Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease
Abstract
:1. Introduction
2. Results
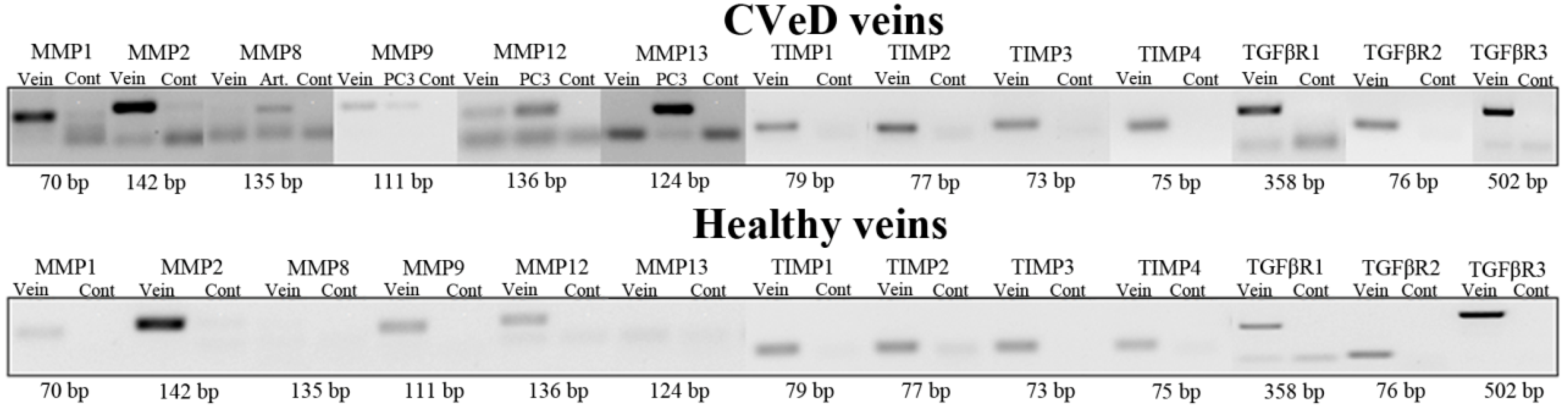
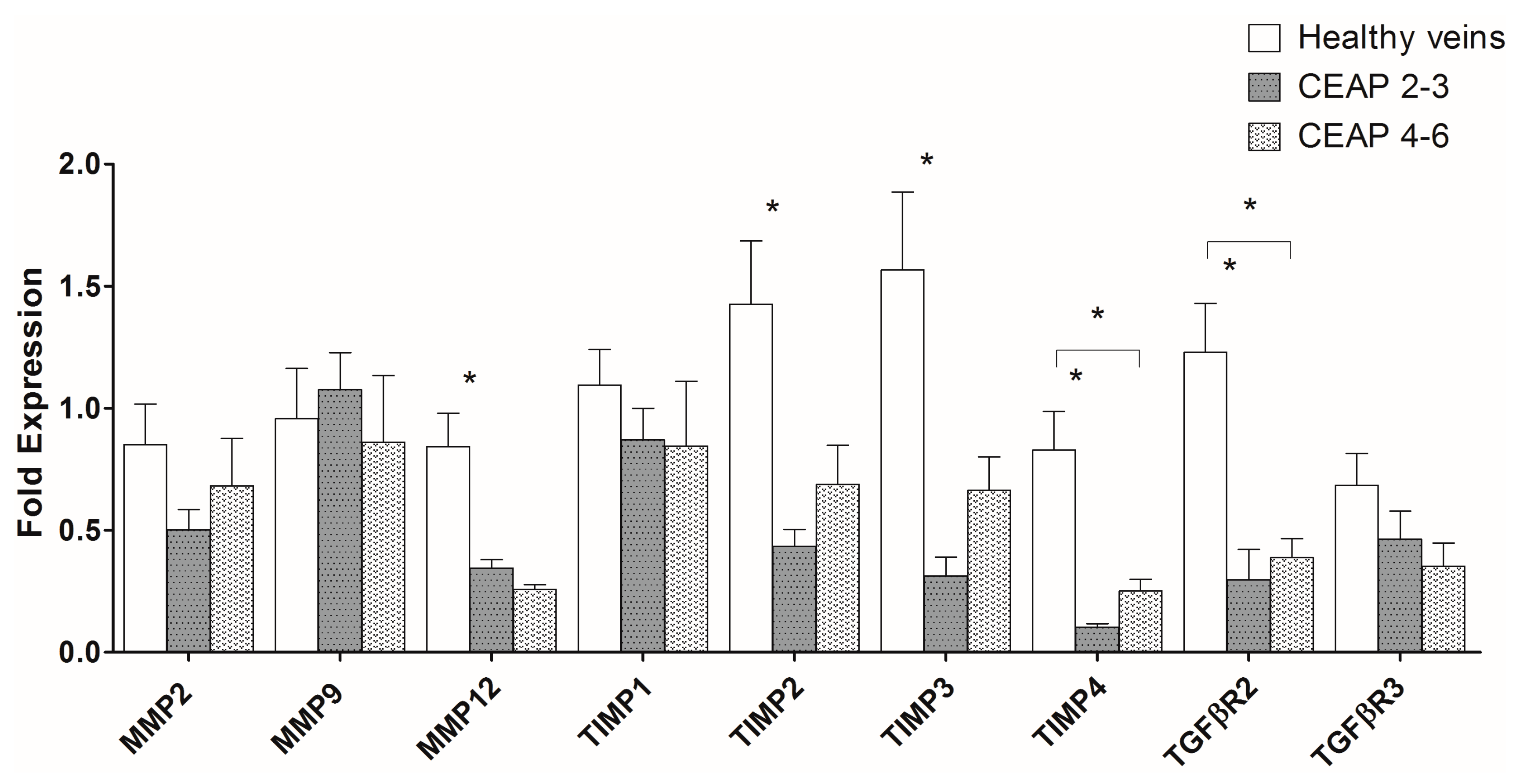
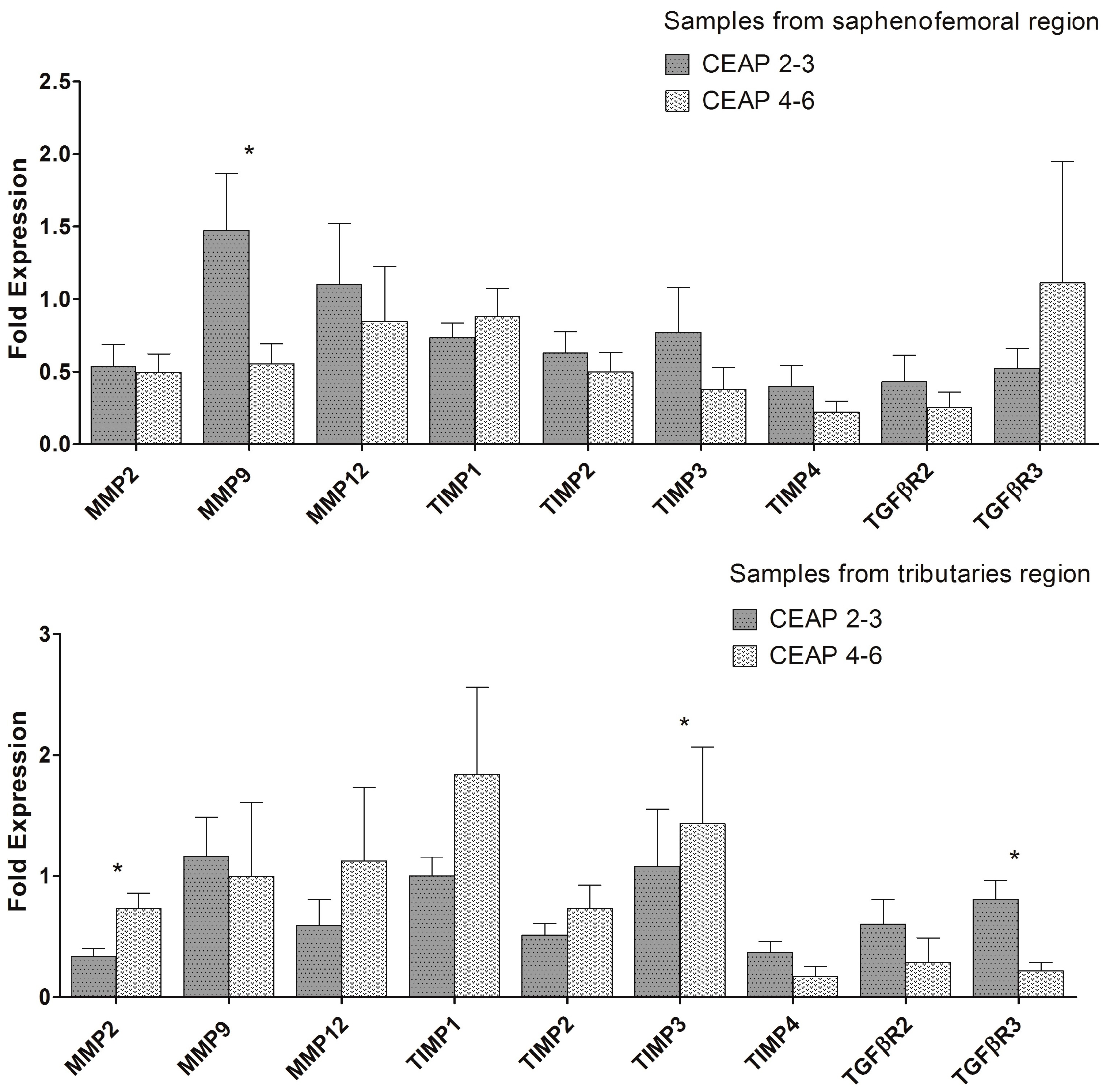
2.1. MMP, TIMP, and TGFβR Gene Expression in Healthy and Varicose Vein Walls
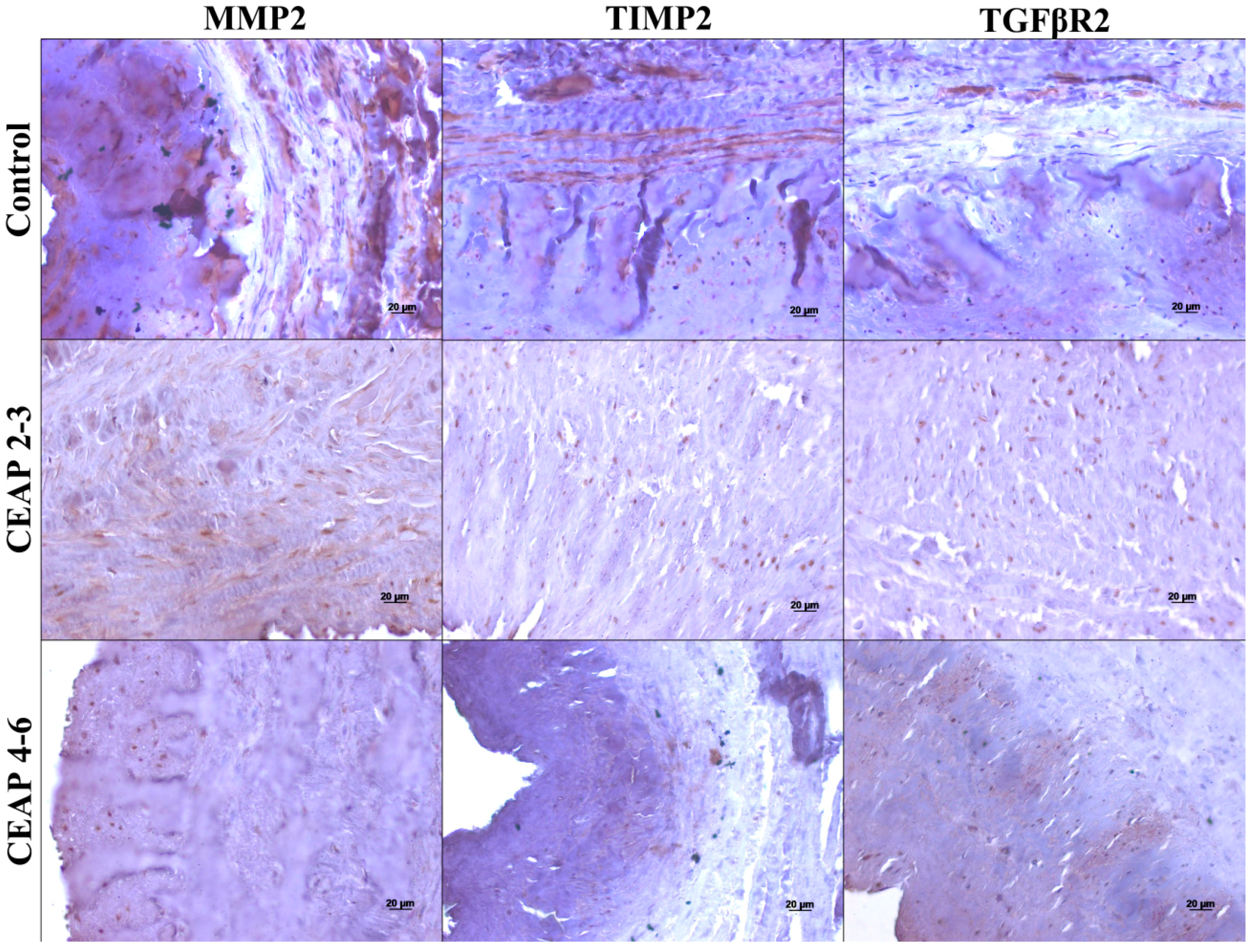
3.2. MMP, TIMP, and TGFβR Immunoreactivity in Healthy and Varicose Vein Walls
3. Discussion
4. Materials and Methods
4.1. Specimen Collection
4.2. Conventional and Quantitative Real-Time Polymerase Chain Reaction
4.3. Immunohistochemistry
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta 2010, 1803, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.S.; Crocker, S.J. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am. J. Pathol. 2012, 180, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases in remodeling of lower extremity veins and chronic venous disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299. [Google Scholar] [PubMed]
- Weiss, A.; Attisano, L. The TGF-β superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Bergan, J. Molecular mechanisms in chronic venous insufficiency. Ann. Vasc. Surg. 2007, 21, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.J.; You, R.; Rameshwar, P.; Gorti, R.; DeFouw, D.O.; Phillips, C.K.; Padberg, F.T., Jr.; Silva, M.B., Jr.; Simonian, G.T.; Hobson, R.W., II; et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-β1 gene expression and protein production. J. Vasc. Surg. 1999, 30, 1129–1145. [Google Scholar] [CrossRef]
- Takase, S.; Pascarella, L.; Lerond, L.; Bergan, J.J.; Schmid-Schonbein, G.W. Venous hypertension, inflammation and valve remodeling. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, P.; Soares, A.; Costa Almeida, C.M.; Verde, I. TGF-β1 in vascular wall pathology: Unraveling chronic venous insufficiency pathophysiology. Int. J. Mol. Sci. 2017, 18, 2534. [Google Scholar] [CrossRef] [PubMed]
- Saharay, M.; Shields, D.A.; Georgiannos, S.N.; Porter, J.B.; Scurr, J.H.; Coleridge Smith, P.D. Endothelial activation in patients with chronic venous disease. Eur. J. Vasc. Endovasc. Surg. 1998, 15, 342–349. [Google Scholar] [CrossRef]
- Saito, S.; Trovato, M.J.; You, R.; Lal, B.K.; Fasehun, F.; Padberg, F.T., Jr.; Hobson, R.W., II; Duran, W.N.; Pappas, P.J. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J. Vasc. Surg. 2001, 34, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, L.; Schmid-Schonbein, G.W.; Bergan, J. An animal model of venous hypertension: The role of inflammation in venous valve failure. J. Vasc. Surg. 2005, 41, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease—Part, II. Proteolytic biomarkers in wound healing. Biochim. Biophys. Acta 2016, 1862, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease—Part, I. Inflammatory biomarkers in wound healing. Biochim. Biophys. Acta 2016, 1862, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Ligi, D.; Canale, M.; Raffetto, J.D. Omics profiles in chronic venous ulcer wound fluid: Innovative applications for translational medicine. Expert Rev. Mol. Diagn. 2014, 14, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Woodside, K.J.; Hu, M.; Burke, A.; Murakami, M.; Pounds, L.L.; Killewich, L.A.; Daller, J.A.; Hunter, G.C. Morphologic characteristics of varicose veins: Possible role of metalloproteinases. J. Vasc. Surg. 2003, 38, 162–169. [Google Scholar] [CrossRef]
- Badier-Commander, C.; Verbeuren, T.; Lebard, C.; Michel, J.B.; Jacob, M.P. Increased TIMP/MMP ratio in varicose veins: A possible explanation for extracellular matrix accumulation. J. Pathol. 2000, 192, 105–112. [Google Scholar] [CrossRef]
- Gillespie, D.L.; Patel, A.; Fileta, B.; Chang, A.; Barnes, S.; Flagg, A.; Kidwell, M.; Villavicencio, J.L.; Rich, N.M. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J. Surg. Res. 2002, 106, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Asuwa, N.; Ishii, T.; Ito, K.; Akasaka, Y.; Masuda, T.; Zhang, L.; Kiguchi, H. Collagen alteration in vascular remodeling by hemodynamic factors. Virchows Arch. 2000, 437, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.P.; Cazaubon, M.; Scemama, A.; Prie, D.; Blanchet, F.; Guillin, M.C.; Michel, J.B. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation 2002, 106, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, I.; Urayama, H.; Kasashima, F.; Ohtake, H.; Watanabe, Y. Matrix metalloproteinase-9 and urokinase-type plasminogen activator in varicose veins. Ann. Vasc. Surg. 2003, 17, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, R.; Sobolewski, K.; Wolanska, M.; Gacko, M. Matrix metalloproteinases in the vein wall. Int. Angiol. 2004, 23, 164–169. [Google Scholar] [PubMed]
- Parra, J.R.; Cambria, R.A.; Hower, C.D.; Dassow, M.S.; Freischlag, J.A.; Seabrook, G.R.; Towne, J.B. Tissue inhibitor of metalloproteinase-1 is increased in the saphenofemoral junction of patients with varices in the leg. J. Vasc. Surg. 1998, 28, 669–675. [Google Scholar] [CrossRef]
- Sansilvestri-Morel, P.; Fioretti, F.; Rupin, A.; Senni, K.; Fabiani, J.N.; Godeau, G.; Verbeuren, T.J. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: Does the skin reflect venous matrix changes? Clin. Sci. 2007, 112, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sansilvestri-Morel, P.; Nonotte, I.; Fournet-Bourguignon, M.P.; Rupin, A.; Fabiani, J.N.; Verbeuren, T.J.; Vanhoutte, P.M. Abnormal deposition of extracellular matrix proteins by cultured smooth muscle cells from human varicose veins. J. Vasc. Res. 1998, 35, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sansilvestri-Morel, P.; Rupin, A.; Jullien, N.D.; Lembrez, N.; Mestries-Dubois, P.; Fabiani, J.N.; Verbeuren, T.J. Decreased production of collagen Type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: Possible implication of MMP-3. J. Vasc. Res. 2005, 42, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Maeng, Y.H.; Kim, S.W. Expression of matrix metalloproteinase-2 and -13 and tissue inhibitor of metalloproteinase-4 in varicose veins. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Davies, A.H. Pathogenesis of primary varicose veins. Br. J. Surg. 2009, 96, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, P.; Cairrao, E.; Maia, C.J.; Joao, M.; Almeida, C.M.; Verde, I. Effect of TGF-β1 on MMP/TIMP and TGF-β1 receptors in great saphenous veins and its significance on chronic venous insufficiency. Phlebology 2017, 32, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Wrana, J.L.; Sodek, J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-β. J. Biol. Chem. 1989, 264, 1860–1869. [Google Scholar] [PubMed]
- Kang, J.S.; Liu, C.; Derynck, R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009, 19, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Mechanisms of varicose vein formation: Valve dysfunction and wall dilation. Phlebology 2008, 23, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Nwomeh, B.C.; Liang, H.X.; Cohen, I.K.; Yager, D.R. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J. Surg. Res. 1999, 81, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, F.; Grassia, G.; Hu, Y.; Zhang, Z.; Xing, Q.; Yin, X.; Maddaluno, M.; Drung, B.; Schmidt, B.; et al. Matrix metalloproteinase-8 promotes vascular smooth muscle cell proliferation and neointima formation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Hingorani, A.; Ascher, E. Overexpression of transforming growth factor-β1 correlates with increased synthesis of nitric oxide synthase in varicose veins. J. Vasc. Surg. 2005, 41, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Mendieta, C.; Garcia-Honduvilla, N.; Corrales, C.; Bellon, J.M.; Bujan, J. TGF-β1 upregulation in the aging varicose vein. J. Vasc. Surg. 2007, 44, 192–201. [Google Scholar]
- Pocock, E.S.; Alsaigh, T.; Mazor, R.; Schmid-Schonbein, G.W. Cellular and molecular basis of Venous insufficiency. Vasc. Cell 2014, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, R.; Malkowski, A.; Gacko, M.; Sobolewski, K. Influence of thrombophlebitis on TGF-β1 and its signaling pathway in the vein wall. Folia Histochem. Cytobiol. 2010, 48, 542–548. [Google Scholar] [PubMed]
- Allison, M.A.; Cushman, M.; Callas, P.W.; Denenberg, J.O.; Jensky, N.E.; Criqui, M.H. Adipokines are associated with lower extremity venous disease: The San Diego population study. J. Thromb. Haemost. 2010, 8, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Badier-Commander, C.; Couvelard, A.; Henin, D.; Verbeuren, T.; Michel, J.B.; Jacob, M.P. Smooth muscle cell modulation and cytokine overproduction in varicose veins—An in situ study. J. Pathol. 2001, 193, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Liu, Z.; ten Dijke, P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rodriguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Aravind, B.; Saunders, B.; Navin, T.; Sandison, A.; Monaco, C.; Paleolog, E.M.; Davies, A.H. Inhibitory effect of TIMP influences the morphology of varicose veins. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Eklof, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Lange, I.G.; Daxenberger, A.; Meyer, H.H. Tissue-specific expression pattern of estrogen receptors (ER): Quantification of ERα and ERβ mRNA with real-time RT-PCR. Acta Pathol. Microbiol. Immunol. Scand. 2001, 109, 345–355. [Google Scholar] [CrossRef]

| Features | Control | CEAP2–3 | CEAP4–6 | |
|---|---|---|---|---|
| Sex | Females | 3 (23.08%) | 14 (73.68%) | 9 (75%) |
| Males | 10 (76.92%) | 5 (26.32%) | 3 (25%) | |
| Age (a) | 67.85 ± 2.679 (54–81) | 56.37 ± 1.764 (40–74) | 59.58 ± 2.930 (45–77) | |
| BMI (kg/m2) | 25.28 ± 0.935 (20.89–29.07) | 28.26 ± 1.072 (22.83–37.46) | 28.82 ± 1.232 (23.15–35.55) | |
| Pregnancies (No.) | 2.33 ± 1.856 (0–6) | 2.07 ± 0.322 (0–4) | 2.89 ± 0.351 (2–5) | |
| CEAP | 2 | - | 2 (6.45%) | - |
| 3 | - | 17 (54.84%) | - | |
| 4 | - | - | 10 (32.25%) | |
| 5 | - | - | 1 (3.23%) | |
| 6 | - | - | 1 (3.23%) | |
| Region | Tunica | Group | MMP2 | TIMP2 | TGFβR2 |
|---|---|---|---|---|---|
| Tibiotarsal junction | Intima | Controls | +++ | ++ | + |
| CEAP2–3 | + | − | −/+ | ||
| CEAP4–6 | + | + | + | ||
| Media | Controls | +++ | ++ | + | |
| CEAP2–3 | + | + | −/+ | ||
| CEAP4–6 | + | + | − | ||
| Adventitia | Controls | +++ | − | −/+ | |
| CEAP2–3 | − | −/+ | − | ||
| CEAP4–6 | − | − | − | ||
| Saphenofemoral junction | Intima | CEAP2–3 | ++ | +/++ | + |
| CEAP4–6 | + | + | + | ||
| Media | CEAP2–3 | ++ | +/++ | −/+ | |
| CEAP4–6 | −/+ | + | −/+ | ||
| Adventitia | CEAP2–3 | − | ++ | − | |
| CEAP4–6 | − | −/+ | − | ||
| Tributary | Intima | CEAP2–3 | + | − | −/+ |
| CEAP4–6 | + | −/+ | − | ||
| Media | CEAP2–3 | + | + | −/+ | |
| CEAP4–6 | − | −/+ | −/+ | ||
| Adventitia | CEAP2–3 | − | −/+ | − | |
| CEAP4–6 | − | − | − |
| Gene | Primer Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|
| β-actin | Sense: CAT CCT CAC CCT GAA GTA CCC | 202 |
| Antisense: AGC CTG GAT AGC AAC GTA CAT G | ||
| TIMP1 | Sense: GAC GGC CTT CTG CAA TTC C | 79 |
| Antisense: GTA TAA GGT GGT CTG GTT GAC TTC TG | ||
| TIMP2 | Sense: GAG CCT GAA CCA CAG GTA CCA | 77 |
| Antisense: AGG AGA TGT AGC ACG GGA TCA | ||
| TIMP3 | Sense: CCA GGA CGC CTT CTG CAA | 73 |
| Antisense: CCC CTC CTT TAC CAG CTT CTT C | ||
| TIMP4 | Sense: CAG CCT CAG CAG CAC ATC TG | 75 |
| Antisense: GGC CGG AAC TAC CTT CTC ACT | ||
| MMP1 | Sense: AAG ATG AAA GGT GGA CCA ACA ATT | 70 |
| Antisense: CCA AGA GAA TGG CCG AGT TC | ||
| MMP2 | Sense: AAC TAC GAT GAC GAC CGC AAG T | 142 |
| Antisense: AGG TGT AAA TGG GTG CCA TCA | ||
| MMP8 | Sense: CAC TCC CTC AAG ATG ACA TCG A | 135 |
| Antisense: ACG GAG TGT GGT GAT AGC ATC A | ||
| MMP9 | Sense: AGG CGC TCA TGT ACC CTA TGT AC | 111 |
| Antisense: GCC GTG GCT CAG GTT CA | ||
| MMP12 | Sense: CGC CTC TCT GCT GAT GAC ATA C | 136 |
| Antisense: GGT AGT GAC AGC ATC AAA ACT CAA A | ||
| MMP13 | Sense: AAA TTA TGG AGG AGA TGC CCA TT | 124 |
| Antisense: TCC TTG GAG TGG TCA AGA CCT AA | ||
| TGFβR1 | Sense: ACG GCG TTA CAG TGT TCT G | 358 |
| Antisense: GGT GTG GCA GAT ATA GAC C | ||
| TGFβR2 | Sense: GCA GGT GGG AAC TGC AAG AT | 76 |
| Antisense: GAA GGA CTC AAC ATT CTC CAA ATT C | ||
| TGFβR3 | Sense: CTG TTC ACC CGA CCT GAA AT | 502 |
| Antisense: CGT CAG GAG GCA CAC ACT TA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serralheiro, P.; Novais, A.; Cairrão, E.; Maia, C.; Costa Almeida, C.M.; Verde, I. Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease. Int. J. Mol. Sci. 2018, 19, 6. https://doi.org/10.3390/ijms19010006
Serralheiro P, Novais A, Cairrão E, Maia C, Costa Almeida CM, Verde I. Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease. International Journal of Molecular Sciences. 2018; 19(1):6. https://doi.org/10.3390/ijms19010006
Chicago/Turabian StyleSerralheiro, Pedro, António Novais, Elisa Cairrão, Cláudio Maia, Carlos M. Costa Almeida, and Ignacio Verde. 2018. "Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease" International Journal of Molecular Sciences 19, no. 1: 6. https://doi.org/10.3390/ijms19010006
APA StyleSerralheiro, P., Novais, A., Cairrão, E., Maia, C., Costa Almeida, C. M., & Verde, I. (2018). Variability of MMP/TIMP and TGF-β1 Receptors throughout the Clinical Progression of Chronic Venous Disease. International Journal of Molecular Sciences, 19(1), 6. https://doi.org/10.3390/ijms19010006







