Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium
Abstract
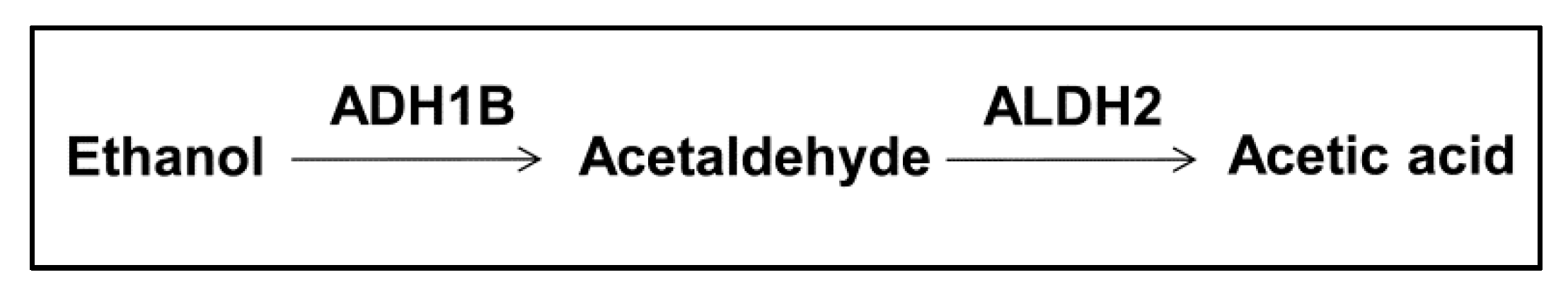
1. Acetaldehyde, Acetaldehyde Metabolism, and Risk of Cancers
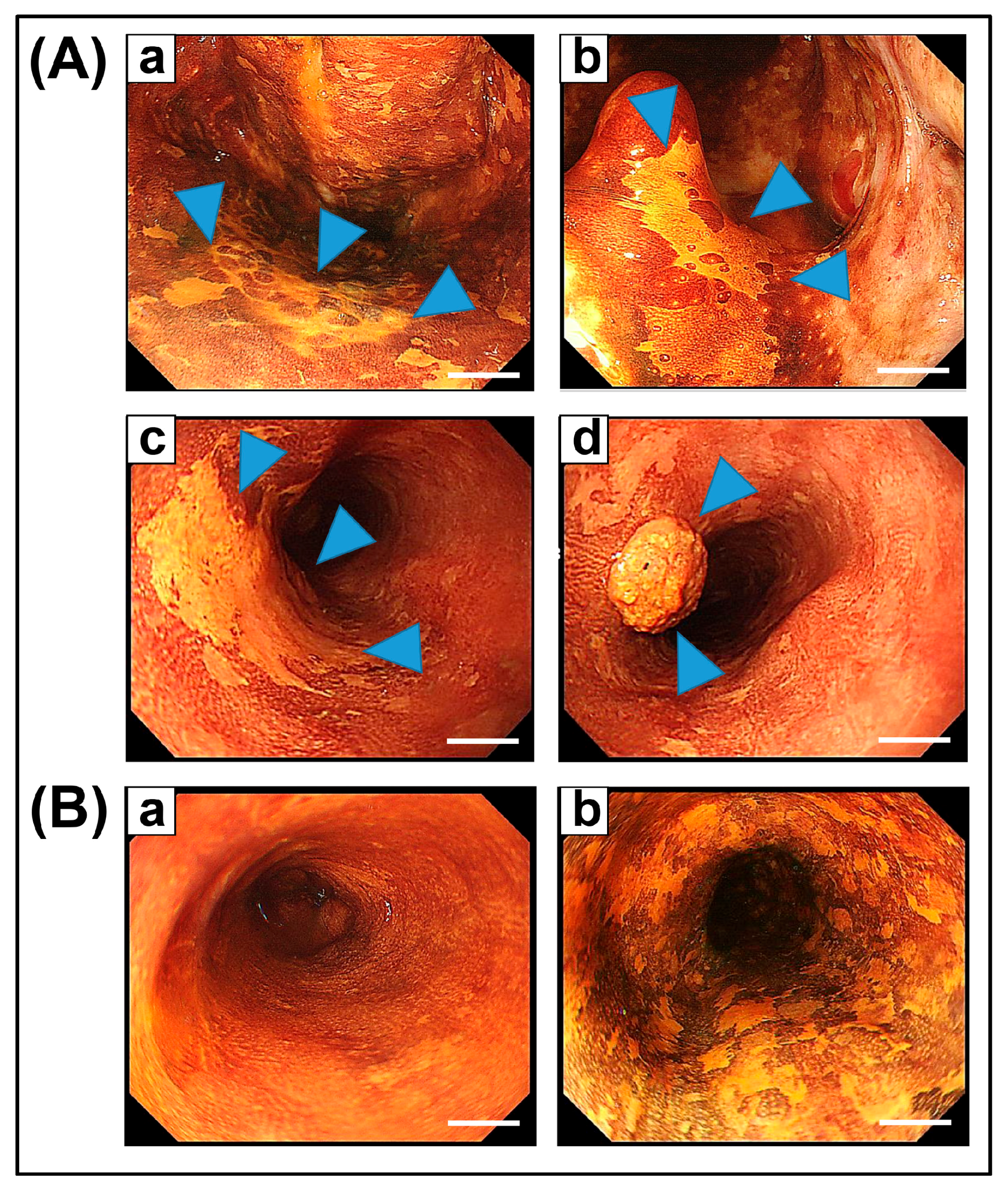
2. Field Cancerization in the Esophagus, and Head and Neck
3. Blood and Salivary Acetaldehyde Level after Alcohol Intake
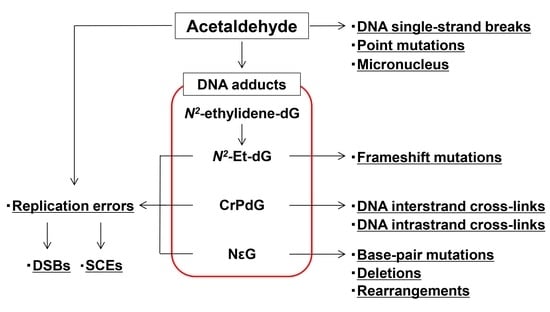
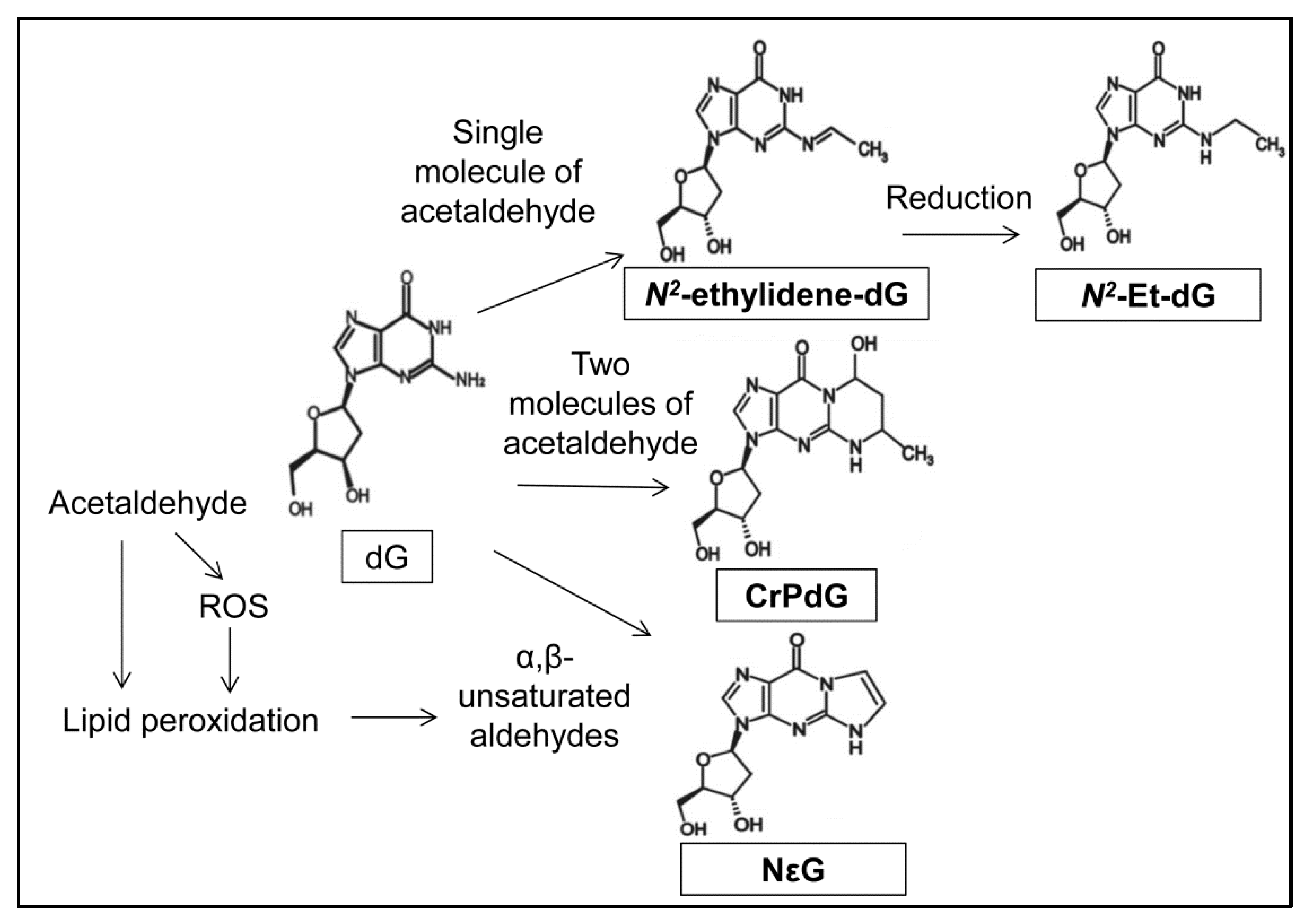
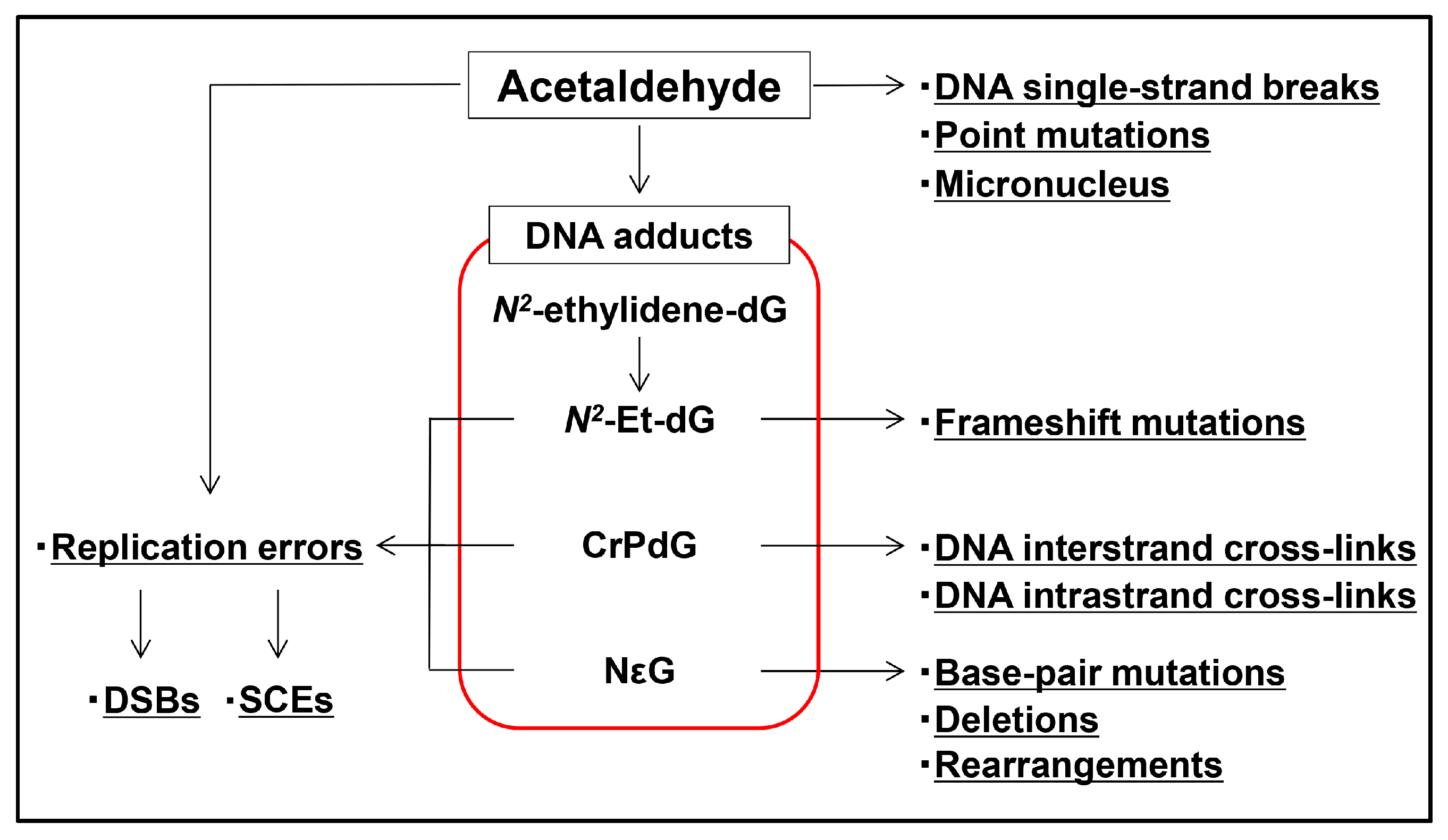
4. Acetaldehyde Reacts with DNA to Form DNA Adducts
5. DNA Adducts Induce Severe DNA Damage
6. Carcinogenic Effects of Acetaldehyde
7. Repair Pathways of Acetaldehyde-Mediated DNA Damage
8. Prevention of Acetaldehyde-Mediated DNA Damage
9. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH1B | Alcohol dehydrogenase 1B |
| ALDH2 | Aldehyde dehydrogenase 2 |
| BER | Base excision repair |
| CrPdG | α-S- and α-R-methyl-γ-hydroxy-1, N2-propano-2′-deoxyguanosine |
| dG | Deoxyguanosine |
| DSB | Double-strand break |
| ESCC | Esophageal squamous cell carcinoma |
| FA | Fanconi anemia |
| HNSCC | Head and neck squamous cell carcinoma |
| HR | Homologous recombination |
| LVL | Lugol-voiding lesion |
| NER | Nucleotide excision repair |
| N2-Et-dG | N2-ethyl-2′-deoxyguanosine |
| N2-ethylidene-dG | N2-ethylidene-2′-deoxyguanosine |
| NεG | 1,N2-etheno-2′-deoxyguanosine |
| PCNA | Proliferating cell nuclear antigen |
| ROS | Reactive oxygen species |
| SCE | Sister chromatid exchange |
| TLS | Translesion DNA synthesis |
| γ-H2AX | Phosphorylated histone H2AX |
References
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Zakhari, S. Acetaldehyde and the genome: Beyond nuclear DNA adducts and carcinogenesis. Environ. Mol. Mutagen. 2014, 55, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Uebelacker, M.; Lachenmeier, D.W. Quantitative determination of acetaldehyde in foods using automated digestion with simulated gastric fluid followed by headspace gas chromatography. J. Autom. Methods Manag. Chem. 2011, 2011, 907317. [Google Scholar] [CrossRef] [PubMed]
- Launoy, G.; Milan, C.; Day, N.E.; Pienkowski, M.P.; Gignoux, M.; Faivre, J. Diet and squamous-cell cancer of the oesophagus: A french multicentre case-control study. Int. J. Cancer 1998, 76, 7–12. [Google Scholar] [CrossRef]
- Salaspuro, V.J.; Hietala, J.M.; Marvola, M.L.; Salaspuro, M.P. Eliminating carcinogenic acetaldehyde by cysteine from saliva during smoking. Cancer Epidemiol. Biomark. Prev. 2006, 15, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Salaspuro, M.P. Acetaldehyde, microbes, and cancer of the digestive tract. Crit. Rev. Clin. Lab. Sci. 2003, 40, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Hitomi, Y.; Ohtsu, A.; Shimada, H.; Kashiwase, Y.; Sasaki, H.; Yoshida, S.; Esumi, H. Acetaldehyde production by non-pathogenic neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int. J. Cancer 2000, 88, 342–350. [Google Scholar] [CrossRef]
- Linderborg, K.; Joly, J.P.; Visapaa, J.P.; Salaspuro, M. Potential mechanism for calvados-related oesophageal cancer. Food Chem. Toxicol. 2008, 46, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Linderborg, K.; Salaspuro, M.; Vakevainen, S. A single sip of a strong alcoholic beverage causes exposure to carcinogenic concentrations of acetaldehyde in the oral cavity. Food Chem. Toxicol. 2011, 49, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Enoch, M.A.; Goldman, D.; Li, T.K.; Yokoyama, A. The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009, 6, e50. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Hamajima, N.; Shinoda, M.; Hatooka, S.; Inoue, M.; Takezaki, T.; Tajima, K. Gene-environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis 2001, 22, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J. Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk: A meta-analysis. World J. Gastroenterol. 2010, 16, 4210. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Muramatsu, T.; Omori, T.; Yokoyama, T.; Matsushita, S.; Higuchi, S.; Maruyama, K.; Ishii, H. Alcohol and aldehyde dehydrogenase gene polymorphisms and oropharyngolaryngeal, esophageal and stomach cancers in japanese alcoholics. Carcinogenesis 2001, 22, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Salaspuro, M. ALDH2-deficiency as genetic epidemiologic and biochemical model for the carcinogenicity of acetaldehyde. Regul. Toxicol. Pharmacol. 2017, 86, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Neumark, Y.D.; Friedlander, Y.; Durst, R.; Leitersdorf, E.; Jaffe, D.; Ramchandani, V.A.; O’Connor, S.; Carr, L.G.; Li, T.K. Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male jewish population. Alcohol Clin. Exp. Res. 2004, 28, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Wu, Q.; Li, Q.; Chen, D.; Xu, L.; Zhang, C.; Zhang, M.; Ye, L. Gene—Environment interactions on the risk of esophageal cancer among Asian populations with the G48A polymorphism in the alcohol dehydrogenase-2 gene: A meta-analysis. Tumour Biol. 2014, 35, 4705–4717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, N.; Miao, L.; Yuan, H.; Wang, R.; Jiang, H. Alcohol dehydrogenase-1B Arg47His polymorphism is associated with head and neck cancer risk in Asian: A meta-analysis. Tumour Biol. 2015, 36, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Takase, S.; Yasuhara, M.; Takada, A. Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes. Alcohol Clin. Exp. Res. 1991, 15, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Hao, W.; Fujita, Y.; Funayama, A.; Miyauchi, Y.; Hashimoto, K.; Miyamoto, K.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; et al. Aldehyde-stress resulting from ALDH2 mutation promotes osteoporosis due to impaired osteoblastogenesis. J. Bone Miner. Res. 2012, 27, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Mizukami, T.; Yokoyama, T. Genetic polymorphisms of alcohol dehydrogense-1B and aldehyde dehydrogenase-2, alcohol flushing, mean corpuscular volume, and aerodigestive tract neoplasia in japanese drinkers. Adv. Exp. Med. Biol. 2015, 815, 265–279. [Google Scholar] [PubMed]
- Harada, S.; Agarwal, D.P.; Goedde, H.W. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet (Lond. Engl.) 1981, 2, 982. [Google Scholar] [CrossRef]
- Yoshida, A.; Huang, I.Y.; Ikawa, M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in orientals. Proc. Natl. Acad. Sci. USA 1984, 81, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Matsushita, S.; Murayama, M.; Takagi, S.; Hayashida, M. Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am. J. Psychiatry 1995, 152, 1219–1221. [Google Scholar] [PubMed]
- Goedde, H.W.; Agarwal, D.P.; Fritze, G.; Meier-Tackmann, D.; Singh, S.; Beckmann, G.; Bhatia, K.; Chen, L.Z.; Fang, B.; Lisker, R.; et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum. Genet. 1992, 88, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Boccia, S.; Hashibe, M.; Galli, P.; De Feo, E.; Asakage, T.; Hashimoto, T.; Hiraki, A.; Katoh, T.; Nomura, T.; Yokoyama, A.; et al. Aldehyde dehydrogenase 2 and head and neck cancer: A meta-analysis implementing a mendelian randomization approach. Cancer Epidemiol. Biomark. Prev. 2009, 18, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Muto, M.; Nakane, M.; Hitomi, Y.; Yoshida, S.; Sasaki, S.; Ohtsu, A.; Yoshida, S.; Ebihara, S.; Esumi, H. Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis 2002, 23, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Adachi, Y.; Matsushima, T.; Matsuda, H.; Kuwano, H.; Sugimachi, K. Lugol staining pattern and histology of esophageal lesions. Am. J. Gastroenterol. 1993, 88, 701–705. [Google Scholar] [PubMed]
- Muto, M.; Hironaka, S.; Nakane, M.; Boku, N.; Ohtsu, A.; Yoshida, S. Association of multiple lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest. Endosc. 2002, 56, 517–521. [Google Scholar] [CrossRef]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Katada, C.; Yokoyama, T.; Yano, T.; Kaneko, K.; Oda, I.; Shimizu, Y.; Doyama, H.; Koike, T.; Takizawa, K.; Hirao, M.; et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology 2016, 151, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Tsutsumi, E.; Imazeki, H.; Suwa, Y.; Nakamura, C.; Yokoyama, T. Polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and the blood and salivary ethanol and acetaldehyde concentrations of japanese alcoholic men. Alcohol Clin. Exp. Res. 2010, 34, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, I.; Ohashi, S.; Amanuma, Y.; Hirohashi, K.; Mizumoto, A.; Funakoshi, M.; Tsurumaki, M.; Nakai, Y.; Tanaka, K.; Hanada, M.; et al. Establishment of a quick and highly accurate breath test for ALDH2 genotyping. Clin. Transl. Gastroenterol. 2017, 8, e96. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Tsutsumi, E.; Imazeki, H.; Suwa, Y.; Nakamura, C.; Mizukami, T.; Yokoyama, T. Salivary acetaldehyde concentration according to alcoholic beverage consumed and aldehyde dehydrogenase-2 genotype. Alcohol Clin. Exp. Res. 2008, 32, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; Peng, T.K.; Yin, S.J. Expression and activities of class Ⅳ alcohol dehydrogenase and class Ⅲ aldehyde dehydrogenase in human mouth. Alcohol 1996, 13, 257–262. [Google Scholar] [CrossRef]
- Vakevainen, S.; Tillonen, J.; Agarwal, D.P.; Srivastava, N.; Salaspuro, M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: Strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin. Exp. Res. 2000, 24, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; McIntee, E.J.; Cheng, G.; Shi, Y.; Villalta, P.W.; Hecht, S.S. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 2000, 13, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Vaca, C.E. Development of a 32P-postlabelling method for the analysis of adducts arising through the reaction of acetaldehyde with 2’-deoxyguanosine-3’-monophosphate and DNA. Carcinogenesis 1995, 16, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Vaca, C.E. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis 1997, 18, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; McIntee, E.J.; Wang, M. New DNA adducts of crotonaldehyde and acetaldehyde. Toxicology 2001, 166, 31–36. [Google Scholar] [CrossRef]
- Matsuda, T.; Matsumoto, A.; Uchida, M.; Kanaly, R.A.; Misaki, K.; Shibutani, S.; Kawamoto, T.; Kitagawa, K.; Nakayama, K.I.; Tomokuni, K.; et al. Increased formation of hepatic N2-ethylidene-2’-deoxyguanosine DNA adducts in aldehyde dehydrogenase 2-knockout mice treated with ethanol. Carcinogenesis 2007, 28, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, N.; Chen, L.; Villalta, P.W.; Hochalter, J.B.; Hecht, S.S. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem. Res. Toxicol. 2006, 19, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, H.; Matsumoto, A.; Nishi, R.; Kawamoto, T.; Ichiba, M.; Matsuda, T. Increased formation of gastric N2-ethylidene-2’-deoxyguanosine DNA adducts in aldehyde dehydrogenase-2 knockout mice treated with ethanol. Mutat. Res. 2009, 673, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Muto, M.; Hori, K.; Nagayoshi, H.; Yokoyama, A.; Chiba, T.; Matsuda, T. Combination of ADH1B*2/ALDH2*2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of japanese alcoholics. Cancer Sci. 2012, 103, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Ohashi, S.; Amanuma, Y.; Nakai, Y.; Tsurumaki, M.; Kikuchi, O.; Miyamoto, S.; Oyama, T.; Kawamoto, T.; Chiba, T.; et al. Impairment of aldehyde dehydrogenase 2 increases accumulation of acetaldehyde-derived DNA damage in the esophagus after ethanol ingestion. Am. J. Cancer Res. 2014, 4, 279–284. [Google Scholar] [PubMed]
- Balbo, S.; Meng, L.; Bliss, R.L.; Jensen, J.A.; Hatsukami, D.K.; Hecht, S.S. Kinetics of DNA adduct formation in the oral cavity after drinking alcohol. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Juanes, R.C.; Khariwala, S.; Baker, E.J.; Daunais, J.B.; Grant, K.A. Increased levels of the acetaldehyde-derived DNA adduct N2-ethyldeoxyguanosine in oral mucosa DNA from rhesus monkeys exposed to alcohol. Mutagenesis 2016, 31, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Hashibe, M.; Gundy, S.; Brennan, P.; Canova, C.; Simonato, L.; Merletti, F.; Richiardi, L.; Agudo, A.; Castellsague, X.; et al. N2-ethyldeoxyguanosine as a potential biomarker for assessing effects of alcohol consumption on DNA. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, M.; Villalta, P.W.; Luo, X.; Feuer, R.; Jensen, J.; Hatsukami, D.K.; Hecht, S.S. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chem. Res Toxicol. 2007, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Amanuma, Y.; Ohashi, S.; Itatani, Y.; Tsurumaki, M.; Matsuda, S.; Kikuchi, O.; Nakai, Y.; Miyamoto, S.; Oyama, T.; Kawamoto, T.; et al. Protective role of ALDH2 against acetaldehyde-derived DNA damage in oesophageal squamous epithelium. Sci. Rep. 2015, 5, 14142. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Angeli, J.P.; Freitas, F.P.; Gomes, O.F.; de Oliveira, T.F.; Loureiro, A.P.; di Mascio, P.; Medeiros, M.H. [13C2]-acetaldehyde promotes unequivocal formation of 1,N2-propano-2’-deoxyguanosine in human cells. J. Am. Chem. Soc. 2011, 133, 9140–9143. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Schnetz-Boutaud, N.C.; Weisenseel, J.P.; Marnett, L.J.; Stone, M.P. Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct. Proc. Natl. Acad. Sci. USA 1999, 96, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Minko, I.G.; Kozekov, I.D.; Harris, T.M.; Rizzo, C.J.; Lloyd, R.S.; Stone, M.P. Chemistry and biology of DNA containing 1,N2-deoxyguanosine adducts of the α,β-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem. Res. Toxicol. 2009, 22, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Theruvathu, J.A.; Jaruga, P.; Nath, R.G.; Dizdaroglu, M.; Brooks, P.J. Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005, 33, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Yabushita, H.; Kanaly, R.A.; Shibutani, S.; Yokoyama, A. Increased DNA damage in ALDH2-deficient alcoholics. Chem. Res. Toxicol. 2006, 19, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.P.; di Mascio, P.; Gomes, O.F.; Medeiros, M.H. Trans,trans-2,4-decadienal-induced 1, N2-etheno-2′-deoxyguanosine adduct formation. Chem. Res. Toxicol. 2000, 13, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Whelan, K.A.; Chandramouleeswaran, P.M.; Kagawa, S.; Rustgi, S.L.; Noguchi, C.; Guha, M.; Srinivasan, S.; Amanuma, Y.; Ohashi, S.; et al. ALDH2 modulates autophagy flux to regulate acetaldehyde-mediated toxicity thresholds. Am. J. Cancer Res. 2016, 6, 781–796. [Google Scholar] [PubMed]
- Matsuda, T.; Terashima, I.; Matsumoto, Y.; Yabushita, H.; Matsui, S.; Shibutani, S. Effective utilization of N2-ethyl-2′-deoxyguanosine triphosphate during DNA synthesis catalyzed by mammalian replicative DNA polymerases. Biochemistry 1999, 38, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Matsuda, T.; Fang, T.W.; Suzuki, N.; Kobayashi, J.; Kohda, K.; Shibutani, S. Miscoding potential of the N2-ethyl-2′-deoxyguanosine DNA adduct by the exonuclease-free klenow fragment of escherichia coli DNA polymerase i. Biochemistry 2001, 40, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Upton, D.C.; Wang, X.; Blans, P.; Perrino, F.W.; Fishbein, J.C.; Akman, S.A. Replication of N2-ethyldeoxyguanosine DNA adducts in the human embryonic kidney cell line 293. Chem. Res. Toxicol. 2006, 19, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Perrino, F.W.; Blans, P.; Harvey, S.; Gelhaus, S.L.; McGrath, C.; Akman, S.A.; Jenkins, G.S.; LaCourse, W.R.; Fishbein, J.C. The N2-ethylguanine and the O6-ethyl- and O6-methylguanine lesions in DNA: Contrasting responses from the "bypass" DNA polymerase eta and the replicative DNA polymerase α. Chem. Res. Toxicol. 2003, 16, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Theruvathu, J.A. DNA adducts from acetaldehyde: Implications for alcohol-related carcinogenesis. Alcohol 2005, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kawanishi, M.; Yagi, T.; Matsui, S.; Takebe, H. Specific tandem GG to TT base substitutions induced by acetaldehyde are due to intra-strand crosslinks between adjacent guanine bases. Nucleic Acids Res. 1998, 26, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Wang, H.; Kozekov, I.D.; Kurtz, A.J.; Jacob, J.; Voehler, M.; Smith, J.; Harris, T.M.; Lloyd, R.S.; Rizzo, C.J.; et al. Stereospecific formation of interstrand carbinolamine DNA cross-links by crotonaldehyde- and acetaldehyde-derived α-CH3-γ-OH-1,N2-propano-2’-deoxyguanosine adducts in the 5′-CpG-3′ sequence. Chem. Res. Toxicol. 2006, 19, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.H.; Kanuri, M.; Nechev, L.V.; Harris, T.M.; Lloyd, R.S. Mammalian cell mutagenesis of the DNA adducts of vinyl chloride and crotonaldehyde. Environ. Mol. Mutagen. 2005, 45, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Lao, Y.; Yang, I.Y.; Hecht, S.S.; Moriya, M. Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1, N2-propanodeoxyguanosine DNA adducts in human cells. Mutat. Res. 2006, 608, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Noori, P.; Hou, S.M. Mutational spectrum induced by acetaldehyde in the HPRT gene of human t lymphocytes resembles that in the p53 gene of esophageal cancers. Carcinogenesis 2001, 22, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Guengerich, F.P. Adduct size limits efficient and error-free bypass across bulky N2-guanine DNA lesions by human DNA polymerase eta. J. Mol. Biol. 2005, 352, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Guengerich, F.P. Kinetic evidence for inefficient and error-prone bypass across bulky N2-guanine DNA adducts by human DNA polymerase iota. J. Biol. Chem. 2006, 281, 12315–12324. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Angel, K.C.; Guengerich, F.P. Translesion synthesis across bulky N2-alkyl guanine DNA adducts by human DNA polymerase κ. J. Biol Chem. 2006, 281, 21062–21072. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, S.; Guengerich, F.P. Mutagenicity of site-specifically located 1,N2-ethenoguanine in chinese hamster ovary cell chromosomal DNA. Chem. Res. Toxicol. 1999, 12, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kotova, N.; Vare, D.; Schultz, N.; Gradecka Meesters, D.; Stepnik, M.; Grawe, J.; Helleday, T.; Jenssen, D. Genotoxicity of alcohol is linked to DNA replication-associated damage and homologous recombination repair. Carcinogenesis 2013, 34, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T. The frequency of sister chromatid exchanges in human lymphocytes treated with ethanol and acetaldehyde. Hereditas 1982, 97, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Thompson, L.H. Molecular mechanisms of sister-chromatid exchange. Mutat. Res. 2007, 616, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Khan, A. Acetaldehyde: Genotoxicity and cytotoxicity in human lymphocytes. Mutat. Res. 1995, 337, 9–17. [Google Scholar] [CrossRef]
- Obe, G.; Jonas, R.; Schmidt, S. Metabolism of ethanol in vitro produces a compound which induces sister-chromatid exchanges in human peripheral lymphocytes in vitro: Acetaldehyde not ethanol is mutagenic. Mutat. Res. 1986, 174, 47–51. [Google Scholar] [CrossRef]
- Dellarco, V.L. A mutagenicity assessment of acetaldehyde. Mutat. Res. 1988, 195, 1–20. [Google Scholar] [CrossRef]
- Helander, A.; Lindahl-Kiessling, K. Increased frequency of acetaldehyde-induced sister-chromatid exchanges in human lymphocytes treated with an aldehyde dehydrogenase inhibitor. Mutat. Res. 1991, 264, 103–107. [Google Scholar] [CrossRef]
- Lambert, B.; Chen, Y.; He, S.M.; Sten, M. DNA cross-links in human leucocytes treated with vinyl acetate and acetaldehyde in vitro. Mutat. Res. 1985, 146, 301–303. [Google Scholar] [CrossRef]
- Kayani, M.A.; Parry, J.M. The in vitro genotoxicity of ethanol and acetaldehyde. Toxicol. In Vitro 2010, 24, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Paget, V.; Lechevrel, M.; Sichel, F. Acetaldehyde-induced mutational pattern in the tumour suppressor gene tp53 analysed by use of a functional assay, the fasay (functional analysis of separated alleles in yeast). Mutat. Res. 2008, 652, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.C.; Hao, J.J.; Nagata, Y.; Xu, L.; Shang, L.; Meng, X.; Sato, Y.; Okuno, Y.; Varela, A.M.; Ding, L.W.; et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat. Genet. 2014, 46, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sawada, G.; Niida, A.; Uchi, R.; Hirata, H.; Shimamura, T.; Suzuki, Y.; Shiraishi, Y.; Chiba, K.; Imoto, S.; Takahashi, Y.; et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology 2016, 150, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar]
- Woutersen, R.A.; Appelman, L.M.; van Garderen-Hoetmer, A.; Feron, V.J. Inhalation toxicity of acetaldehyde in rats. Ⅲ. Carcinogenicity study. Toxicology 1986, 41, 213–231. [Google Scholar] [CrossRef]
- Feron, V.J.; Kruysse, A.; Woutersen, R.A. Respiratory tract tumours in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo(a)pyrene or diethylnitrosamine. Eur. J. Cancer Clin. Oncol. 1982, 18, 13–31. [Google Scholar] [CrossRef]
- Noguchi, C.; Grothusen, G.; Anandarajan, V.; Martinez-Lage Garcia, M.; Terlecky, D.; Corzo, K.; Tanaka, K.; Nakagawa, H.; Noguchi, E. Genetic controls of DNA damage avoidance in response to acetaldehyde in fission yeast. Cell Cycle 2017, 16, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, P.; Robins, P.; Lindahl, T.; Sedgwick, B. Demethylation of 3-methylthymine in DNA by bacterial and human DNA dioxygenases. J. Biol. Chem. 2004, 279, 40470–40474. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Pan, J.; Amin, S.; Chung, F.L.; Roy, R. Repair kinetics of trans-4-hydroxynonenal-induced cyclic 1,N2-propanodeoxyguanine DNA adducts by human cell nuclear extracts. Biochemistry 2004, 43, 7514–7521. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Nebert, D.W.; Bruford, E.A.; Thompson, D.C.; Joenje, H.; Vasiliou, V. Update of the human and mouse fanconi anemia genes. Hum. Genomics 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Clauson, C.; Scharer, O.D.; Niedernhofer, L. Advances in understanding the complex mechanisms of DNA interstrand cross-link repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012732. [Google Scholar] [CrossRef] [PubMed]
- Kottemann, M.C.; Smogorzewska, A. Fanconi anaemia and the repair of watson and crick DNA crosslinks. Nature 2013, 493, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; D’Andrea, A.D. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 2009, 43, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Walden, H.; Deans, A.J. The fanconi anemia DNA repair pathway: Structural and functional insights into a complex disorder. Annu. Rev. Biophys. 2014, 43, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.H.; Hinz, J.M. Cellular and molecular consequences of defective fanconi anemia proteins in replication-coupled DNA repair: Mechanistic insights. Mutat. Res. 2009, 668, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Balbo, S.; Crabb, D.; Brooks, P.J. Alcohol metabolism in human cells causes DNA damage and activates the fanconi anemia-breast cancer susceptibility (FA-BRCA) DNA damage response network. Alcohol Clin. Exp. Res. 2011, 35, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Marietta, C.; Thompson, L.H.; Lamerdin, J.E.; Brooks, P.J. Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: Implications for alcohol-related carcinogenesis. Mutat. Res. 2009, 664, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Obe, G.; Natarajan, A.T.; Meyers, M.; Hertog, A.D. Induction of chromosomal aberrations in peripheral lymphocytes of human blood in vitro, and of sces in bone-marrow cells of mice in vivo by ethanol and its metabolite acetaldehyde. Mutat. Res. 1979, 68, 291–294. [Google Scholar] [CrossRef]
- Mechilli, M.; Schinoppi, A.; Kobos, K.; Natarajan, A.T.; Palitti, F. DNA repair deficiency and acetaldehyde-induced chromosomal alterations in CHO cells. Mutagenesis 2008, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lorenti Garcia, C.; Mechilli, M.; Proietti De Santis, L.; Schinoppi, A.; Kobos, K.; Palitti, F. Relationship between DNA lesions, DNA repair and chromosomal damage induced by acetaldehyde. Mutat. Res. 2009, 662, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Langevin, F.; Crossan, G.P.; Rosado, I.V.; Arends, M.J.; Patel, K.J. FANCD2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature 2011, 475, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.J.; Malek, K.; Crabb, D.W. Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. J. Investig. Med. 1996, 44, 42–46. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. Int. J. Mol. Sci. 2017, 18, 1943. https://doi.org/10.3390/ijms18091943
Mizumoto A, Ohashi S, Hirohashi K, Amanuma Y, Matsuda T, Muto M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. International Journal of Molecular Sciences. 2017; 18(9):1943. https://doi.org/10.3390/ijms18091943
Chicago/Turabian StyleMizumoto, Ayaka, Shinya Ohashi, Kenshiro Hirohashi, Yusuke Amanuma, Tomonari Matsuda, and Manabu Muto. 2017. "Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium" International Journal of Molecular Sciences 18, no. 9: 1943. https://doi.org/10.3390/ijms18091943
APA StyleMizumoto, A., Ohashi, S., Hirohashi, K., Amanuma, Y., Matsuda, T., & Muto, M. (2017). Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. International Journal of Molecular Sciences, 18(9), 1943. https://doi.org/10.3390/ijms18091943