Melatonin and Hippo Pathway: Is There Existing Cross-Talk?
Abstract
1. Introduction
2. Melatonin Membrane Receptors
MT1 and MT2 Mediated Signal Transduction
3. Melatonin and Nuclear Receptors: Contrasting Evidence
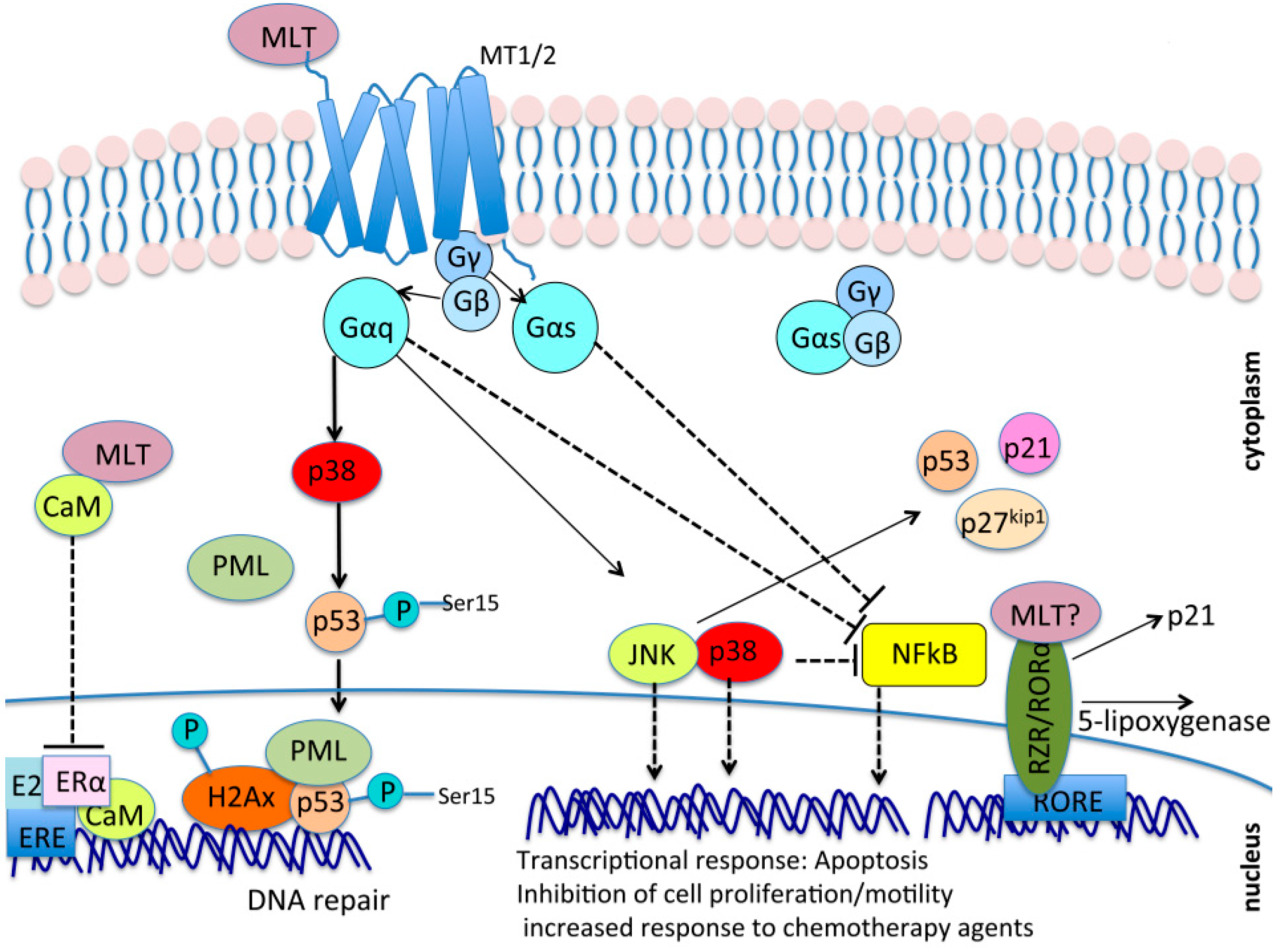
4. Oncoprotective Role of Melatonin: In Vitro Evidence
5. Melatonin Antioxidant Properties
6. Clinical Studies
6.1. Circadian Disruption and Increased Light Exposure Contribute to Increased Cancer Risk
6.2. Low Levels of Endogenous Melatonin or Altered Expression of Its Receptors Are Associated with Increased Cancer Risk
7. A Possible Crosstalk between Melatonin Signaling and the Hippo Tumor Suppressor Pathway
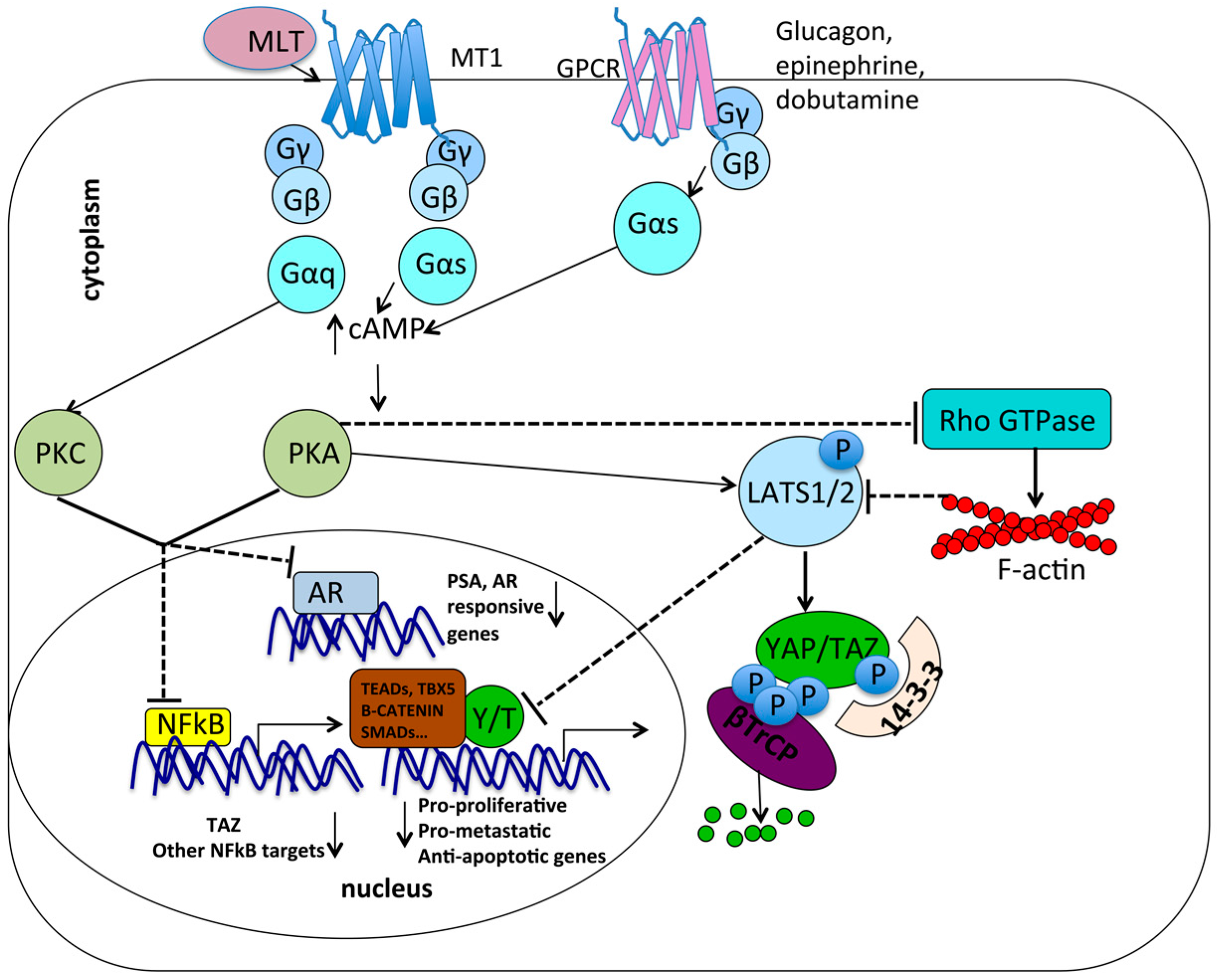
7.1. Gαs May Be a Common Molecular Intermediate between Melatonin Signaling and GPCR/YAP/TAZ Signalng
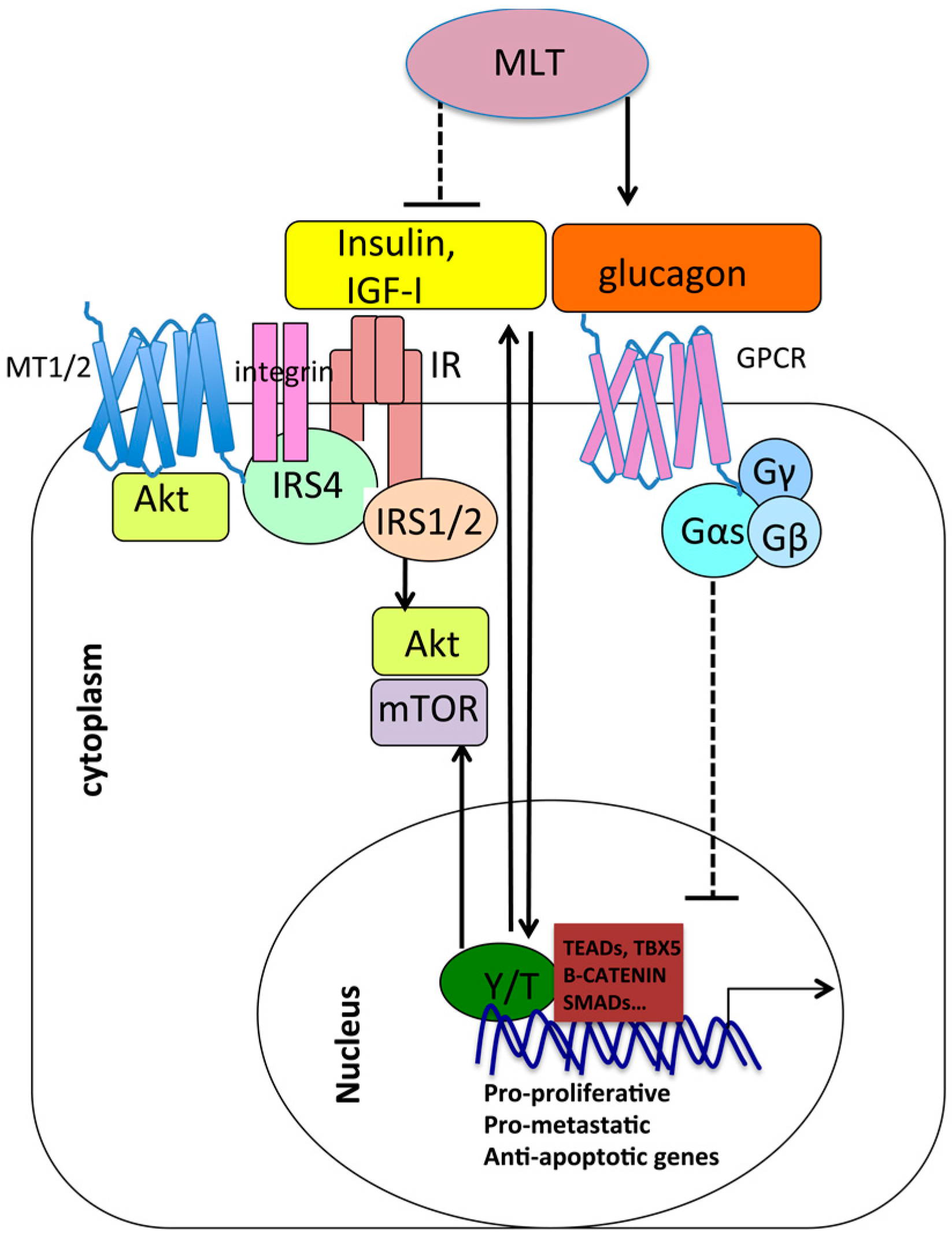
7.2. Metabolic Pathways: Antagonism between Melatonin and YAP/TAZ
7.3. Mechanotransduction and Chemoresistance: Opposite Roles of Melatonin and YAP/TAZ
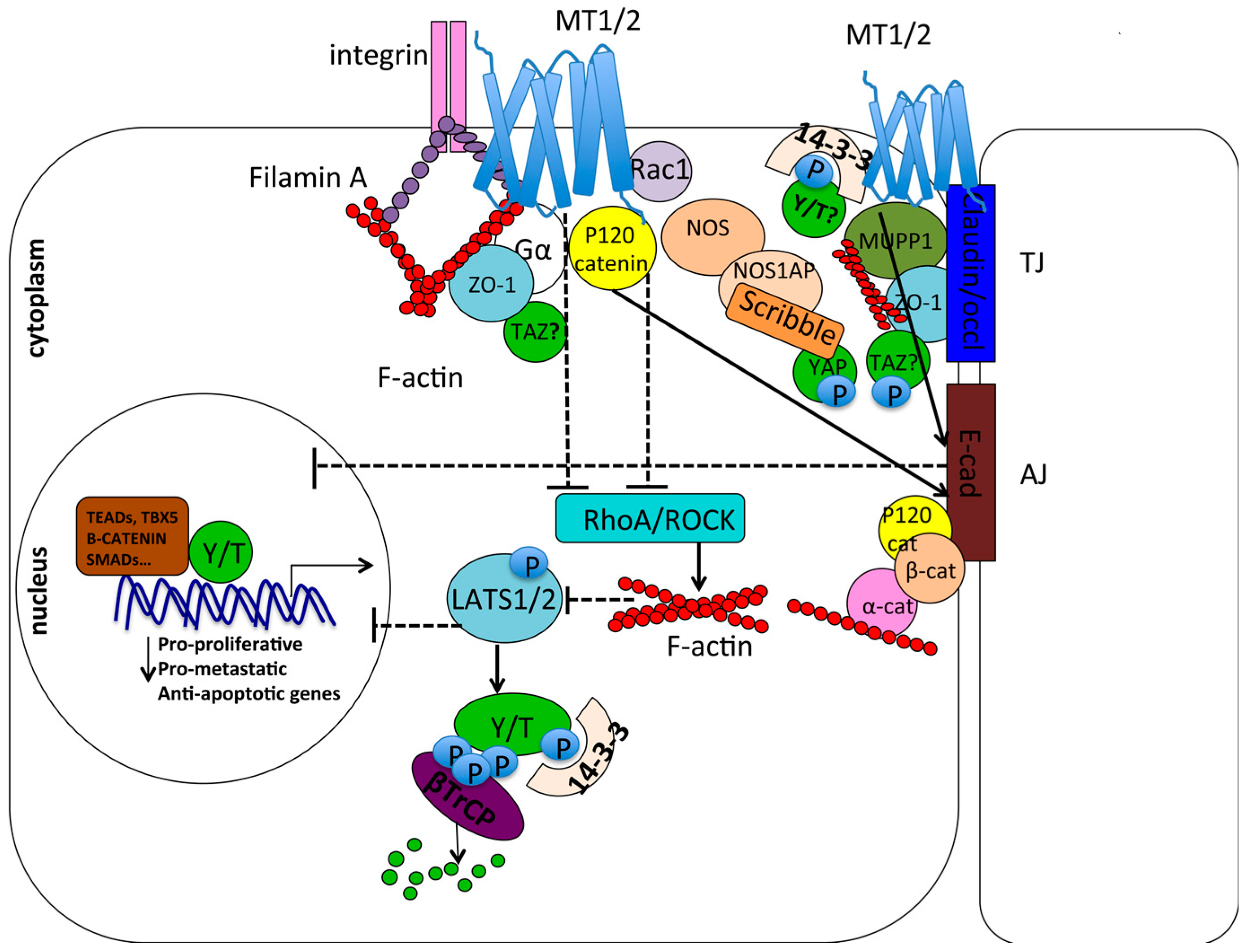
7.4. Cell Contact/Polarity and RhoA/ROCK Signaling
7.5. Opposite Roles of YAP/TAZ and Melatonin in Androgen–Estrogen Receptor Response and Angiogenesis
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Solis, C.A.; Perez-Leon, J.A. Phototransduction mediated by melanopsin in intrinsically photosensitive retinal ganglion cells. Gac. Med. Mex. 2015, 151, 764–776. [Google Scholar] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in aging and disease—Multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar] [PubMed]
- Karolczak, M.; Korf, H.W.; Stehle, J.H. The rhythm and blues of gene expression in the rodent pineal gland. Endocrine 2005, 27, 89–100. [Google Scholar] [CrossRef]
- Klein, D.C. Arylalkylamine N-acetyltransferase: “The timezyme”. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Guerlotte, J.; Greve, P.; Grechez-Cassiau, A.; Iuvone, M.P.; Zatz, M.; Chong, N.W.; Klein, D.C.; Voisin, P. Melatonin synthesis pathway: Circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reprod. Nutr. Dev. 1999, 39, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Moore, R.Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979, 174, 245–262. [Google Scholar] [CrossRef]
- Gastel, J.A.; Roseboom, P.H.; Rinaldi, P.A.; Weller, J.L.; Klein, D.C. Melatonin production: Proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 1998, 279, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Grof, E.; Grof, P.; Brown, G.M.; Arato, M.; Lane, J. Investigations of melatonin secretion in man. Prog. Neuropsychopharmacol. Biol. Psychiatry 1985, 9, 609–612. [Google Scholar] [CrossRef]
- Graham, C.; Cook, M.R.; Kavet, R.; Sastre, A.; Smith, D.K. Prediction of nocturnal plasma melatonin from morning urinary measures. J. Pineal Res. 1998, 24, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Rosner, B.; Willett, W.C.; Laden, F.; Colditz, G.A.; Hankinson, S.E. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol. Biomark. Prev. 2004, 13, 936–943. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Erren, T.C.; Piekarski, C.; Tamura, H.; Manchester, L.C. Light at night, chronodisruption, melatonin suppression, and cancer risk: A review. Crit. Rev. Oncog. 2007, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Deng, Q.; Fan, W.Y.; Wang, W.Y.; Wang, X. Light exposure at night, sleep duration, melatonin, and breast cancer: A dose-response analysis of observational studies. Eur. J. Cancer Prev. 2014, 23, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Jetter, A.; Fink, D.; Seifert, B.; Kullak-Ublick, G.A.; Trojan, A. Urinary excretion of melatonin and association with breast cancer: Meta-analysis and review of the literature. Breast Care 2014, 9, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W. Towards rational and evidence-based use of melatonin in prostate cancer prevention and treatment. J. Pineal Res. 2007, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, C.; Bartsch, H.; Jain, A.K.; Laumas, K.R.; Wetterberg, L. Urinary melatonin levels in human breast cancer patients. J. Neural Transm. 1981, 52, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, L.; Danforth, D.; Lichter, A.; DeMoss, E.; Cohen, M.; Chabner, B.; Lippman, M. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 1982, 216, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Berrino, F.; Krogh, V.; Secreto, G.; Micheli, A.; Venturelli, E.; Sieri, S.; Sempos, C.T.; Cavalleri, A.; Schunemann, H.J.; et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2008, 100, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Berrino, F.; Krogh, V.; Secreto, G.; Micheli, A.; Venturelli, E.; Grioni, S.; Sempos, C.T.; Cavalleri, A.; Schunemann, H.J.; et al. Urinary 6-sulphatoxymelatonin levels and risk of breast cancer in premenopausal women: The ordet cohort. Cancer Epidemiol. Biomark. Prev. 2010, 19, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Warner, E.T.; Eliassen, A.H.; Brown, S.B.; Beck, A.H.; Hankinson, S.E.; Schernhammer, E.S. Urinary melatonin in relation to postmenopausal breast cancer risk according to melatonin 1 receptor status. Cancer Epidemiol. Biomark. Prev. 2017, 26, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.L. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Seely, D.; Wu, P.; Fritz, H.; Kennedy, D.A.; Tsui, T.; Seely, A.J.; Mills, E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012, 11, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Schmidt, K.; Baum, M. Complementary/alternative therapies for the treatment of breast cancer. A systematic review of randomized clinical trials and a critique of current terminology. Breast J. 2006, 12, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M. Melatonin and circadian control in mammals. Experientia 1989, 45, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Gobbi, G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: A novel target in psychopharmacology. J. Psychiatry Neurosci. 2014, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Rombauts, L. Melatonin: Shedding light on infertility?—A review of the recent literature. J. Ovarian Res. 2014, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 2006, 119, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [PubMed]
- Mathes, A.M. Hepatoprotective actions of melatonin: Possible mediation by melatonin receptors. World J. Gastroenterol. 2010, 16, 6087–6097. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Horie, N.; Mitome, M.; Shinohara, K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res. 2007, 42, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J.; Qualls, C.R.; Lisansky, E.J.; Peake, G.T. Elevated rectal temperature produced by all-night bright light is reversed by melatonin infusion in men. J. Appl. Physiol. 1991, 71, 2178–2182. [Google Scholar] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 2016, 145, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, N.J.; Friedman, P.A. GPCR signaling and trafficking: The long and short of it. Trends Endocrinol. Metab. 2017, 28, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Legros, C.; Devavry, S.; Caignard, S.; Tessier, C.; Delagrange, P.; Ouvry, C.; Boutin, J.A.; Nosjean, O. Melatonin MT(1) and MT(2) receptors display different molecular pharmacologies only in the G-protein coupled state. Br. J. Pharmacol. 2014, 171, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: Iuphar review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Offermanns, S. Mammalian g proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, M.; Wilkie, T.M.; Simon, M.I. Diversity of the G-protein family: Sequences from five additional α subunits in the mouse. Proc. Natl. Acad. Sci. USA 1989, 86, 7407–7409. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, L. Receptor-to-effector signaling through g proteins: Roles for β γ dimers as well as α subunits. Cell 1992, 71, 1069–1072. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R.; Godson, C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharmacol. Sci. 1996, 17, 100–102. [Google Scholar] [CrossRef]
- Masana, M.I.; Dubocovich, M.L. Melatonin receptor signaling: Finding the path through the dark. Sci. STKE 2001, 2001, pe39. [Google Scholar] [CrossRef] [PubMed]
- Brydon, L.; Roka, F.; Petit, L.; de Coppet, P.; Tissot, M.; Barrett, P.; Morgan, P.J.; Nanoff, C.; Strosberg, A.D.; Jockers, R. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol. Endocrinol. 1999, 13, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Brydon, L.; Petit, L.; de Coppet, P.; Barrett, P.; Morgan, P.J.; Strosberg, A.D.; Jockers, R. Polymorphism and signalling of melatonin receptors. Reprod. Nutr. Dev. 1999, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Jarzynka, M.J.; Passey, D.K.; Ignatius, P.F.; Melan, M.A.; Radio, N.M.; Jockers, R.; Rasenick, M.M.; Brydon, L.; Witt-Enderby, P.A. Modulation of melatonin receptors and G-protein function by microtubules. J. Pineal Res. 2006, 41, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Yuan, L.; Chen, Q.; Dong, C.; Mao, L.; Rowan, B.; Frasch, T.; Hill, S.M. The Gα and Gαq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J. Pineal Res. 2008, 45, 476–488. [Google Scholar] [CrossRef] [PubMed]
- New, D.C.; Tsim, S.T.; Wong, Y.H. G protein-linked effector and second messenger systems involved in melatonin signal transduction. Neurosignals 2003, 12, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W.; Pang, B.; Tam, C.W.; Yao, K.M. Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Gα(s) and Gα(q) proteins. J. Pineal Res. 2010, 49, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Shiu, S.Y.W. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: Potential implications for therapeutic strategies against prostate cancer. J. Pineal Res. 2011, 51, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.; Leung, W.Y.; Tam, C.W.; Liu, V.W.; Yao, K.M. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(KIP1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor κ B (NF-κB): Potential implications on prostate cancer chemoprevention and therapy. J. Pineal Res. 2013, 54, 69–79. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.K.; Yung, L.Y.; Chan, J.S.; Chan, J.H.; Wong, C.S.; Wong, Y.H. Gα(14) links a variety of G(i)- and G(s)-coupled receptors to the stimulation of phospholipase c. Br. J. Pharmacol. 2001, 132, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.; Ross, A.W.; Barrett, P.; Hastings, M.H.; Morgan, P.J. Melatonin regulates the phosphorylation of CREB in ovine pars tuberalis. J. Neuroendocrinol. 1994, 6, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Niles, L.P.; Hashemi, F. Picomolar-affinity binding and inhibition of adenylate cyclase activity by melatonin in syrian hamster hypothalamus. Cell. Mol. Neurobiol. 1990, 10, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.J.; Barrett, P.; Howell, H.E.; Helliwell, R. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994, 24, 101–146. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; MacKenzie, R.S.; McKeon, R.M.; Carroll, E.A.; Bordt, S.L.; Melan, M.A. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil. Cytoskelet. 2000, 46, 28–42. [Google Scholar] [CrossRef]
- Godson, C.; Reppert, S.M. The Mel1a melatonin receptor is coupled to parallel signal transduction pathways 1. Endocrinology 1997, 138, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Roka, F.; Brydon, L.; Waldhoer, M.; Strosberg, A.D.; Freissmuth, M.; Jockers, R.; Nanoff, C. Tight association of the human Mel(1a)-melatonin receptor and G(i): Precoupling and constitutive activity. Mol. Pharmacol. 1999, 56, 1014–1024. [Google Scholar] [PubMed]
- Wan, Q.; Man, H.Y.; Liu, F.; Braunton, J.; Niznik, H.B.; Pang, S.F.; Brown, G.M.; Wang, Y.T. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 1999, 2, 401–403. [Google Scholar] [PubMed]
- Liu, V.W.S.; Yau, W.L.; Tam, C.W.; Yao, K.M.; Shiu, S.Y.W. Melatonin inhibits androgen receptor splice variant-7 (ar-v7)-induced nuclear factor-kappa b (nf-kappab) activation and nf-kappab activator-induced ar-v7 expression in prostate cancer cells: Potential implications for the use of melatonin in castration-resistant prostate cancer (crpc) therapy. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Tam, C.W.; Mo, C.W.; Yao, K.M.; Shiu, S.Y.W. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22RV1 human prostate cancer cells: Implications for prostate cancer chemoprevention. J. Pineal Res. 2007, 42, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Chan, K.W.; Liu, V.W.S.; Pang, B.; Yao, K.M.; Shiu, S.Y.W. Melatonin as a negative mitogenic hormonal regulator of human prostate epithelial cell growth: Potential mechanisms and clinical significance. J. Pineal Res. 2008, 45, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Lai, F.P.; Lo, R.K.; Voyno-Yasenetskaya, T.A.; Stanbridge, E.J.; Wong, Y.H. Melatonin MT1 and MT2 receptors stimulate c-jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell Signal 2002, 14, 249–257. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Zhang, Y.; Chen, X.; Lai, X.; Shao, J.; Shi, Y.; Zhou, N. Melatonin receptor type 1 signals to extracellular signal-regulated kinase 1 and 2 via Gi and Gs dually coupled pathways in Hek-293 cells. Biochemistry 2014, 53, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Becker-Andre, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; Andre, E.; Missbach, M.; Saurat, J.H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994, 269, 28531–28534. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Wiesenberg, I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: An unexpected relationship. J. Pineal Res. 1995, 18, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Wiesenberg, I.; Missbach, M.; Kahlen, J.P.; Schrader, M.; Carlberg, C. Transcriptional activation of the nuclear receptor RZR α by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 1995, 23, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Wiesenberg, I.; Missbach, M.; Carlberg, C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor. Neurol. Neurosci. 1998, 12, 143–150. [Google Scholar] [PubMed]
- Acuna-Castroviejo, D.; Reiter, R.J.; Menendez-Pelaez, A.; Pablos, M.I.; Burgos, A. Characterization of high-affinity melatonin binding sites in purified cell nuclei of rat liver. J. Pineal Res. 1994, 16, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, X.Y.; Wu, D.H.; Xu, Y. ROR nuclear receptors: Structures, related diseases, and drug discovery. Acta Pharmacol. Sin. 2015, 36, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V.; McBroom, L.D.; Flock, G. Determinants of target gene specificity for RORα1: Monomeric DNA binding by an orphan nuclear receptor. Mol. Cell. Biol. 1995, 15, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V.; Tini, M.; Flock, G.; Ong, E.; Evans, R.M.; Otulakowski, G. Isoform-specific amino-terminal domains dictate DNA-binding properties of RORα, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994, 8, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Yan, Z.H.; Hirose, T.; Giguere, V.; Jetten, A.M. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR γ and characterization of its response element. Gene 1996, 181, 199–206. [Google Scholar] [CrossRef]
- Schrader, M.; Danielsson, C.; Wiesenberg, I.; Carlberg, C. Identification of natural monomeric response elements of the nuclear receptor RZR/ROR. They also bind coup-TF homodimers. J. Biol. Chem. 1996, 271, 19732–19736. [Google Scholar] [CrossRef] [PubMed]
- Winrow, C.J.; Capone, J.P.; Rachubinski, R.A. Cross-talk between orphan nuclear hormone receptor RZRα and peroxisome proliferator-activated receptor α in regulation of the peroxisomal hydratase-dehydrogenase gene. J. Biol. Chem. 1998, 273, 31442–31448. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M.; Carrillo-Vico, A.; Guerrero, J.M.; Winczyk, K.; Pawlikowski, M. Expression of melatonin MT(1) and MT(2) receptors, and RORα(1) receptor in transplantable murine colon 38 cancer. Neuro Endocrinol. Lett. 2002, 23 (Suppl. 1), 55–60. [Google Scholar] [PubMed]
- Winczyk, K.; Pawlikowski, M.; Guerrero, J.M.; Karasek, M. Possible involvement of the nuclear RZR/ROR-α receptor in the antitumor action of melatonin on murine colon 38 cancer. Tumour Biol. 2002, 23, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maurino, S.; Pozo, D.; Calvo, J.R.; Guerrero, J.M. Correlation between nuclear melatonin receptor expression and enhanced cytokine production in human lymphocytic and monocytic cell lines. J. Pineal Res. 2000, 29, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Pozo, D.; Garcia-Maurino, S.; Osuna, C.; Molinero, P.; Calvo, J.R. Involvement of nuclear receptors in the enhanced IL-2 production by melatonin in jurkat cells. Ann. N. Y. Acad Sci. 2000, 917, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.M.; Pozo, D.; Garcia-Maurino, S.; Carrillo, A.; Osuna, C.; Molinero, P.; Calvo, J.R. Nuclear receptors are involved in the enhanced IL-6 production by melatonin in u937 cells. Biol. Signals Recept. 2000, 9, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schrader, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human b lymphocytes. J. Biol. Chem. 1995, 270, 7037–7040. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ram, P.T.; Yuan, L.; Spriggs, L.L.; Hill, S.M. Transcriptional repression of RORα activity in human breast cancer cells by melatonin. Mol. Cell. Endocrinol. 2001, 176, 111–120. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, L.; Dai, J.; Lai, L.; Mao, L.; Xiang, S.; Rowan, B.; Hill, S.M. Melatonin inhibits mitogenic cross-talk between retinoic acid-related orphan receptor α (RORα) and ERα in MCF-7 human breast cancer cells. Steroids 2010, 75, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Melatonin downregulates nuclear receptor RZR/RORγ expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol. Lett. 2016, 12, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, E843. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.A.; Reiter, R.J.; Lupi Junior, L.A. Melatonin as a promising agent to treat ovarian cancer: Molecular mechanisms. Carcinogenesis 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, M.; Chen, Y.; Zhao, Q.; Song, C.; Yan, Y.; Jin, Y.; Huang, Z.; Lin, C.; Wu, J. Melatonin induces cell apoptosis in ags cells through the activation of jnk and p38 mapk and the suppression of nuclear factor-κB: A novel therapeutic implication for gastric cancer. Cell. Physiol. Biochem. 2015, 37, 2323–2338. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Yoo, Y.M. Melatonin induces apoptotic cell death via p53 in lncap cells. Korean J. Physiol. Pharmacol. 2010, 14, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.; Li, Z.; Zhou, Z.; Zheng, C.; Lin, L.; Tan, B.; Huang, M.; Fan, M. Melatonin induces cell apoptosis in mia PACA-2 cells via the suppression of nuclear factor-κB and activation of ERK and JNK: A novel therapeutic implication for pancreatic cancer. Oncol. Rep. 2016, 36, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.; Negrin, G.; Estevez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-Mel-1 cells. J. Pineal Res. 2010, 49, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Garcia-Palomo, A.; Martin-Renedo, J.; Piva, M.; Gonzalez-Gallego, J.; Mauriz, J.L. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HEPG2 cell line: Role of the MT1 receptor. J. Pineal Res. 2011, 51, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 2012, 31, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Mori, F.; Marani, M.; Grasso, G.; Cambria, M.A.; Blandino, G.; Muti, P.; Strano, S. Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 2013, 34, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Ferraiuolo, M.; Santoro, R.; Sacconi, A.; Goeman, F.; Pallocca, M.; Pulito, C.; Korita, E.; Fanciulli, M.; Muti, P.; et al. Multitargeting activity of MIR-24 inhibits long-term melatonin anticancer effects. Oncotarget 2016, 7, 20532–20548. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing pi3k/AKT and Nf-κB/INOS signaling pathways. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Fu, L.; Tang, Z.; Zhang, C.; Qin, L.; Wang, J.; Yu, Z.; Shi, D.; Xiao, X.; Xie, F.; et al. Melatonin inhibits AP-2β/HTERT, NF-κB/Cox-2 and AKT/ERK and activates caspase/cyto c signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 2016, 7, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Li, H.; Tian, T.; Lu, Y.X.; Bai, L.; Chen, L.Z.; Sheng, H.; Mo, H.Y.; Zeng, J.B.; Deng, W.; et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-κB activation. J. Pineal Res. 2016, 60, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lee, L.M.; Lee, W.J.; Chu, C.Y.; Tan, P.; Yang, Y.C.; Chen, W.Y.; Yang, S.F.; Hsiao, M.; Chien, M.H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing AKT-MAPKS pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, A.; Li, X.; Du, Z.; Li, H.; Wang, H.; Yang, H.; Fang, Z. Melatonin inhibits tumorigenicity of glioblastoma stem-like cells via the AKT-EZH2-STAT3 signaling axis. J. Pineal Res. 2016, 61, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Pang, B.; Gu, G.; Gao, T.; Zhang, R.; Pang, Q.; Liu, Q. Melatonin inhibits glioblastoma stem-like cells through suppression of EZH2-notch1 signaling axis. Int. J. Biol. Sci. 2017, 13, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Y.; Reiter, R.J.; Pan, Y.; Chen, D.; Liu, Y.; Pu, X.; Jiang, L.; Li, Z. Inhibition of ERK1/2 signaling pathway is involved in melatonin’s antiproliferative effect on human MG-63 osteosarcoma cells. Cell. Physiol. Biochem. 2016, 39, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Konakchieva, R.; Todorov, P. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil. Steril. 2011, 96, e159. [Google Scholar] [CrossRef] [PubMed]
- Molpeceres, V.; Mauriz, J.L.; Garcia-Mediavilla, M.V.; Gonzalez, P.; Barrio, J.P.; Gonzalez-Gallego, J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- GarciaMaurino, S.; GonzalezHaba, M.G.; Calvo, J.R.; RafiiElIdrissi, M.; SanchezMargalet, V.; Goberna, R.; Guerrero, J.M. Melatonin enhances IL-2, IL-6, and IFN-γ production by human circulating CD4(+) cells—A possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 1997, 159, 574–581. [Google Scholar]
- Lardone, P.J.; Carrillo-Vico, A.; Naranjo, M.C.; de Felipe, B.; Vallejo, A.; Karasek, M.; Guerrero, J.M. Melatonin synthesized by jurkat human leukemic T cell line is implicated in IL-2 production. J. Cell. Physiol. 2006, 206, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Han, E.; Park, H.J.; Kim, H.J.; Hong, M.S.; Hong, S.J.; Yoon, K.S.; Kang, I.; Cho, Y.H.; Chung, J.H.; et al. Microarray analysis of transcription factor gene expression in melatonin-treated human peripheral blood mononuclear cells. J. Pineal Res. 2006, 40, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Lardone, P.J.; Carrillo-Vico, A.; Molinero, P.; Rubio, A.; Guerrero, J.M. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: Significance in IL-2 production. Cell. Mol. Life Sci. 2009, 66, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Sun, L.Z.Y.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Vigore, L.; Messina, G.; Brivio, F.; Fumagalli, L.; Rovelli, F.; G, D.I.F.; Lissoni, P. Psychoneuroendocrine modulation of regulatory T lymphocyte system: In vivo and in vitro effects of the pineal immunomodulating hormone melatonin. In Vivo 2010, 24, 787–789. [Google Scholar] [PubMed]
- Liu, H.; Xu, L.; Wei, J.E.; Xie, M.R.; Wang, S.E.; Zhou, R.X. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat. Rec. 2011, 294, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Disis, M.L.; Salazar, L.G. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol. Immunother. 2007, 56, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Fernandez, R.; Guezmes, A.; Sanchez-Barcelo, E.J. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998, 58, 4383–4390. [Google Scholar] [PubMed]
- Wu, S.M.; Lin, W.Y.; Shen, C.C.; Pan, H.C.; Keh-Bin, W.; Chen, Y.C.; Jan, Y.J.; Lai, D.W.; Tang, S.C.; Tien, H.R.; et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated c/EBPβ and NFκB cleavage. J. Pineal Res. 2016, 60, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Ndo, N.; Colombo, J.; Lopes, J.R.; Gelaleti, G.B.; Moschetta, M.G.; Sonehara, N.M.; Hellmen, E.; Zanon Cde, F.; Oliani, S.M.; Zuccari, D.A. Effect of melatonin in epithelial mesenchymal transition markers and invasive properties of breast cancer stem cells of canine and human cell lines. PLoS ONE 2016, 11, e0150407. [Google Scholar]
- Zhou, Q.; Gui, S.; Zhou, Q.; Wang, Y. Melatonin inhibits the migration of human lung adenocarcinoma a549 cell lines involving JNK/MAPK pathway. PLoS ONE 2014, 9, e101132. [Google Scholar] [CrossRef] [PubMed]
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.S.; Varma, N.R.; Shankar, A.; Coimbra, V.B.; et al. Melatonin decreases breast cancer metastasis by modulating rho-associated kinase protein-1 expression. J. Pineal Res. 2016, 60, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Ndo, N.; Rodrigues, R.V.; Jardim-Perassi, B.V.; Moschetta, M.G.; Lopes, J.R.; Colombo, J.; Zuccari, D.A. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med. Chem. 2014, 14, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Arnosti, D.; Trosko, J.E.; Tai, M.H.; Zuccari, D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer 2016, 7, 209–217. [Google Scholar] [PubMed]
- Lopes, J.R.; da Silva Kavagutti, M.; Medeiros, F.A.; De Campos Zuccari, D.A. Evaluation of melatonin effect on human breast cancer stem cells using a three-dimensional growth method of mammospheres. Anticancer Agents Med. Chem. 2016. [Google Scholar] [CrossRef]
- Nooshinfar, E.; Bashash, D.; Safaroghli-Azar, A.; Bayati, S.; Rezaei-Tavirani, M.; Ghaffari, S.H.; Akbari, M.E. Melatonin promotes ATO-induced apoptosis in MCF-7 cells: Proposing novel therapeutic potential for breast cancer. Biomed. Pharmacother. 2016, 83, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Chen, D.L.; Wang, D.S.; Chen, L.Z.; Mo, H.Y.; Sheng, H.; Bai, L.; Wu, Q.N.; Yu, H.E.; Xie, D.; et al. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of ERK and AKT pathway. Cell Death Dis. 2016, 7, e2432. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Ivanova, D.; Bakalova, R.; Aoki, I.; Higashi, T. Synergistic cytotoxicity of melatonin and new-generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res. 2017, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Yang, Z.P. Effects of melatonin combined with cis-platinum or methotrexate on the proliferation of osteosarcoma cell line SAOS-2. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015, 37, 215–220. [Google Scholar] [PubMed]
- Fan, L.; Sun, G.; Ma, T.; Zhong, F.; Lei, Y.; Li, X.; Wei, W. Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing chop and decreasing survivin. J. Pineal Res. 2013, 55, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Maschio-Signorini, L.B.; Gelaleti, G.B.; Moschetta, M.G.; Borin, T.F.; Jardim-Perassi, B.V.; Lopes, J.R.; Lacerda, J.Z.; Roela, R.A.; Bordin, N.A.; Correa, L.A.; et al. Melatonin regulates angiogenic and inflammatory proteins in MDA-MB-231 cell line and in co-culture with cancer-associated fibroblasts. Anticancer Agents Med. Chem. 2016, 16, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jang, W.J.; Yi, E.Y.; Jang, J.Y.; Jung, Y.; Jeong, J.W.; Kim, Y.J. Melatonin suppresses tumor angiogenesis by inhibiting Hif-1α stabilization under hypoxia. J. Pineal Res. 2010, 48, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yu, M.; Peng, X.; Dong, L.; Yang, Z. Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J. Pineal Res. 2012, 52, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and Hif-1α induced by COCL2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Cui, P.L.; Yao, S.W.; Xu, Y.Q.; Yang, Z.X. Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin. J. Cancer Res. 2012, 24, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Gonzalez-Gonzalez, A.; Alonso-Gonzalez, C.; Menendez-Menendez, J.; Martinez-Campa, C.; Cos, S. Melatonin inhibits angiogenesis in SH-SY5Y human neuroblastoma cells by downregulation of VEGF. Oncol. Rep. 2017, 37, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Ordonez, R.; Benet, M.; Jover, R.; Garcia-Palomo, A.; Mauriz, J.L.; Gonzalez-Gallego, J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HEPG2 liver cancer cells. Br. J. Cancer 2013, 109, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Lourenco, M.R.; Doho, G.M.; Grigolo, I.H.; Gelaleti, G.B.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Pires de Campos Zuccari, D.A. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer Agents Med. Chem. 2016, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Arbab, A.S.; Ferreira, L.C.; Borin, T.F.; Varma, N.R.; Iskander, A.S.; Shankar, A.; Ali, M.M.; Pires de Campos Zuccari, D.A. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE 2014, 9, e85311. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, V.; Gonzalez, A.; Alonso-Gonzalez, C.; Martinez-Campa, C.; Cos, S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc. Res. 2013, 87, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.; Rahbarghazi, R.; Nabat, E.; Movassaghpour, A.A.; Shanehbandi, D.; Faramarzian Azimi Maragheh, B.; Matluobi, D.; Barazvan, B.; Kazemi, M.; Samadi, N.; et al. The impact of different extracellular matrices on melatonin effect in proliferation and stemness properties of ovarian cancer cells. Biomed. Pharmacother. 2017, 87, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Maciel, J.M.; Ferreira, L.C.; Silva, R.F.D.; Zuccari, D.A. Effects of melatonin on Hif-1α and vegf expression and on the invasive properties of hepatocarcinoma cells. Oncol. Lett. 2016, 12, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Rimler, A.; Lupowitz, Z.; Zisapel, N. Differential regulation by melatonin of cell growth and androgen receptor binding to the androgen response element in prostate cancer cells. Neuro Endocrinol. Lett. 2002, 23 (Suppl. 1), 45–49. [Google Scholar] [PubMed]
- Rimler, A.; Culig, Z.; Lupowitz, Z.; Zisapel, N. Nuclear exclusion of the androgen receptor by melatonin. J. Steroid. Biochem. Mol. Biol. 2002, 81, 77–84. [Google Scholar] [CrossRef]
- Rimler, A.; Culig, Z.; Levy-Rimler, G.; Lupowitz, Z.; Klocker, H.; Matzkin, H.; Bartsch, G.; Zisapel, N. Melatonin elicits nuclear exclusion of the human androgen receptor and attenuates its activity. Prostate 2001, 49, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Spriggs, L.L.; Simon, M.A.; Muraoka, H.; Blask, D.E. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992, 64, 249–256. [Google Scholar] [CrossRef]
- Cos, S.; Sanchez-Barcelo, E.J. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histol. Histopathol. 2000, 15, 637–647. [Google Scholar] [PubMed]
- Sanchez-Barcelo, E.J.; Cos, S.; Mediavilla, D.; Martinez-Campa, C.; Gonzalez, A.; Alonso-Gonzalez, C. Melatonin-estrogen interactions in breast cancer. J. Pineal Res. 2005, 38, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, B.; Garcia Pedrero, J.M.; Martinez-Campa, C.; Zuazua, P.; Lazo, P.S.; Ramos, S. Melatonin, an endogenous-specific inhibitor of estrogen receptor α via calmodulin. J. Biol. Chem. 2004, 279, 38294–38302. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Cheng, Q.; Guardiola-Lemaitre, B.; Schuster-Klein, C.; Dong, C.; Lai, L.; Hill, S.M. In vitro and in vivo antitumor activity of melatonin receptor agonists. J. Pineal Res. 2010, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Frasch, T.; Xiang, S.; Yuan, L.; Duplessis, T.; Mao, L. Molecular mechanisms of melatonin anticancer effects. Integr. Cancer Ther. 2009, 8, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Oprea-Ilies, G.; Haus, E.; Sackett-Lundeen, L.; Liu, Y.; McLendon, L.; Busch, R.; Adams, A.; Cohen, C. Expression of melatonin receptors in triple negative breast cancer (tnbc) in african american and caucasian women: Relation to survival. Breast Cancer Res. Treat. 2013, 137, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Collins, A.R.; Dai, J.; Dubocovich, M.L.; Hill, S.M. MT(1) Melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol. Cell. Endocrinol. 2002, 192, 147–156. [Google Scholar] [CrossRef]
- Collins, A.; Yuan, L.; Kiefer, T.L.; Cheng, Q.; Lai, L.; Hill, S.M. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003, 189, 49–57. [Google Scholar] [CrossRef]
- Nemeth, C.; Humpeler, S.; Kallay, E.; Mesteri, I.; Svoboda, M.; Rogelsperger, O.; Klammer, N.; Thalhammer, T.; Ekmekcioglu, C. Decreased expression of the melatonin receptor 1 in human colorectal adenocarcinomas. J. Biol. Regul. Homeost. Agents 2011, 25, 531–542. [Google Scholar] [PubMed]
- Leon, J.; Casado, J.; Carazo, A.; Sanjuan, L.; Mate, A.; Munoz de Rueda, P.; de la Cueva, P.; Quiles, R.; Ruiz, S.; Ruiz-Extremera, A.; et al. Gender-related invasion differences associated with mRNA expression levels of melatonin membrane receptors in colorectal cancer. Mol. Carcinog. 2012, 51, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Itoh, M.T.; Kondo, H.; Okuma, Y.; Sato, S.; Kanishi, Y.; Hamada, N.; Kiguchi, K.; Ishizuka, B. Melatonin binding sites in estrogen receptor-positive cells derived from human endometrial cancer. J. Pineal Res. 2003, 35, 71–74. [Google Scholar] [PubMed]
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Involvement of nuclear receptor RZR/RORγ in melatonin-induced Hif-1α inactivation in SGC-7901 human gastric cancer cells. Oncol. Rep. 2015, 34, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Winczyk, K.; Pawlikowski, M.; Karasek, M. Melatonin and RZR/ROR receptor ligand CGP 52608 induce apoptosis in the murine colonic cancer. J. Pineal Res. 2001, 31, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Petranka, J.; Baldwin, W.; Biermann, J.; Jayadev, S.; Barrett, J.C.; Murphy, E. The oncostatic action of melatonin in an ovarian carcinoma cell line. J. Pineal Res. 1999, 26, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Narendhirakannan, R.T.; Hannah, M.A. Oxidative stress and skin cancer: An overview. Indian J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Minini, M.; Bizzarri, M. Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Lecour, S. Melatonin has multiorgan effects. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Hardeland, R. Inflammaging, metabolic syndrome and melatonin: A call for treatment studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gusdon, A.M.; Qu, S. Effects of melatonin on cardiovascular diseases: Progress in the past year. Curr. Opin. Lipidol. 2016, 27, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Quera Salva, M.A.; Hartley, S. Mood disorders, circadian rhythms, melatonin and melatonin agonists. J. Cent. Nerv. Syst. Dis. 2012, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Meng, X.; Li, Y.; Zhou, Y.; Xu, D.P.; Li, S.; Li, H.B. Effects of melatonin on liver injuries and diseases. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.A. Profound bilateral blindness and the incidence of breast cancer. Epidemiology 1991, 2, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Klerman, E.B.; Zeitzer, J.M.; Duffy, J.F.; Khalsa, S.B.; Czeisler, C.A. Absence of an increase in the duration of the circadian melatonin secretory episode in totally blind human subjects. J. Clin. Endocrinol. Metab. 2001, 86, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.; Carome, E.; Kubulins, V.; Hansler, R. Nighttime use of special spectacles or light bulbs that block blue light may reduce the risk of cancer. Med. Hypotheses 2009, 73, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Flynn-Evans, E.E.; Stevens, R.G.; Tabandeh, H.; Schernhammer, E.S.; Lockley, S.W. Total visual blindness is protective against breast cancer. Cancer Causes Control 2009, 20, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Kliukiene, J.; Tynes, T.; Andersen, A. Risk of breast cancer among norwegian women with visual impairment. Br. J. Cancer 2001, 84, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Blakeman, V.; Williams, J.L.; Meng, Q.J.; Streuli, C.H. Circadian clocks and breast cancer. Breast Cancer Res. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 2014, 64, 207–218. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Fall, K.; Rider, J.R.; Lockley, S.W.; Schernhammer, E.; Mucci, L.A. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Kozaki, K.; Tsuda, H.; Suzuki, E.; Pimkhaokham, A.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Amagasa, T.; Inazawa, J.; et al. Frequent silencing of a putative tumor suppressor gene melatonin receptor 1 a (MTNR1A) in oral squamous-cell carcinoma. Cancer Sci. 2008, 99, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, K.; Pula, B.; Zemla, A.; Owczarek, T.; Wojnar, A.; Rys, J.; Ambicka, A.; Podhorska-Okolow, M.; Ugorski, M.; Dziegiel, P. Expression of melatonin receptor mt1 in cells of human invasive ductal breast carcinoma. J. Pineal Res. 2013, 54, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of yap and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of yap oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor suppressor lats1 is a negative regulator of oncogene yap. J. Biol. Chem. 2008, 283, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Kanai, F.; Marignani, P.A.; Sarbassova, D.; Yagi, R.; Hall, R.A.; Donowitz, M.; Hisaminato, A.; Fujiwara, T.; Ito, Y.; Cantley, L.C.; et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000, 19, 6778–6791. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol. Cell. Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, A.; Kaneko, K.J.; Shu, H.; Zhao, Y.; DePamphilis, M.L. TEAD/Tef Transcription factors utilize the activation domain of YAP65, a SRC/YES-associated protein localized in the cytoplasm. Genes Dev. 2001, 15, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X.; Samavarchi-Tehrani, P.; Narimatsu, M.; Weiss, A.; Cockburn, K.; Larsen, B.G.; Rossant, J.; Wrana, J.L. The crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-Smad pathway. Dev. Cell 2010, 19, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by lats and CK1 regulates yap stability through SCF(β-Trcp). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zha, Z.Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The hippo tumor pathway promotes taz degradation by phosphorylating a phosphodegron and recruiting the SCF{β}-Trcp e3 ligase. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Battilana, G.; Cordenonsi, M.; Piccolo, S. YAP/TAZ as therapeutic targets in cancer. Curr. Opin. Pharmacol. 2016, 29, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Yang, J.; DeRan, M.; Wu, C.; Su, A.I.; Bonamy, G.M.; Liu, J.; Peters, E.C.; Wu, X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of yap. Chem. Biol. 2012, 19, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Yu, F.X.; Gong, R.; Brown, J.H.; Guan, K.L. Regulation of the Hippo-yap pathway by protease-activated receptors (pars). Genes Dev. 2012, 26, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun. Signal. 2013, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.O.; Shin, S.H.; Seo, E.J.; Kwon, Y.W.; Heo, S.C.; Kim, K.H.; Yoon, M.S.; Suh, D.S.; Kim, J.H. Taz mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells. Cell. Physiol. Biochem. 2013, 32, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the hippo-yap pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Lee, S.; Kuninaka, S.; Saya, H.; Lee, H.; Lee, S.; Lim, D.S. CAMP/PKA signalling reinforces the lats-yap pathway to fully suppress yap in response to actin cytoskeletal changes. EMBO J. 2013, 32, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhang, Y.; Park, H.W.; Jewell, J.L.; Chen, Q.; Deng, Y.; Pan, D.; Taylor, S.S.; Lai, Z.C.; Guan, K.L. Protein kinase a activates the hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013, 27, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Shin, K.K.; Kim, Y.J.; Song, J.S.; Kim, J.M.; Bae, Y.C.; Kim, C.D.; Jung, J.S. NF-κB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing taz expression. J. Cell. Physiol. 2010, 223, 168–177. [Google Scholar] [PubMed]
- Brunn, G.J.; Hudson, C.C.; Sekulic, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C., Jr.; Abraham, R.T. Phosphorylation of the translational repressor PHAS-i by the mammalian target of rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.B.; Jaeschke, A.; Saitoh, M.; Fowler, B.; Kozma, S.C.; Thomas, G. Mammalian tor: A homeostatic atp sensor. Science 2001, 294, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Shamji, A.F.; Nghiem, P.; Schreiber, S.L. Integration of growth factor and nutrient signaling: Implications for cancer biology. Mol. Cell 2003, 12, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by AKT and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Tee, A.R.; Logsdon, M.N.; Blenis, J.; Cantley, L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/AKT pathway. Mol. Cell 2002, 10, 151–162. [Google Scholar] [CrossRef]
- Hao, F.; Xu, Q.; Zhao, Y.; Stevens, J.V.; Young, S.H.; Sinnett-Smith, J.; Rozengurt, E. Insulin receptor and gpcr crosstalk stimulates yap via PI3K and PKD in pancreatic cancer cells. Mol. Cancer Res. 2017, 15, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jeong, K.; Jiang, H.; Guo, W.; Gu, C.; Lu, Y.; Liang, J. YAP/TAZ Regulates the insulin signaling via IRS1/2 in endometrial cancer. Am. J. Cancer Res. 2016, 6, 996–1010. [Google Scholar] [PubMed]
- Tumaneng, K.; Schlegelmilch, K.; Russell, R.C.; Yimlamai, D.; Basnet, H.; Mahadevan, N.; Fitamant, J.; Bardeesy, N.; Camargo, F.D.; Guan, K.L. Yap mediates crosstalk between the hippo and PI(3)K-TOR pathways by suppressing pten via MIR-29. Nat. Cell Biol. 2012, 14, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.A.; Squatrito, M.; Northcott, P.; Awan, A.; Holland, E.C.; Taylor, M.D.; Nahle, Z.; Kenney, A.M. Oncogenic yap promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated AKT activation. Oncogene 2012, 31, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, P.; von Gise, A.; Gu, F.; Ma, Q.; Chen, J.; Guo, H.; van Gorp, P.R.; Wang, D.Z.; Pu, W.T. PI3KCB links hippo-yap and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ. Res. 2015, 116, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, Z.D.; Li, X.; Aziz, K.E.; Gan, B.; Johnson, R.L.; Chen, J. AMPK modulates hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015, 17, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Sohn, E.J.; Shin, E.A.; Lee, D.; Kim, B.; Jung, D.B.; Kim, J.H.; Yun, M.; Lee, H.J.; Park, Y.K.; et al. Melatonin suppresses the expression of 45s preribosomal rna and upstream binding factor and enhances the antitumor activity of puromycin in MDA-MB-231 breast cancer cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 879746. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jia, Z.; Zhang, X.; Hou, J.; Wang, L.; Hao, S.; Ruan, X.; Yu, Z.; Zheng, Y. Involvement of melatonin in autophagy-mediated mouse hepatoma H22 cell survival. Int. Immunopharmacol. 2012, 12, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, T.J.; Yoo, Y.M. Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/AKT/mTOR pathway in B16F10 melanoma cells. PLoS ONE 2014, 9, e92627. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/AKT/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Bahr, I.; Muhlbauer, E. Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef] [PubMed]
- Daulat, A.M.; Maurice, P.; Froment, C.; Guillaume, J.L.; Broussard, C.; Monsarrat, B.; Delagrange, P.; Jockers, R. Purification and identification of G protein-coupled receptor protein complexes under native conditions. Mol. Cell. Proteom. 2007, 6, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Daulat, A.M.; Maurice, P.; Jockers, R. Recent methodological advances in the discovery of GPCR-associated protein complexes. Trends Pharmacol. Sci. 2009, 30, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Maurice, P.; Daulat, A.M.; Broussard, C.; Mozo, J.; Clary, G.; Hotellier, F.; Chafey, P.; Guillaume, J.L.; Ferry, G.; Boutin, J.A.; et al. A generic approach for the purification of signaling complexes that specifically interact with the carboxyl-terminal domain of G protein-coupled receptors. Mol. Cell. Proteom. 2008, 7, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Santinon, G.; Pocaterra, A.; Dupont, S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016, 26, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; MM, A.S.; Acuna-Castroviejo, D.; Fernandez-Vazquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Cosgrove, B.D.; Heo, S.J.; Shurden, Z.E.; Mauck, R.L. Cytoskeletal to nuclear strain transfer regulates yap signaling in mesenchymal stem cells. Biophys. J. 2015, 108, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Wang, L.; Wang, C.Y.; Yu, J.; Guan, K.L. Cell detachment activates the hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012, 26, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yong, K.M.; Villa-Diaz, L.G.; Zhang, X.; Chen, W.; Philson, R.; Weng, S.; Xu, H.; Krebsbach, P.H.; Fu, J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 2014, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.G.; Gumbiner, B.M. Adhesion to fibronectin regulates hippo signaling via the FAK-SRC-PI3K pathway. J. Cell Biol. 2015, 210, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Benham-Pyle, B.W.; Pruitt, B.L.; Nelson, W.J. Cell adhesion. Mechanical strain induces e-cadherin-dependent yap1 and β-catenin activation to drive cell cycle entry. Science 2015, 348, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Cottrill, K.A.; Lu, Y.; Haeger, C.M.; Dieffenbach, P.; Annis, S.; Hale, A.; Bhat, B.; Kaimal, V.; Zhang, Y.Y.; et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-mir-130/301 circuit. Cell Rep. 2015, 13, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and yap-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; DuFort, C.C.; Rossier, O.; Bainer, R.; Mouw, J.K.; Godula, K.; Hudak, J.E.; Lakins, J.N.; Wijekoon, A.C.; Cassereau, L.; et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 2014, 511, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Rubashkin, M.G.; Cassereau, L.; Bainer, R.; DuFort, C.C.; Yui, Y.; Ou, G.; Paszek, M.J.; Davidson, M.W.; Chen, Y.Y.; Weaver, V.M. Force engages vinculin and promotes tumor progression by enhancing pi3k activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014, 74, 4597–4611. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Yui, Y.; Damiano, L.; Bainer, R.O.; Lakins, J.N.; Acerbi, I.; Ou, G.; Wijekoon, A.C.; Levental, K.R.; Gilbert, P.M.; et al. Tissue mechanics modulate microrna-dependent pten expression to regulate malignant progression. Nat. Med. 2014, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Pelissier, F.A.; Zhang, H.; Lakins, J.; Weaver, V.M.; Park, C.; LaBarge, M.A. Microenvironment rigidity modulates responses to the her2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol. Biol. Cell 2015, 26, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- Bartucci, M.; Dattilo, R.; Moriconi, C.; Pagliuca, A.; Mottolese, M.; Federici, G.; Benedetto, A.D.; Todaro, M.; Stassi, G.; Sperati, F.; et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 2015, 34, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sabnis, A.J.; Chan, E.; Olivas, V.; Cade, L.; Pazarentzos, E.; Asthana, S.; Neel, D.; Yan, J.J.; Lu, X.; et al. The hippo effector yap promotes resistance to raf- and mek-targeted cancer therapies. Nat. Genet. 2015, 47, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, H.S.; Lee, D.; Yoo, G.; Kim, T.; Jeon, H.; Yeo, M.K.; Lee, C.S.; Moon, J.Y.; Jung, S.S.; et al. Hippo pathway effector yap inhibition restores the sensitivity of egfr-tki in lung adenocarcinoma having primary or acquired egfr-tki resistance. Biochem. Biophys. Res. Commun. 2016, 474, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, Z.; Rodriguez-Barrueco, R.; Borczuk, A.; Liu, H.; Yu, J.; Silva, J.M.; Cheng, S.K.; Perez-Soler, R.; Halmos, B. Functional genomics screen identifies yap1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget 2016, 7, 28976–28988. [Google Scholar] [CrossRef] [PubMed]
- Anton-Tay, F.; Ramirez, G.; Martinez, I.; Benitez-King, G. In vitro stimulation of protein kinase c by melatonin. Neurochem. Res. 1998, 23, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G. PKC Activation by melatonin modulates vimentin intermediate filament organization in n1e-115 cells. J. Pineal Res. 2000, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G.; Hernandez, M.E.; Tovar, R.; Ramirez, G. Melatonin activates PKC-α but not pkc-epsilon in n1e-115 cells. Neurochem. Int. 2001, 39, 95–102. [Google Scholar] [CrossRef]
- Koopman, M.G.; Koomen, G.C.; Krediet, R.T.; de Moor, E.A.; Hoek, F.J.; Arisz, L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin. Sci. 1989, 77, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.A.; Studier, E.H.; Stallone, J.N.; Kennedy, C.M. Effects of melatonin on water metabolism and renal function in male syrian hamsters (mesocricetus auratus). J. Pineal Res. 1992, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; Sheetz, M.P. Cellular mechanotransduction: Filamin a strains to regulate motility. Curr. Biol. 2011, 21, R916–R918. [Google Scholar] [CrossRef] [PubMed]
- Szymaniak, A.D.; Mahoney, J.E.; Cardoso, W.V.; Varelas, X. CRUMBS3-Mediated polarity directs airway epithelial cell fate through the hippo pathway effector yap. Dev. Cell 2015, 34, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Thompson, B.J. Yap and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. Bioessays 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M.; Kim, N.G. The hippo-yap signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Genevet, A.; Wehr, M.C.; Brain, R.; Thompson, B.J.; Tapon, N. Kibra is a regulator of the salvador/warts/hippo signaling network. Dev. Cell 2010, 18, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zheng, Y.; Dong, J.; Klusza, S.; Deng, W.M.; Pan, D. Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with merlin and expanded. Dev. Cell 2010, 18, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Lu, Q.; Wang, L.H.; Liu, C.Y.; Lei, Q.; Guan, K.L. Angiomotin is a novel hippo pathway component that inhibits yap oncoprotein. Genes Dev. 2011, 25, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Gajewski, K.M.; Hamaratoglu, F.; Bossuyt, W.; Sansores-Garcia, L.; Tao, C.; Halder, G. The apical-basal cell polarity determinant crumbs regulates hippo signaling in drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Gurvich, N.; Perna, F.; Farina, A.; Voza, F.; Menendez, S.; Hurwitz, J.; Nimer, S.D. L3MBTL1 Polycomb protein, a candidate tumor suppressor in del(20q12) myeloid disorders, is essential for genome stability. Proc. Natl. Acad. Sci. USA 2010, 107, 22552–22557. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Zheng, Y.; Yin, F.; Yu, J.; Huang, J.; Hong, Y.; Wu, S.; Pan, D. The apical transmembrane protein crumbs functions as a tumor suppressor that regulates hippo signaling by binding to expanded. Proc. Natl. Acad. Sci. USA 2010, 107, 10532–10537. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.S.; Huang, J.; Hong, Y.; Moberg, K.H. Crumbs regulates salvador/warts/hippo signaling in drosophila via the ferm-domain protein expanded. Curr. Biol. 2010, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin signalling regulates yap and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Das Thakur, M.; Feng, Y.; Jagannathan, R.; Seppa, M.J.; Skeath, J.B.; Longmore, G.D. Ajuba lim proteins are negative regulators of the hippo signaling pathway. Curr. Biol. 2010, 20, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Babcock, J.T.; Wells, C.D.; Quilliam, L.A. Lkb1 tumor suppressor regulates amp kinase/mTOR-independent cell growth and proliferation via the phosphorylation of yap. Oncogene 2013, 32, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Remue, E.; Meerschaert, K.; Oka, T.; Boucherie, C.; Vandekerckhove, J.; Sudol, M.; Gettemans, J. TAZ interacts with zonula occludens-1 and -2 proteins in a pdz-1 dependent manner. FEBS Lett. 2010, 584, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Remue, E.; Meerschaert, K.; Vanloo, B.; Boucherie, C.; Gfeller, D.; Bader, G.D.; Sidhu, S.S.; Vandekerckhove, J.; Gettemans, J.; et al. Functional complexes between yap2 and zo-2 are pdz domain-dependent, and regulate yap2 nuclear localization and signalling. Biochem. J. 2010, 432, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.G.; Koh, E.; Chen, X.; Gumbiner, B.M. E-cadherin mediates contact inhibition of proliferation through hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA 2011, 108, 11930–11935. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, N.; Figel, S.A.; Wilson, K.E.; Morrison, C.D.; Gelman, I.H.; Zhang, J. Ptpn14 interacts with and negatively regulates the oncogenic function of yap. Oncogene 2013, 32, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, P.Z.; Moon, S.Y.; Thoreson, M.A.; Mariner, D.J.; Crawford, H.C.; Zheng, Y.; Reynolds, A.B. Inhibition of rhoa by p120 catenin. Nat. Cell Biol. 2000, 2, 637–644. [Google Scholar] [PubMed]
- Grosheva, I.; Shtutman, M.; Elbaum, M.; Bershadsky, A.D. P120 catenin affects cell motility via modulation of activity of rho-family gtpases: A link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 2001, 114, 695–707. [Google Scholar] [PubMed]
- Noren, N.K.; Liu, B.P.; Burridge, K.; Kreft, B. P120 catenin regulates the actin cytoskeleton via rho family gtpases. J. Cell Biol. 2000, 150, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Chen, H.C.; Chen, S.Y.; Tseng, S.C. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J. Cell Sci. 2012, 125, 3636–3648. [Google Scholar] [CrossRef] [PubMed]
- Ireton, R.C.; Davis, M.A.; van Hengel, J.; Mariner, D.J.; Barnes, K.; Thoreson, M.A.; Anastasiadis, P.Z.; Matrisian, L.; Bundy, L.M.; Sealy, L.; et al. A novel role for p120 catenin in e-cadherin function. J. Cell Biol. 2002, 159, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Ireton, R.C.; Reynolds, A.B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003, 163, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Allison, D.F.; Buckley, K.M.; Kottke, M.D.; Vincent, P.A.; Faundez, V.; Kowalczyk, A.P. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003, 163, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Graves, H.K.; Moya, I.M.; Tao, C.; Hamaratoglu, F.; Gladden, A.B.; Halder, G. Differential regulation of the hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc. Natl. Acad. Sci. USA 2015, 112, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sagi, D.; Hall, A. Ras and rho gtpases: A family reunion. Cell 2000, 103, 227–238. [Google Scholar] [CrossRef]
- Parri, M.; Chiarugi, P. Rac and rho gtpases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Arthur, W.T.; Quilliam, L.A.; Cooper, J.A. Rap1 promotes cell spreading by localizing rac guanine nucleotide exchange factors. J. Cell Biol. 2004, 167, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Stiffler, M.A.; Chen, J.R.; Grantcharova, V.P.; Lei, Y.; Fuchs, D.; Allen, J.E.; Zaslavskaia, L.A.; MacBeath, G. Pdz domain binding selectivity is optimized across the mouse proteome. Science 2007, 317, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, J.L.; Daulat, A.M.; Maurice, P.; Levoye, A.; Migaud, M.; Brydon, L.; Malpaux, B.; Borg-Capra, C.; Jockers, R. The pdz protein mupp1 promotes gi coupling and signaling of the mt1 melatonin receptor. J. Biol. Chem. 2008, 283, 16762–16771. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, Y.; Itoh, M.; Sasaki, H.; Furuse, M.; Tsukita, S. Multi-pdz domain protein 1 (mupp1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J. Biol. Chem. 2002, 277, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.N.; Schwesinger, C.; Denker, B.M. Zonula occludens-1 is a scaffolding protein for signaling molecules. Gα(12) directly binds to the src homology 3 domain and regulates paracellular permeability in epithelial cells. J. Biol. Chem. 2002, 277, 24855–24858. [Google Scholar] [CrossRef] [PubMed]
- Sabath, E.; Negoro, H.; Beaudry, S.; Paniagua, M.; Angelow, S.; Shah, J.; Grammatikakis, N.; Yu, A.S.; Denker, B.M. Gα12 regulates protein interactions within the mdck cell tight junction and inhibits tight-junction assembly. J. Cell Sci. 2008, 121, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Clattenburg, L.; Wigerius, M.; Qi, J.; Rainey, J.K.; Rourke, J.L.; Muruganandan, S.; Sinal, C.J.; Fawcett, J.P. Nos1ap functionally associates with yap to regulate hippo signaling. Mol. Cell. Biol. 2015, 35, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.; Clattenburg, L.; Muruganandan, S.; Bullock, M.; MacIsaac, K.; Wigerius, M.; Williams, B.A.; Graham, M.E.; Rigby, M.H.; Trites, J.R.; et al. The hippo component yap localizes in the nucleus of human papilloma virus positive oropharyngeal squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2017, 46, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sun, J.; Lau, A.; Curtis, S.; Goldsmith, J.; Fox, V.L.; Wei, C.; Frazier, M.; Samson, O.; Wong, K.K.; et al. A genetic screen identifies an lkb1-mark signalling axis controlling the hippo-yap pathway. Nat. Cell Biol. 2014, 16, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Haldar, C.; Ortmann, O. Antiestrogens modulate mt1 melatonin receptor expression in breast and ovarian cancer cell lines. Oncol. Rep. 2006, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Powzaniuk, M.; McElwee-Witmer, S.; Vogel, R.L.; Hayami, T.; Rutledge, S.J.; Chen, F.; Harada, S.; Schmidt, A.; Rodan, G.A.; Freedman, L.P.; et al. The lats2/kpm tumor suppressor is a negative regulator of the androgen receptor. Mol. Endocrinol. 2004, 18, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Lit, L.C.; Scott, S.; Zhang, H.; Stebbing, J.; Photiou, A.; Giamas, G. Lats2 is a modulator of estrogen receptor α. Anticancer Res. 2013, 33, 53–63. [Google Scholar] [PubMed]
- Britschgi, A.; Duss, S.; Kim, S.; Couto, J.P.; Brinkhaus, H.; Koren, S.; De Silva, D.; Mertz, K.D.; Kaup, D.; Varga, Z.; et al. The hippo kinases lats1 and 2 control human breast cell fate via crosstalk with ERα. Nature 2017, 541, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, Y.; Chen, L.; Cheng, H.; Mu, C.; Li, J.; Gao, R.; Zhou, C.; Cao, L.; Liu, J.; et al. Hypoxia regulates hippo signalling through the siah2 ubiquitin e3 ligase. Nat. Cell Biol. 2015, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Piccolo, S. Eradicating tumor drug resistance at its yap-biomechanical roots. EMBO J. 2016, 35, 459–461. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardo, F.L.; Muti, P.; Blandino, G.; Strano, S. Melatonin and Hippo Pathway: Is There Existing Cross-Talk? Int. J. Mol. Sci. 2017, 18, 1913. https://doi.org/10.3390/ijms18091913
Sardo FL, Muti P, Blandino G, Strano S. Melatonin and Hippo Pathway: Is There Existing Cross-Talk? International Journal of Molecular Sciences. 2017; 18(9):1913. https://doi.org/10.3390/ijms18091913
Chicago/Turabian StyleSardo, Federica Lo, Paola Muti, Giovanni Blandino, and Sabrina Strano. 2017. "Melatonin and Hippo Pathway: Is There Existing Cross-Talk?" International Journal of Molecular Sciences 18, no. 9: 1913. https://doi.org/10.3390/ijms18091913
APA StyleSardo, F. L., Muti, P., Blandino, G., & Strano, S. (2017). Melatonin and Hippo Pathway: Is There Existing Cross-Talk? International Journal of Molecular Sciences, 18(9), 1913. https://doi.org/10.3390/ijms18091913




