Carry a Potent and Functionally Active Angiogenic Signal in Subjects with Crohn’s Disease. International Journal of Molecular Sciences, 19(10), 2921. https://doi.org/10.3390/ijms19102921









