Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation
Abstract
:1. Introduction
2. Results
2.1. Vancouver and Manchester Scar Scales at 6 and 12 Months
2.1.1. Vancouver Scar Scale (VSS)
2.1.2. Manchester Scar Scale (MSS)
2.2. Longitudinal Data from a Cutometer, Mexameter, Moisture Meter, and Color Meter (Table 2, Table 3 and Table 4)
2.2.1. Cutometer
2.2.2. Mexameter
2.2.3. Moisture Meter
2.2.4. Color Meter
3. Discussion
4. Materials and Methods
4.1. Moisture Meter
4.2. Color Meter
4.3. Cutometer
4.4. Mexameter
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CEA | Cultured Epithelial Autografts |
| GEE | generalized estimating equation |
| GLMM | generalized linear mixed model |
| JACE® | Commercially processed CEA (J-TEC Autologous Cultured Epidermis, JACE®, Japan Tissue Engineering CO., Ltd. (J-TEC) |
References
- Thompson, P.; Herndon, D.N.; Abston, S.; Rutan, T. Effect of early excision on patients with major thermal injury. J. Trauma 1987, 27, 205–207. [Google Scholar] [CrossRef] [PubMed]
- White, C.E.; Renz, E.M. Advances in surgical care: Management of severe burn injury. Crit. Care Med. 2008, 36 (Suppl. S7), S318–S324. [Google Scholar] [CrossRef] [PubMed]
- Hefton, J.M.; Madden, M.R.; Finkelstein, J.L.; Shires, G.T. Grafting of burn patients with allografts of cultured epidermal cells. Lancet 1983, 2, 428–430. [Google Scholar] [CrossRef]
- Cirodde, A.; Leclerc, T.; Jault, P.; Duhamel, P.; Lataillade, J.J.; Bargues, L. Cultured epithelial autografts in massive burns: A single-center retrospective study with 63 patients. Burns 2011, 37, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lineaweaver, W.C.; Sailes, F.C.; Kisner, C.; Zhang, F. Clinical application of cultured epithelial autografts on acellular dermal matrices in the treatment of extended burn injuries. Ann. Plast. Surg. 2014, 73, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Matsushima, A.; Ueyama, M.; Kumagai, N. Application of the cultured epidermal autograft “JACE®” for treatment of severe burns: Results of a 6-year multicenter surveillance in Japan. Burns 2016, 42, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Muramatsu, H.; Nakano, M.; Ito, H.; Inoie, M.; Tomizuka, Y.; Inoue, M.; Yoshimoto, S. Experience of using cultured epithelial autografts for the extensive burn wounds in eight patients. Ann. Plast. Surg. 2014, 73, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Correa, J.A.; de Oliveira, A.; LaSalle, L.; Perrault, I. Longitudinal burn scar quantification. Burns 2014, 40, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Correa, J.A.; Rachelska, G.; Armour, A.; LaSalle, L. Quantitative measurement of hypertrophic scar: Interrater reliability and concurrent validity. J. Burn Care Res. 2008, 29, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Saris, D.B.; Vanlauwe, J.; Victor, J.; Almqvist, K.F.; Verdonk, R.; Bellemans, J.; Luyten, F.P. Treatment of symptomatic cartilage defects of the knee. Am. J. Sports Med. 2009, 37 (Suppl. S1), 10S–19S. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Rzehak, P.; Szabó, É.; Jakobik, V.; Weck, M.; Weyermann, M.; Grallert, H.; Rothenbacher, D.; Heinrich, J.; Brenner, H.; et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 2011, 93, 382–391. [Google Scholar] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Vandeput, J.; Nelissen, M.; Tanner, J.C.; Boswick, J. A review of skin meshers. Burns 1995, 21, 364–370. [Google Scholar] [CrossRef]
- Gardien, K.L.; Marck, R.E.; Bloemen, M.C.; Waaijman, T.; Gibbs, S.; Ulrich, M.M.; Middelkoop, E.; Dutch Outback Study Group. Outcome of Burns Treated With Autologous Cultured Proliferating Epidermal Cells: A Prospective Randomized Multicenter Intrapatient Comparative Trial. Cell Transplant. 2016, 25, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Watanabe, T.; Sakamoto, A.; Yukawa, K.; Morimoto, K. Studies on the Physical Surface Area of Japanese. Jpn. J. Hyg. 1968, 23, 443–456. [Google Scholar] [CrossRef]
- Sullivan, T.; Smith, J.; Kermode, J.; McIver, E.; Courtemanche, D.J. Rating the burn scar. J. Burn Care Rehabil. 1990, 11, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Beausang, E.; Floyd, H.; Dunn, K.W.; Orton, C.I.; Ferguson, M.W. A new quantitative scale for clinical scar assessment. Plast. Reconstr. Surg. 1998, 102, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Akita, S.; Yoshimoto, H.; Houbara, S.; Hirano, A. Lipid-colloid dressing shows improved reepithelialization, pain relief, and corneal barrier function in split-thickness skin-graft donor wound healing. Int. J. Low Extrem. Wounds 2014, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Preece, S.J.; Claridge, E. Spectral filter optimization for the recovery of parameters which describe human skin. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, S.W.; Choi, T.H.; Kim, N.G.; Lee, K.S.; Kim, J.R.; Lee, S.I.; Kang, D.; Han, K.H.; Son, D.G.; et al. The evaluation of relevant factors influencing skin graft changes in color over time. Dermatol. Surg. 2008, 34, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Kobayashi, K.; Isomura, I.; Tsuji, T.; Krutmann, J. Ultraviolet A1 (340–400 nm) phototherapy for scleroderma in systemic sclerosis. J. Am. Acad. Dermatol. 2000, 43, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Draaijers, L.J.; Botman, Y.A.; Tempelman, F.R.; Kreis, R.W.; Middelkoop, E.; van Zuijlen, P.P. Skin elasticity meter or subjective evaluation in scars: A reliability assessment. Burns 2004, 30, 109–114. [Google Scholar] [CrossRef] [PubMed]
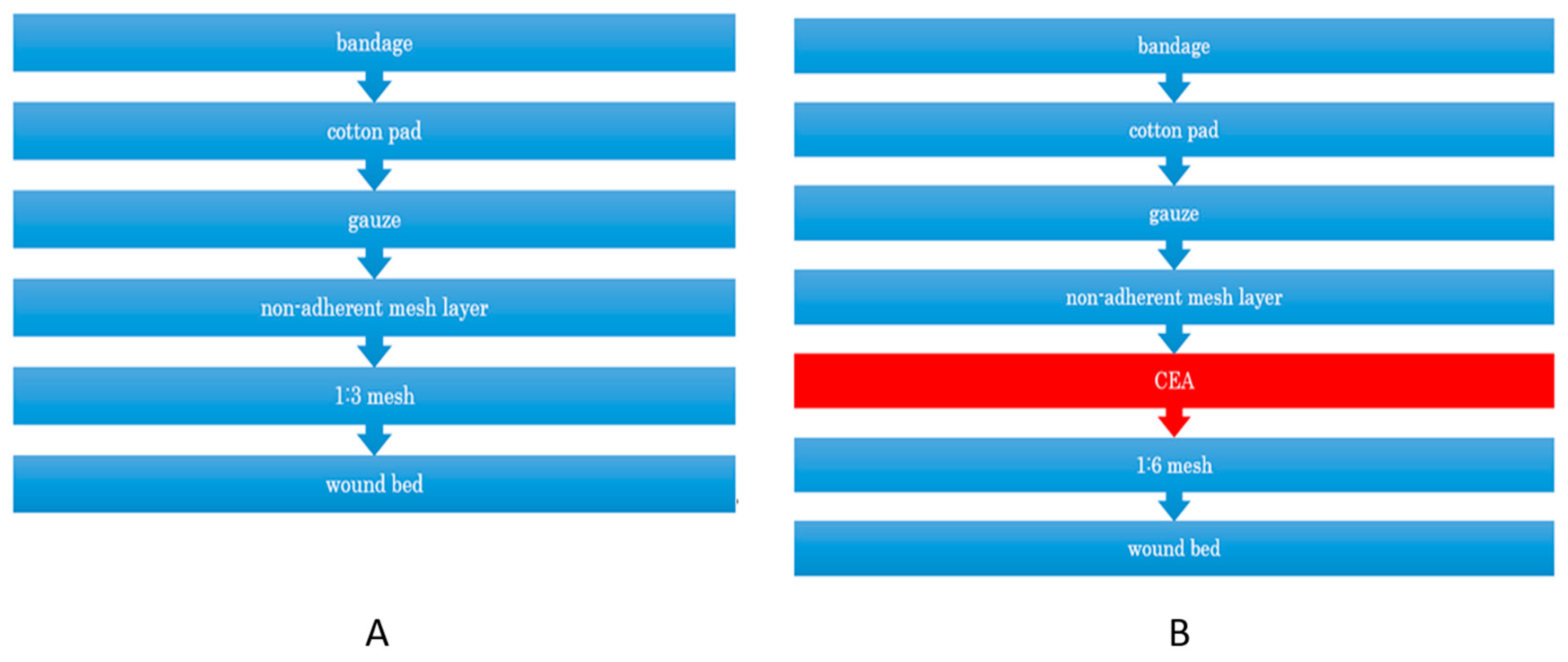
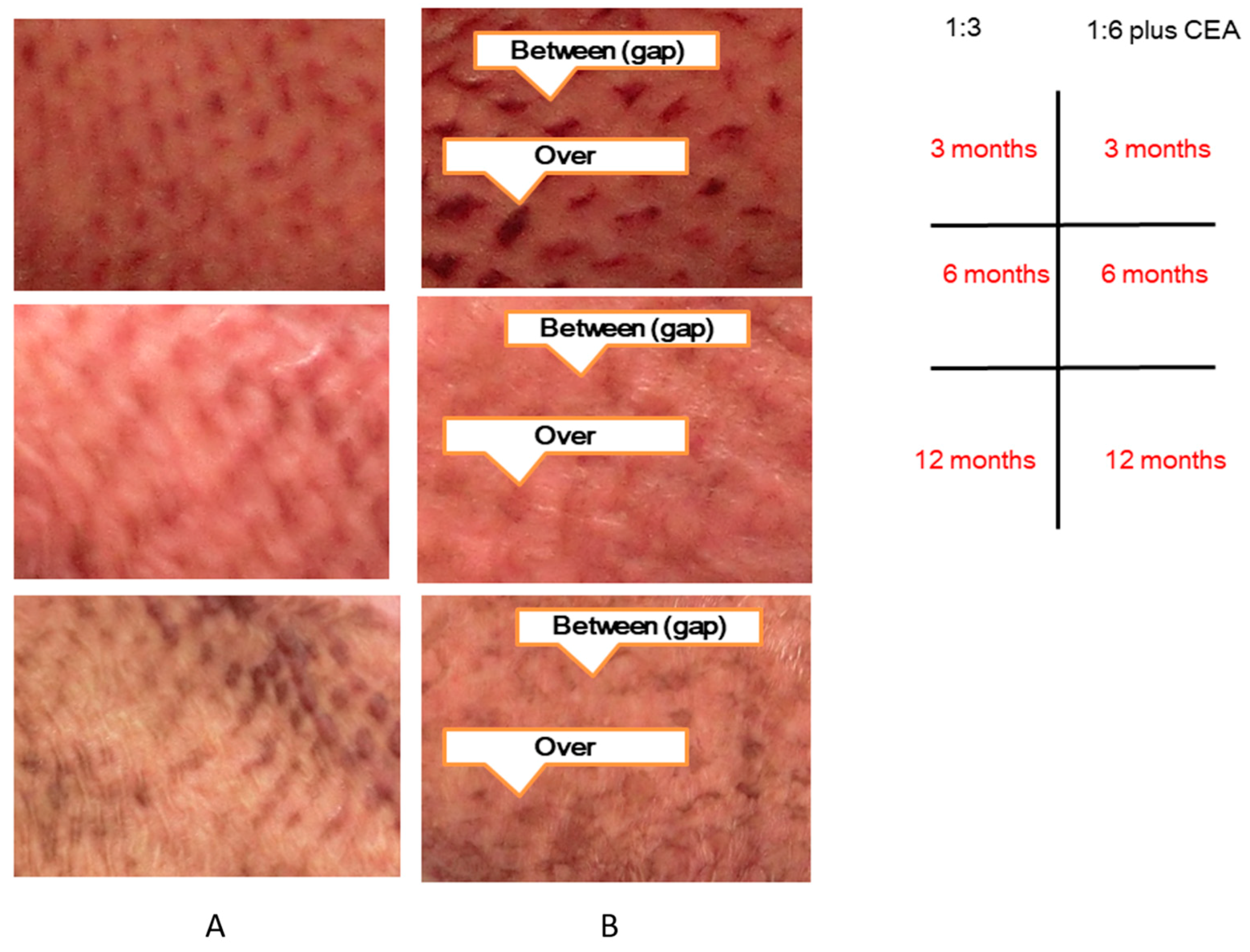
| Graft Type | 6 Months | 12 Months | ||
|---|---|---|---|---|
| VSS (Mean ± SE) | MSS (Mean ± SE) | VSS (Mean ± SE) | MSS (Mean ± SE) | |
| 1:6 gap + CEA | 6.6 (0.60) | 12.7 (0.81) | 5.6 (041) | 10.7 (0.70) |
| 1:6 over + CEA | 4.1 (0.50) ** | 8.7 (0.40) ** | 2.6 (0.40) ** | 7.3 (0.41) ** |
| 1:3 skin grafting | 5.8 (0.50) | 10.9 (0.61) | 5.1 (0.20) | 10.2 (0.40) |
| Linearity | Intercept | Slope | Selected Statistics | |
|---|---|---|---|---|
| Model 1 | non-linear | fixed | fixed | GEE |
| Model 2 | linear | fixed | fixed | GEE |
| Model 3 | linear | random | random | GLMM |
| Model 4 | linear | random | fixed | GLMM |
| Parameter | Over 1:6 Mesh Plus CEA (Mean ± SE) | Gap 1:6 Mesh Plus CEA (Mean ± SE) | 1:3 Mesh (Mean ± SE) |
|---|---|---|---|
| 3 Months | |||
| Maximal extensibility (R0) | 67.0 (±13.52) | 62.6 (±8.48) | 69.7 (±5.58) |
| Viscoelasticity (R7) | 126.6 (±18.67) | 93.7 (±13.06) | 108.2 (±14.87) |
| Melanin Index | 97.9 (±12.24) | 118.1 (±15.80) | 123.4 (±35.54) |
| Hemoglobin Index | 133.2 (±11.89) | 155.2 (±13.68) | 144.7 (±14.16) |
| Transepidermal Water Loss (TEWL) | 177.7 (±49.34) | 160.3 (±32.44) | 191.9 (±50.31) |
| Clarity | 84.9 (±4.01) | 79.9 (±3.21) | 85.9 (±3.23) |
| Red | 172.2 (±21.65) | 181.7 (±19.87) | 179.7 (±25.21) |
| Yellow | 72.7 (±4.30) | 66.9 (±3.99) | 75.9 (±5.60) |
| 6 Months | |||
| Maximal extensibility (R0) | 70.1 (±11.06) | 67.5 (±9.48) | 75.3 (±6.00) |
| Viscoelasticity (R7) | 118.0 (±16.20) | 87.3 (±11.23) | 95.1 (±12.53) |
| Melanin Index | 107.1 (±16.25) | 121.2 (±17.18) | 124.8 (±30.55) |
| Hemoglobin Index | 135.2 (±12.38) | 150.2 (±13.73) | 141.0 (±13.83) |
| Transepidermal Water Loss (TEWL) | 158.4 (±37.52) | 155.1 (±20.30) | 170.5 (±40.77) |
| Clarity | 86.8 (±3.74) | 82.7 (±3.15) | 87.8 (±2.82) |
| Red | 161.6 (±20.63) | 182.8 (±17.61) | 166.6 (±24.01) |
| Yellow | 77.2 (±4.98) | 70.6 (±3.73) | 80.4 (±5.22) |
| 12 Months | |||
| Maximal extensibility (R0) | 76.2 (±10.66) | 77.5 (±15.15) | 86.4 (±8.11) |
| Viscoelasticity (R7) | 100.7 (±15.78) | 74.4 (±9.54) | 68.7 (±9.08) |
| Melanin Index | 125.6 (±26.41) | 127.4 (±21.68) | 127.7 (±24.02) |
| Hemoglobin Index | 139.1 (±15.74) | 140.1 (±20.33) | 133.6 (±15.99) |
| Transepidermal Water Loss (TEWL) | 120.0 (±18.52) | 144.7 (±33.15) | 127.7 (±25.01) |
| Clarity | 90.6 (±3.58) | 88.2 (±3.35) | 91.8 (±2.38) |
| Red | 140.3 (±20.85) | 185.1 (±22.83) | 140.3 (±23.32) |
| Yellow | 86.3 (±6.61) | 77.9 (±4.16) | 89.2 (±5.90) |
| Selected Model | Comparision | Statistics among Groups | |
|---|---|---|---|
| Maximal extensibility (R0) by a cutometer | Model 4 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.7446 |
| Over 1:6 vs. 1:3 | n.s. p = 0.9919 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.5604 | ||
| Viscoelasticity (R7) by a cutometer | Model 2 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.2059 |
| Over 1:6 vs. 1:3 | n.s. p = 0.6296 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.3683 | ||
| Melanin index by a mexameter | Model 4 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.1653 |
| Over 1:6 vs. 1:3 | n.s. p = 0.4409 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.8756 | ||
| Hemoglobin index by a mexameter | Model 2 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.1670 |
| Over 1:6 vs. 1:3 | n.s. p = 0.3930 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.6004 | ||
| Transepidermal Water Loss (TEWL) by a moisture meter | Model 2 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.6926 |
| Over 1:6 vs. 1:3 | n.s. p = 0.8510 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.5432 | ||
| Clarity by a color meter | Model 4 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.3013 |
| Over 1:6 vs. 1:3 | n.s. p = 0.8910 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.1992 | ||
| Red by a color meter | Model 2 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.9463 |
| Over 1:6 vs. 1:3 | n.s. p = 0.7801 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.7376 | ||
| Yellow by color meter | Model 2 | Over 1:6 vs. Gap 1:6 | n.s. p = 0.4162 |
| Over 1:6 vs. 1:3 | n.s. p = 0.6559 | ||
| Gap 1:6 vs. 1:3 | n.s. p = 0.3040 |
| Inclusion Criteria |
|---|
| 20 years of age or older |
| Acute full-thickness burn wounds that require widely meshed skin grafting |
| Minimal TBSA of 30% with full thickness wounds |
| Minimal study wound area of 100 cm2 |
| Maximal study wound area of 300 cm2 |
| Informed consent |
| Exclusion criteria |
| Immunocompromised patients or immunosuppressed physical conditions |
| Non-compliance by the patient, judged by medical experts |
| Active infected wounds |
| Known drug allergy |
| Case | Age | Sex | Burn Depth and Percent | TBSA | PBI | How to Reconstruct “Dermis“ | Body Surface Area (m2) | Frequency of Grafts | Number of Sheets | Total CEA Covered BSA (%) | Percent Epithelialization at Four Weeks (%) | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | DDB 5% DB 40% | 45 | 98 | Terudermis | 1.441 | 2 | 20 + 20 | 22.2 | 100 | survive |
| 2 | 59 | M | DDB 30% DB 10% | 40 | 84 | none | 1.6705 | 1 | 7 | 3.4 | 100 | survive |
| 3 | 31 | M | DDB 30% DB 10% | 40 | 56 | none | 1.8623 | 1 | 20 | 8.6 | 100 | survive |
| 4 | 41 | M | DDB 10% DB 40% | 50 | 86 | Integra® | 1.9984 | 1 | 32 + 12 | 17.1 | 50 | survive |
| 5 | 63 | M | DDB 20% DB 25% | 45 | 98 | Integra® | 1.5958 | 2 | 20 + 20 | 20.1 | 100 | survive |
| 6 | 53 | F | DDB 35% DB 5% | 40 | 78 | Integra® | 1.42 | 1 | 22 | 12.4 | 95 | survive |
| 7 | 70 | M | DDB 10% DB 39% | 49 | 114 | Integra® | 1.7985 | 2 | 24 + 11 | 15.6 | 50 | deseased at 8 months |
| 8 | 46 | M | DDB 5% DB 45% | 50 | 91 | Integra® | 1.98 | 2 | 30 + 30 | 24.2 | 95 | survive |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, S.; Hayashida, K.; Yoshimoto, H.; Fujioka, M.; Senju, C.; Morooka, S.; Nishimura, G.; Mukae, N.; Kobayashi, K.; Anraku, K.; et al. Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation. Int. J. Mol. Sci. 2018, 19, 57. https://doi.org/10.3390/ijms19010057
Akita S, Hayashida K, Yoshimoto H, Fujioka M, Senju C, Morooka S, Nishimura G, Mukae N, Kobayashi K, Anraku K, et al. Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation. International Journal of Molecular Sciences. 2018; 19(1):57. https://doi.org/10.3390/ijms19010057
Chicago/Turabian StyleAkita, Sadanori, Kenji Hayashida, Hiroshi Yoshimoto, Masaki Fujioka, Chikako Senju, Shin Morooka, Gozo Nishimura, Nobuhiko Mukae, Kazuo Kobayashi, Kuniaki Anraku, and et al. 2018. "Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation" International Journal of Molecular Sciences 19, no. 1: 57. https://doi.org/10.3390/ijms19010057
APA StyleAkita, S., Hayashida, K., Yoshimoto, H., Fujioka, M., Senju, C., Morooka, S., Nishimura, G., Mukae, N., Kobayashi, K., Anraku, K., Murakami, R., Hirano, A., Oishi, M., Ikenoya, S., Amano, N., Nakagawa, H., & Nagasaki University plastic surgeons group. (2018). Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation. International Journal of Molecular Sciences, 19(1), 57. https://doi.org/10.3390/ijms19010057







