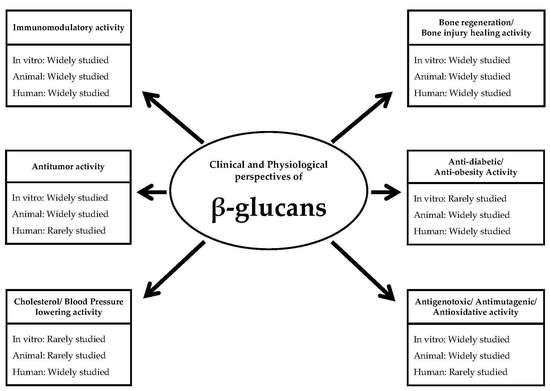
Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future
Abstract
1. Introduction
2. Antitumor Effects of β-Glucans
2.1. Antitumor Effects of β-Glucans—In Vitro Studies
2.2. Antitumor Effects of β-Glucans—Animal Studies
2.3. Antitumor Effects of β-Glucans—Clinical Studies
3. Immunomodulating Effects of β-Glucans
3.1. Immunomodulating Effects of β-Glucans—In Vitro Studies
3.2. Immunomodulating Effects of β-Glucans—Animal Studies
3.3. Immunomodulating Effects of β-Glucans—Clinical Studies
4. Bone Regeneration/Bone Injury Healing Effects of β-Glucans
4.1. Bone Regeneration/Bone Injury Healing Effects of β-Glucans—In Vitro Studies
4.2. Bone Regeneration/Bone Injury Healing Effects of β-Glucans—Animal Studies
4.3. Bone Regeneration/Bone Injury Healing Effects of β-Glucans—Clinical Studies
5. Anti-Diabetic/Anti-Obesity Effects of β-Glucans
5.1. Anti-Diabetic/Anti-Obesity Effect of β-Glucans—In Vitro Studies
5.2. Anti-Diabetic/Anti-Obesity Effect of β-Glucans—Animal Studies
5.3. Anti-Diabetic/Anti-Obesity Effect of β-Glucans—Clinical Studies
6. Cholesterol and Blood Pressure Lowering Effects of β-Glucans
6.1. Cholesterol and Blood Pressure Lowering Effects of β-Glucans—In Vitro Studies
6.2. Cholesterol and Blood Pressure Lowering Effects of β-Glucans—Animal Studies
6.3. Cholesterol and Blood Pressure Lowering Effects of β-Glucans—Human Studies
7. Antigenotoxic/Antimutagenic/Antioxidative Effects of β-Glucans
7.1. Antigenotoxic/Antimutagenic/Antioxidative Effects of β-Glucans—In Vitro Studies
7.2. Antigenotoxic/Antimutagenic/Antioxidative Effects of β-Glucans—Animal Studies
7.3. Antigenotoxic/Antimutagenic/Antioxidative Effects of β-Glucans—Clinical Studies
8. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Harada, T.; Ohno, N. Contribution of dectin-1 and granulocyte macrophage-colony stimulating factor (GM-CSF) to immunomodulating actions of β-glucan. Int. Immunopharmacol. 2008, 8, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.C.B.; Young, T.R.; Carroll, J.A.; Rathmann, R.J.; Johnson, B.J. Yeast cell wall supplementation alters aspects of the physiological and acute phase responses of crossbred heifers to an endotoxin challenge. Innate Immun. 2013, 19, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kuczaj, M.; Preś, J.; Zachwieja, A.; Twardoń, J.; Orda, J.; Dobicki, A. Effect of supplementing dairy cows with live yeasts cells and dried brewer’s yeasts on milk chemical composition, somatic cell count and blood biochemical indices. Vet. Med. 2014, 17, 6. [Google Scholar]
- Klasing, K.C.; Korver, D.R. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. J. Anim. Sci. 1997, 75, 58–67. [Google Scholar]
- Auinger, A.; Riede, L.; Bothe, G.; Busch, R.; Gruenwald, J. Yeast (1,3)-(1,6)-β-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 2013, 52, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Physiological effects of different types of β-glucan. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2007, 151, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [PubMed]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Vetvicka, V. β-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Kossaczka, Z.; Jiang, B.; Bussey, H. A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1→6)-β-glucan synthesis. Genetics 1993, 133, 4837–4849. [Google Scholar]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Stuart, I.M.; Loi, L.; Fincher, G.B. Immunological comparison of (1→3,1→4)-β-glucan endohydrolases in germinating cereals. J. Cereal Sci. 1987, 6, 45–52. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Demirbas, A. β-Glucan and mineral nutrient contents of cereals grown in Turkey. Food Chem. 2005, 90, 773–777. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Uhlen, A.K.; Brathen, E.; Sahlstrøm, S.; Knutsen, S.H. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem. 2006, 94, 348–358. [Google Scholar] [CrossRef]
- Bacic, A.; Fincher, G.B.; Stone, B.A. Chemistry, Biochemistry, and Biology of (1→3)-β-Glucans and Related Polysaccharides, 1st ed.; Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Teas, J. The dietary intake of Laminaria, a brown seaweed, and breast cancer prevention. Nutr. Cancer 1983, 4, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [PubMed]
- Ripsin, C.M.; Keenan, J.M.; Jacobs, D.R.; Elmer, P.J.; Welch, R.R.; Van Horn, L.; Liu, K.; Turnbull, W.H.; Thye, F.W.; Kestin, M.; et al. Oat products and lipid lowering: A meta-analysis. J. Am. Med. Assoc. 1992, 267, 3317–3325. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, H.J.; Lee, Y.Y.; Cho, K.-H.; Roh, Y.K. Biomedical issues of dietary fiber β-Glucan. J. Korean Med. Sci. 2006, 21, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.F.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Chen, J. Recent advances in the studies of β-glucans for cancer therapy. Anti-Cancer Agents Med. Chem. 2013, 13, 679–680. [Google Scholar] [CrossRef]
- Vetvicka, V.; Pinatto-Botelho, M.F.; Santos, A.A.D.; De Oliveira, C.A.F. Evaluation of a special combination of glucan with organic selenium derivative in different murine tumor model. Anticancer Res. 2014, 34, 6939–6944. [Google Scholar] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Lipids significantly reduced by diets containing Barley in moderately hypercholesterolemic men. J. Am. Coll. Nutr. 2004, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am. J. Clin. Nutr. 2004, 80, 1185–1193. [Google Scholar] [PubMed]
- Liatis, S.; Tsapogas, P.; Chala, E.; Dimosthenopoulos, C.; Kyriakopoulos, K.; Kapantais, E.; Katsilambros, N. The consumption of bread enriched with β-glucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadokova, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma 2008, 55, 387–393. [Google Scholar] [PubMed]
- Daou, C.; Zhang, H. Oat β-Glucan: Its role in health promotion and prevention of diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Murphy, E.A.; Davis, J.M.; Carmichael, M.D. Immune modulating effects of β-glucan. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.C.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Banovcin, P.; Rennerova, Z.; Majtan, J. β-glucans in the treatment and prevention of allergic diseases. Allergol. Immunopathol. 2014, 42, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Urbancikova, I.; Banovcin, P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients 2017, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.E.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. β-glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. β-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc. Health Risk Manag. 2008, 4, 126–1272. [Google Scholar] [CrossRef]
- Hou, T.-Y.; Wang, S.-H.; Liang, S.-X.; Jiang, W.-X.; Luo, D.-D.; Huang, D.-H. The screening performance of serum 1,3 β-d-glucan in patients with invasive fungal diseases: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0131602. [Google Scholar] [CrossRef] [PubMed]
- Hallfrisch, J.; Behall, K.M. Physiological responses of men and women to barley and oat extracts (nu-trimX). I. Breath hydrogen, methane, and gastrointestinal symptoms. Cereal Chem. 2003, 80, 76–79. [Google Scholar] [CrossRef]
- Chen, M.; Seviour, R. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Kirkwood, A.; Misaki, A.; Nelson, T.; Scalettie, J.; Smith, F. Structure of a new glucan. Chem. Ind. 1963, 41, 820–822. [Google Scholar]
- Kikumoto, S.; Miyazima, T.; Kimura, K.; Okubo, S.; Komatsu, N. Polysaccharide produced by Schizophyllum commune, part II. Chemical structure of an extracellular polysaccharide. Nippon Nougeikagaku Kaishi 1971, 45, 162–168. [Google Scholar] [CrossRef]
- Garcia-Lora, A.; Martinez, M.; Pedrinaci, S.; Garrido, F. Different regulation of PKC isoenzymes and MAPK by PSK and IL-2 in the proliferative and cytotoxic activities of the NKL human natural killer cell line. Cancer Immunol. Immunother. 2003, 52, 59–64. [Google Scholar] [PubMed]
- Tada, R.; Harada, T.; Nagi-Miura, N.; Adachi, Y.; Nakajima, M.; Toshiro, Y.; Ohno, N. NMR characterization of the structure of a β-(1→3)-d-glucan isolate from cultured fruit bodies of Sparassis Crispa. Carbohydr. Res. 2007, 342, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Kritzman, G.; Chet, I.; Henis, Y. Isolation of extracellular polysaccharides from Sclerotium rolfsii. Can. J. Bot. 1979, 57, 1855–1859. [Google Scholar] [CrossRef]
- Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Production of scleroglucan from Sclerotium rolfsii MTCC 2156. Bioresour. Technol. 2006, 97, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Misaki, A.; Kawaguchi, K.; Miyaji, H.; Nagae, H.; Hokkoku, S.; Kakuta, M.; Sasaki, T. Structure of pestalotan, a highly branched (1/3)-β-d-glucan elaborated by Pestalotia sp. 815, and the enhancement of its antitumor activity by polyol modification of the side chains. Carbohydr. Res. 1984, 129, 209–227. [Google Scholar] [CrossRef]
- Schmid, F.; Stone, B.A.; McDougall, B.M.; Bacic, A.; Martin, K.L.; Brownlee, R.T.; Chai, E.; Seviour, R.J. Structure of epiglucan, a highly side-chain/branched (1/3;1/6)-β-glucan from the micro fungus Epicoccum nigrum Ehrenb. Ex Schlecht. Carbohydr. Res. 2001, 331, 163–171. [Google Scholar] [CrossRef]
- Warsi, S.A.; Whelan, W.J. Structure of pachyman, the polysaccharide component of Poria cocos. Chem. Ind. 1957, 48, 1573–1575. [Google Scholar]
- Wang, Y.; Zhang, M.; Ruan, D.; Shashkov, A.S.; Kilcoyne, M.; Savage, A.V.; Zhang, L. Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos. Carbohydr. Res. 2004, 339, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Hara, C.; Kumazawa, Y.; Inagaki, K.; Kaneko, M.; Kiho, T.; Ukai, S. Mitogenic and colony-stimulating factor-inducing activities of polysaccharide fractions from the fruit bodies of Dictyophora indusiata FISCH. Chem. Pharm. Bull. 1991, 39, 1615–1616. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, K.; Kraus, J.; Franz, G.; Röper, H. Structural investigations of glucans from cultures of Glomerella cingulata Spaulding & von Schrenck. Carbohydr. Res. 1991, 217, 153–161. [Google Scholar] [PubMed]
- Gomaa, K.; Kraus, J.; Rosskopf, F.; Röper, H.; Franz, G. Antitumour and immunological activity of a β (1→3/1→6) glucan from Glomerella cingulata. J. Cancer Res. Clin. Oncol. 1992, 118, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Adachi, Y.; Suzuki, I.; Sato, K.; Oikawa, S.; Yadomae, T. Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. 1986, 34, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Sone, Y.; IsodaJohmura, M.; Misaki, A. Isolation and chemical characterization of polysaccharides from Iwatake, Gyrophara esculenta Miyoshi. Biosci. Biotechnol. Biochem. 1996, 60, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Munz, C.; Steinman, R.M.; Fujii, S. Dendritic cell maturation by innate lymphocytes: Coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 2005, 202, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Maeda, Y.Y.; Hamuro, J.; Sasaki, T.; Fukuoka, F. Inhibition of mouse sarcoma 180 by polysaccharide from Lentinus edodes (Berk.) Sing. Nature 1969, 222, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar] [PubMed]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yadomae, T.; Sugiura, M.; Ito, H.; Fujii, K.; Naruse, S.; Kunihisa, M. Chemical structure of antitumor polysaccharide, coriolan, produced by Coriolus versicolor. Chem. Pharm. Bull. 1974, 22, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, S.; Akuawa, Y.; Endo, F. Effects of Lentinus edodes, Grifola frondosa and Pleurotus ostreatus administration on cancer outbreak, and activities of macrophages and lymphocytes in mice treated with a carcinogen, N-butyl-N-butanolnitrosoamine. Immunopharmacol. Immunotoxicol. 1997, 19, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheung, P.C.K.; Zhang, L. Evaluation of mushroom dietary fiber (nonstarch polysaccharides) from sclerotia of Pleurotus tuber-regium as a potential antitumor agent. J. Agric. Food Chem. 2001, 49, 5059–5062. [Google Scholar] [CrossRef] [PubMed]
- Kulicke, W.-M.; Lettau, A.I.; Thielking, H. Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-β-d-glucans. Carbohydr. Res. 1997, 297, 135–143. [Google Scholar] [CrossRef]
- Misaki, A.; Kakuta, M.; Sasaki, T.; Tanaka, M.; Miyaji, H. Studies on interrelation of structure and antitumor effects of polysaccharides: Antitumor action of periodate-modified, branched (1→3)-β-d-glucan of Auricularia auricula-judae, and other polysaccharides containing (1→3)-glycosidic linkages. Carbohydr. Res. 1981, 92, 115–129. [Google Scholar] [CrossRef]
- Defaye, J.; Kohlmunzer, S.; Sodzawiczny, K.; Wong, E. Structure of an antitumor, water-soluble d-glucan from the carpophores of Tylopilus felleus. Carbohydr. Res. 1988, 173, 316–323. [Google Scholar] [CrossRef]
- Grzybek, J.; Zgorniak-Nowosielska, I.; Kasprowicz, A.; Zawilinska, B.; Kohlmunzer, S. Antitumor activity of fungal glucan tylopilan and Propionibacterium acnes preparation. Acta Soc. Bot. Pol. 1994, 63, 293–298. [Google Scholar] [CrossRef]
- Kitamura, S.; Hori, T.; Kurita, K.; Takeo, K.; Hara, C.; Itoh, W.; Tabata, K.; Elgsaeter, A.; Stokke, B.T. An antitumor, branched (1→3)-β-d-glucan from a water extract of fruiting bodies of Cryptoporus volvatus. Carbohydr. Res. 1994, 263, 111–121. [Google Scholar] [CrossRef]
- Bell, W.; Kaesbauer, J.; Kraus, J.; Franz, G. Pythium aphanidermatum: Culture, cell wall composition, and isolation and structure of antitumor storage and solubilised cell wall (1→3), (1→6)-β-d-glucans. Carbohydr. Res. 1992, 231, 293–307. [Google Scholar]
- Sone, Y.; Okuda, R.; Wada, N.; Kishida, E.; Misaki, A. Structures and antitumor activities of the polysaccharides isolated from fruiting body and the growing culture of mycelium of Ganoderma lucidum. Agric. Biol. Chem. 1985, 49, 2641–2653. [Google Scholar]
- Hung, W.-T.; Wang, S.-H.; Chen, C.-H.; Yang, W.-B. Structure determination of β-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules 2008, 13, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Oshiman, K.; Fujimiya, Y.; Ebina, T.; Suzuki, I.; Noji, M. Orally administered β-1,6-d-polyglucose extracted from Agaricus blazei results in tumor regression in tumor-bearing mice. Planta Med. 2002, 68, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Suzuki, M.; Kanayama, N.; et al. Suppressing effects of daily oral supplementation of β-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J. Cancer Res. Clin. Oncol. 2005, 131, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Miyazato, A.; Abe, Y.; Zhang, T.; Nakamura, K.; Inden, K.; Tanaka, M.; Tanno, D.; Miyasaka, T.; Ishii, K.; et al. Activation of myeloid dendritic cells by deoxynucleic acids from Cordyceps sinensis via a Toll-like receptor 9-dependent pathway. Cell Immunol. 2010, 263, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pao, H.Y.; Pan, B.S.; Leu, S.F.; Huang, B.M. Cordycepin stimulated steroidogenesis in MA-10 mouse leydig tumor cells through the protein kinase C Pathway. J. Agric. Food Chem. 2012, 60, 4905–4913. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Kang, M.Y.; Kim, J.H.; Nam, S.H.; Friedman, M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011, 59, 9861–9869. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kimura, A.T.; Sugitachi, A.A.; Matsuurac, B.A.N. Anti-angiogenic and anti-metastatic effects of β-1,3-d-glucan purified from hanabiratake, Sparassis crispa. Biol. Pharm. Bull. 2009, 32, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.-K.; Ku, S.-K.; Choi, J.-S.; Kim, J.-W. Effect of polycan, a β-glucan originating from Aureobasidium, on a high-fat diet-induced hyperlipemic hamster model. Exp. Ther. Med. 2015, 9, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Cho, H.-R.; Choi, J.-S.; Kim, J.-W. Effects of polycan on calcium bioavailability in two different rat models of osteoporosis. Toxicol. Environ. Health Sci. 2015, 7, 35–42. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, J.W.; Kim, K.Y.; Choi, S.H.; Ku, S.K. Polycan, a β-glucan from Aureobasidium pullulans SM-2001, mitigates ovariectomy-induced osteoporosis in rats. Exp. Ther. Med. 2016, 12, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Sovrani, V.; De Jesus, L.I.; Simas-Tosin, F.F.; Smiderle, F.R.; Iacomini, M. Structural characterization and rheological properties of a gel-like β-d-glucan from Pholiota nameko. Carbohydr. Polym. 2017, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, Y.; Aoki, M.; Mikami, Y. Purification and some properties of an exo-β-1,3-glucanase from Porodisculus pendulus. J. Ferment. Technol. 1985, 63, 405–409. [Google Scholar]
- Whistler, R.L.; Bushway, A.A.; Singn, P.P.; Nakahara, W.; Tokuzen, R. Noncytotoxic, antitumor polysaccharides. Adv. Carbohydr. Chem. Biochem. 1976, 32, 235–274. [Google Scholar] [PubMed]
- Sato, M.; Sano, H.; Iwaki, D.; Kudo, K.; Konishi, M.; Takahashi, H.; Takahashi, T.; Imaizumi, H.; Asai, Y.; Kuroki, Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan induced NF-kappa B activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003, 171, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mariani, C.L.; Rajon, D.; Bova, F.J.; Streit, W.J. Nonspecific immunotherapy with intratumoral lipopolysaccharide and zymosan A but not GM-CSF leads to an effective anti-tumor response in subcutaneous RG-2 gliomas. J. Neurooncol. 2007, 85, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Lin, N.; Zan, D.; Yuan, J.J.; Cai, D.L. Effect of zymosan on antioxidant and immune function of S180 tumor-bearing mice. Cell Biochem. Biophys. 2011, 60, 225–229. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, B.W.; Albina, J.E.; Reichner, J.S. The effect of PGG β-glucan on neutrophil chemotaxis in vivo. J. Leukoc. Biol. 2006, 79, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.E.; Wagner, S.; Li, B.; Liu, J.; Hansen, R.; Reca, R.; Wu, W.; Surma, E.Z.; Laber, D.A.; Ratajczak, M.Z.; et al. Mobilization of hematopoietic progenitor cells by yeast-derived β-glucan requires activation of matrix metalloproteinase-9. Stem Cells 2008, 26, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Lebron, F.; Vassallo, R.; Puri, V.; Limper, A.H. Pneumocystis carinii cell wall β-glucans initiate macrophage inflammatory responses through NF-kappaB activation. J. Biol. Chem. 2003, 278, 25001–25008. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Cai, Y.; Gunn, L.; Ding, C.; Li, B.; Kloecker, G.; Qian, K.; Vasilakos, J.; Saijo, S.; Iwakura, Y.; et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood 2011, 117, 6825–6836. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J.; Kim, T.J.; Lee, H.; Shin, K.S.; Yun, Y.P.; Moon, W.K.; Kim, D.W.; Lee, K.H. Anti-tumor metastatic activity of β-glucan purified from mutated Saccharomyces cerevisiae. Int. Immunopharmacol. 2008, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary yeast β-1,3/1,6-d-glucan. Nutr. J. 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandula, J.; Machová, E.; Hribalová, V. Mitogenic activity of particulate yeast β-(1→3)-d-glucan and its water-soluble derivatives. Int. J. Biol. Macromol. 1995, 17, 323–326. [Google Scholar] [CrossRef]
- Nakanishi, L.; Kimura, K.; Suzuki, T.; Ishikawa, M.; Banno, L.; Sakane, T.; Harada, T. Demonstration of curdlan-type polysaccharide and some other β-1,3-glucan in microorganisms with aniline blue. J. Gen. Appl. Microbiol. 1976, 22, 1–11. [Google Scholar] [CrossRef]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1/3)-β-d-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, M.; Ionescu, C.; Caraiani, T.; Căùărică, A.; Marinescu, M.C.; Zăhărăchescu, V.; Ghera, D.; Gheorghiu, E.; Stan, A.; Soare, M.; et al. Curdlan-type polysaccharide obtained using a strain of Agrobacterium rhizogenes. Rom. Biotechnol. Lett. 2009, 14, 4530–4537. [Google Scholar]
- West, T.P. Elevated curdlan production by a mutant of Agrobacterium sp. ATCC 31749. J. Basic Microbiol. 2009, 29, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Sung, K.J.; Cho, M.C.; Choi, W.A.; Yang, Y.; Lim, J.S.; Yoon, D.Y. Antitumor effect of soluble β-1,3-glucan from Agrobacterium sp. R259 KCTC 1019. J. Microbiol. Biotechnol. 2007, 17, 1513–1520. [Google Scholar] [PubMed]
- Elyakova, L.A.; Pavlov, G.M.; Isakov, V.V.; Zaitseva, I.; Stepchenova, T.A. Molecular characteristics of laminarin subfractions. Khimiya Prir. Soedin. 1994, 2, 296–298. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Reason, A.J.; Haslam, S.M.; McDowell, R.A.; Chizhov, O.S.; Usov, A.I. Structural analysis of laminarans by MALDI and FAB mass spectrometry. Carbohydr. Res. 1998, 310, 203–210. [Google Scholar] [CrossRef]
- Wang, M.C.; Bartnicki-Garcia, S. Novel phosphoglucans from the cytoplasm of Phytophthora palmivora and their selective occurrence in certain life cycle stages. J. Biol. Chem. 1973, 248, 4112–4118. [Google Scholar] [PubMed]
- Wang, M.C.; Bartnicki-Garcia, S. Distribution of mycolaminarans and cell wall β-glucans in the life cycle of Phytophthora. Exp. Mycol. 1980, 4, 269–280. [Google Scholar] [CrossRef]
- Archibald, A.R.; Cunningham, W.L.; Manners, D.J.; Stark, J.R.; Ryley, J.F. Metabolism of the protozoa, X. The molecular structure of the reserve polysaccharides from Ochromonas malhamensis and Peranema trichophorum. Biochem. J. 1963, 88, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef] [PubMed]
- Storseth, T.R.; Hansen, K.; Skjermo, J.; Krane, J. Characterization of a β-d-(1,3)-glucan from the marine diatom Chaetoceros mulleri by high resolution magic-angle spinning NMR spectroscopy on whole algal cells. Carbohydr. Res. 2004, 339, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Dvorak, B.; Vetvickova, J.; Richter, J.; Krizan, J.; Sima, P.; Yvin, J.-C. Orally administered marine (1→3)-β-d-glucan phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol. 2007, 40, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.E.; Stone, B.A. Structure of the paramylon from Euglena gracilis. Biochim. Biophys. Acta 1960, 44, 161–163. [Google Scholar] [CrossRef]
- Kreger, D.R.; van der Veer, J. Paramylon in a chrysophyte. Plant Biol. 1970, 19, 401–402. [Google Scholar] [CrossRef]
- Ford, C.W.; Percival, E. The carbohydrates of Phaeodactylum tricornutum. Preliminary examination of the organism, and characterization of low molecular weight material and of a glucan. J. Chem. Soc. 1965. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lakhdara, N.; Lazaridou, A.; Biliaderis, C.G.; Izydorczyk, M.S. Water extractable (1→3,1→4)-β-d-glucans from barley and oats: An intervarietal study on their structural features and rheological behaviour. J. Cereal Sci. 2005, 42, 213–224. [Google Scholar] [CrossRef]
- Bohm, N.; Kulicke, W.M. Rheological studies of barley (1→3)(1→4)-β-glucan in concentrated solution: Mechanistic and kinetic investigation of the gel formation. Carbohydr. Res. 1999, 315, 302–311. [Google Scholar] [CrossRef]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, R.G.; Slavin, J.L. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.E.; Tapsell, L.C.; Batterham, M.J.; O’Shea, J.; Thorne, R.; Beck, E.; Tosh, S.M. Effect of 6 weeks’ consumption of β-glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br. J. Nutr. 2012, 107, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wood, P.J.; Blackwell, B.A.; Nikiforuk, J. Physicochemical properties and structural characterization by two-dimensional NMR spectroscopy of wheat β-d-glucan-Comparison with other cereal β-d-glucans. Carbohydr. Polym. 2000, 41, 249–258. [Google Scholar] [CrossRef]
- Li, W.; Cui, S.W.; Wang, Q. Solution and conformational properties of wheat β-d-glucans studied by light scattering and viscometry. Biomacromolecules 2006, 7, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Berovic, M.; Habijanic, J.; Zore, I.; Wraber, B.; Hodzar, D.; Boh, B.; Pohleven, F. Submerged cultivation of Ganoderma lucidum biomass and immunostimulatory effects of fungal polysaccharides. J. Biotecnol. 2003, 103, 77–86. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Matuo, R.; Silva, A.F.; Matiazi, H.J.; Mantovani, M.S.; Ribeiro, L.R. Protective effect of β-glucan extracted from Saccharomyces cerevisiae, against DNA damage and cytotoxicity in wild-type (K1) and repair-deficient (xrs5) CHO cells. Toxicol. In Vitro 2007, 21, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Law, H.K.; Lin, Z.B.; Lau, Y.L.; Chan, G.C. Response of human dendritic cells to different immunomodulatory polysaccharides derived from mushroom and barley. Int. Immunol. 2007, 19, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Hashimoto, K.; Takagi, R.; Mizuno, Y.; Okazaki, Y.; Tanaka, Y.; Matsushita, S. Curdlan induces DC-mediated Th17 polarization via Jagged1 activation in human dendritic cells. Allergol. Int. 2010, 59, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, A.; Kulbacka, J.; Rembialkowska, N.; Pilat, J.; Oledzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat β-glucan-An in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.K.; Fung, K.P.; Choy, Y.M. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xuelian, L.; Xu, X.; Zeng, F. Correlation between antitumoral activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Yvin, J.C. Effects of marine β-glucan on immune reaction. Int. Immunopharmacol. 2004, 4, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miura, T.; Saito, K.; Nishijima, M.; Miyazaki, T.; Yadomae, T. Physicochemical characteristics and antitumor activities of a highly branched fungal (1,3)-β-d-Glucan, OL-2, isolated from Omphalia lapidescens. Chem. Pharm. Bull. 1992, 40, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nishijima, M.; Ohno, N.; Yadomae, T.; Miyazaki, T. Structure and antitumor activity of the less-branched derivatives of an alkali-soluble glucan isolated from Omphalia lapidescens. (Studies on Fungal Polysaccharide. XXXVIII). Chem. Pharm. Bull. 1992, 40, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miura, N.N.; Nakajima, M.; Yadomae, T. Antitumor 1,3-β-glucan from cultured fruit body of Sparassis crispa. Biol. Pharm. Bull. 2000, 23, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Motoi, M.; Yadomae, T. Antitumoral β-glucan from cultured fruit body of Agaricus blazei. Biol. Pharm. Bull. 2001, 24, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ebina, T.; Fujimiya, Y. Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumor system in mice. Biotherapy 1998, 11, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, M.; Hansen, R.; Ding, C.; Cramer, D.E.; Yan, J. Therapeutic potential of various β-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol. Ther. 2009, 8, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Kodoi, R.; Nonaka, Y. Lentinan from shiitake mushroom (Lentinus edodes) suppresses expression of cytochrome P450 1A subfamily in the mouse liver. Biofactors 2004, 21, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Weitberg, A.B. A phase I/II trial of β-(1,3)/(1,6) d-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 2008, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Esfahani, A.; Jafarabadi, M.A.; Ziaei, J.E.; Movassaghpourakbari, A.; Farrin, N. Effect of β-glucan on quality of life in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Adv. Pharm. Bull. 2014, 4, 471–477. [Google Scholar] [PubMed]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S.; Crowley, J.J.; Tufts, J.C.; Zimmerman, J.; Mackin, W.; Adams, D.S. PGG-glucan, a soluble β-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity and activates an NF-kappa B-like factor in human PMN: Evidence for a glycosphingolipid β-(1,3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NFkappaB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Chaung, H.-C.; Huang, T.-C.; Yu, J.-H.; Wuc, M.-L.; Chung, W.-B. Immunomodulatory effects of β-glucans on porcine alveolar macrophages and bone marrow haematopoietic cell-derived dendritic cells. Vet. Immunol. Immunopathol. 2009, 131, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. β-glucans are involved in immune-modulation of THP-1 macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1,6 glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Crabohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Terayama, K.; Mandeville, R.; Brousseau, P.; Kournikakis, B.; Ostroff, G. Pilot study: Orally administered yeast β-1,3-glucan prophylactically protects against anthrax infection and cancer in mice. J. Am. Nutraceut. Assoc. 2002, 5, 1–6. [Google Scholar]
- Sakurai, T.; Hashimoto, K.; Suzuki, I.; Ohno, N.; Oikawa, S.; Masuda, A.; Yadomae, T. Enhancement of murine alveolar macrophage functions by orally administered β-glucan. Int. J. Immunopharmacol. 1992, 14, 821–830. [Google Scholar] [CrossRef]
- Di Luzio, N.R.; Williams, D.L.; Mcnamee, R.B.; Edwards, B.F.; Kitahama, A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int. J. Cancer 1979, 24, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A.; Kastello, M.D.; Harrigton, D.G.; Crabs, C.L.; Peters, C.J.; Jemski, J.V.; Scott, G.H.; Di Luzio, N.R. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect. Immun. 1980, 30, 51–57. [Google Scholar] [PubMed]
- Hotta, H.; Hagiwara, K.; Tabata, K.; Ito, W.; Homma, M. Augmentation of protective immune responses against Sendai virus infection by fungal polysaccharide schizophyllan. Int. J. Immunopharmacol. 1993, 5, 55–60. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Kernodle, D. Synergism between poly-(1→6)-β-d-glucopyranose glucana and cefazolin in prophylaxis of staphylococcal wound infection in guinea pig model. Antimicrobiol. Agents Chemother. 1998, 42, 2449–2451. [Google Scholar]
- Yun, C.H.; Estrada, A.; Kessel, A.V.; Gajadhar, A.; Redmond, M.; Laearveld, B. Immunomodulatory effects of a oat-β-glucan administered intragastrically or parentally on mice infected with Eimeria verminoformis. Microbiol. Immunol. 1998, 42, 457–465. [Google Scholar] [PubMed]
- Hetland, G.; Ohno, N.; Aaberge, I.S.; Løvik, M. Protective effect of β-glucan against systemic Streptococcus pneumoniae infection in mice. FEMS Immunol. Med. Microbiol. 2000, 27, 111–116. [Google Scholar] [CrossRef]
- Hasegawa, A.; Yamada, M.; Dombo, M.; Fukushima, R.; Matsuura, N.; Sugitachi, A. Sparassis crispa as biological response modifier. Gan To Kagaku Ryoho 2004, 11, 1761–1763. [Google Scholar]
- Sener, G.; Eksioglu-Demiralp, E.; Cetiner, M.; Ercan, E.; Yegen, B.C. β-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immunomodulatory effects. Eur. J. Pharmacol. 2006, 542, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvicka, J. Immunological effects of yeast- and mushroom-derived β-glucans. J. Med. Food 2008, 11, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vancikova, Z. Anti-stress action of several orally-given β-glucans. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 235–238. [Google Scholar] [CrossRef] [PubMed]
- McCormack, E.; Skavland, J.; Mujic, M.; Bruserud, O.; Gjertsen, B.T. Lentinan: Hematopoietic, immunological, and efficacy studies in a syngeneic model of acute myeloid leukemia. Nutr. Cancer 2010, 62, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.K.; Maiti, S.; Bhutia, S.K.; Maiti, T.K. Immunostimulatory properties of a polysaccharide isolated from Astraeus hygrometricus. J. Med. Food 2010, 13, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arachida, R.; Asayama, Y.; Yabuta, Y.; Takeucki, T. Oral administration of paramylon, a β-1,3-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-Q.; Reza, M.A.; Lee, J.-S.; Gebru, E.; Jang, S.-H.; Choi, M.-J.; Lee, S.-J.; Damte, D.; Kim, J.-C.; Park, S.-C. Immunomodulatory activities and subacute toxicity of a novel β-glucan from Paenibacillus polymyxa JB115 in rats. Immunopharmacol. Immunotoxicol. 2011, 33, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Beynen, A.C.; Saris, D.H.J.; Paap, P.M.; Altena, F.V.; Visser, E.A.; Middelkoop, J.; De Jong, L.; Staats, M. Dietary β-1,3/1,6-glucans reduce clinical signs of canine atopy. Am. J. Anim. Vet. Sci. 2011, 6, 146–152. [Google Scholar]
- Hong, H.; Kim, C.-J.; Kim, J.-D.; Seo, J.-H. β-glucan reduces exercise-induced stress through down regulation of c-Fos and c-Jun expression in the brains of exhausted rats. Mol. Med. Rep. 2014, 9, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Babineau, T.J.; Hackford, A.; Kenler, A.; Bistrian, B.; Forse, R.A.; Fairchild, P.G.; Heard, S.; Keroack, M.; Caushaj, P.; Benotti, P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch. Surg. 1994, 129, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Meira, D.A.; Pereira, P.C.M.; Marcondes-Machado, J.; Barravieira, R.P.; Pellegrino, J.R.J.; Rezkallah-Iwasso, M.T.; Peracoli, M.T.S.; Castilho, L.M.; Thomzaini, I.; Silva, C.L.; et al. The use of glucan as immunostimulant in treatment of paracoccidiomycosis. Am. J. Trop. Med. Hyg. 1996, 55, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Holck, P.; Sletmoen, M.; Stokke, B.T.; Permin, H.; Norn, S. Potentiation of histamine release by microfungal (1,3)- and (1,6)-β-d-glucans. Basic Clin. Pharmacol. Toxicol. 2007, 101, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Sarinho, E.; Medeiros, D.; Schor, D.; Silva, A.R.; Sales, V.; Motta, M.E.; Costa, A.; Azoubel, A.; Rizzo, J.A. Production of interleukin-10 in asthmatic children after β-1–3-glucan. Allergol. Immunopathol. (Madr.) 2009, 37, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, K.R.; Purhonen, A.-K.; Salmenkallio-Marttila, M.; Lahteenmaki, L.; Laaksonen, D.E.; Herzig, K.-H.; Uusitupa, M.I.J.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.C.; Breslin, W.L.; Davidson, T.; Adams, A.; McFarlin, B.K. Baker’s yeast β-glucan supplementation increases monocytes and cytokined post-exercise: Implications for infection risk? Br. J. Nutr. 2013, 109, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Kim, Y.S.; Lee, Y.J.; Ahn, H.Y.; Kim, M.; Kim, M.; Cho, M.J.; Cho, Y.; Lee, J.H. Effect of immune-enhancing enteral nutrition enriched with or without β-glucan on immunomodulation in critically ill patients. Nutrients 2016, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.; Talbott, J. Effect of β 1,3/1,6 glucan on upper respiratory tract infection symptoms and mood state in marathon athletes. J. Sport Sci. Med. 2009, 8, 509–515. [Google Scholar]
- Talbott, S.; Talbott, J. β 1,3/1,6 glucan decreases upper respiratory tract infection symptoms and improves psychological well-being in moderate to highly-stressed subjects. Agro Food Ind. Hi-Tech 2010, 21, 21–24. [Google Scholar]
- Talbott, S.M.; Talbott, J.A. Baker’s yeast β-glucan supplement reduces upper respiratory symptoms and improved mood state in stressed women. J. Am. Coll. Nutr. 2012, 31, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Richter, J.; Svozil, V.; Dobiášová, L.R.; Král, V. Placebo-driven clinical trials of yeast-derived β-(1,3) glucan in children with chronic respiratory problems. Ann. Transl. Med. 2013, 1, 26. [Google Scholar] [PubMed]
- Vetvicka, V.; Richter, J.; Svozil, V.; Dobiasova, K.R.; Kral, V. Placebo-driven clinical trials of transfer point glucan#300 in children with chronic respiratory problems: Antibody production. Am. J. Immunol. 2013, 9, 43–47. [Google Scholar]
- Vetvicka, V.; Richter, J.; Svozil, V.; Dobiasova, L.R.; Kral, V. Placebo-driven clinical trials of transfer point glucan#300 in children with chronic respiratory problems: III. Clinical findings. Am. J. Immunol. 2013, 9, 88–93. [Google Scholar]
- Richter, J.; Svozil, V.; Kral, V.; Rajnohova Dobiasova, L.; Stiborova, I.; Vetvicka, V. Clinical trials of yeast-derived β-(1,3) glucan in children: Effects on innate immunity. Ann. Transl. Med. 2014, 2, 15. [Google Scholar] [PubMed]
- Richter, J.; Kral, V.; Svozil, V.; Dobiasova, L.R.; Pohorska, J.; Stiborova, I.; Vetvicka, V. Effects of transfer point glucan#300 supplementation on children exposed to passive smoking-placebo-driven double-blind clinical trials. J. Nutr. Health Sci. 2014, 1, 1–8. [Google Scholar]
- Richter, J.; Svozil, V.; Kral, V.; Dobiasova, L.R.; Vetvicka, V. β-glucan affects mucosal immunity in children with chronic respiratory problems under physical stress: Clinical trials. Ann. Transl. Med. 2015, 3, 52. [Google Scholar] [PubMed]
- Jesenak, M.; Sanislo, L.; Kuniakova, R.; Rennerova, Z.; Buchanec, J.; Banovcin, P. Imunoglukan P4H® in the prevention of recurrent respiratory infections in childhood. Cesk Pediatra 2010, 73, 639–647. [Google Scholar]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Hrubisko, M.; Majtan, J.; Rennerova, Z.; Banovcin, P. Anti-allergic effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Phytother. Res. 2014, 28, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Urbancek, S.; Majtan, J.; Banovcin, P.; Hercogova, J. β-Glucan-based cream (containing pleuran isolated from Pleurotus ostreatus) in supportive treatment of mild-to moderate atopic dermatitis. J. Dermatol. Treat. 2015. [Google Scholar] [CrossRef]
- Grau, S.J.; Sirvent, P.L.; Ingles, M.M.; Urgell, R.M. β-glucans from Pleurotus ostreatus for prevention of recurrent respiratory tract infections. Acta Pediatr. Esp. 2015, 73, 186–193. [Google Scholar]
- Pasnik, J.; Slemp, A.; Cywinska-Bernas, A.; Zeman, K.; Jesenak, M. Preventive effect of pleuran (β-glucan isolated from Pleurotus ostreatus) in children with recurrent respiratory tract infections-Open-label prospective study. Curr. Pediatr. Res. 2017, 21, 99–104. [Google Scholar]
- Turnbull, J.L.; Patchen, M.L.; Scadden, D.T. The polysaccharide, PGG-glucan, enhances human myelopoiesis by direct action independent of and additive to early-acting cytokines. Acta Haematol. 1999, 102, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Kim, J.-W.; Kim, K.-Y.; Cho, H.-R.; Ha, Y.-M.; Ku, S.K.; Cho, K.K.; Choi, I.S. In vitro activities of polycalcium, a mixture of polycan and calcium lactate-gluconate, on osteoclasts and osteoblasts. J. Life Sci. 2011, 21, 199–1203. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, J.W.; Jung, G.-W.; Moon, S.-B.; Cho, H.-R.; Sung, S.H.; Jung, J.J.; Kwon, Y.S.; Ku, S.K.; Sohn, J.-H. Effect of a β-glucan from Aureobasidium on TGF-β1-modulated in vitro dermal wound repair. Toxicol. Environ. Health Sci. 2016, 8, 12–18. [Google Scholar] [CrossRef]
- Przekora, A.; Palka, K.; Ginalska, G. Biomedical potential of Chitosan/HA and Chitosan/β-1,3-glucan/HA biomaterials as scaffolds for bone regeneration-A comparative study. Mater. Sci. Eng. C 2016, 58, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, A.A.; El-Gohr, A.A.; El-Nahas, S.M.; Noshy, M.M. β-glucan inhibits the genotoxicity of cyclophosphamide, adramycin and cisplatin. Mutat. Res. 2003, 541, 45–53. [Google Scholar] [CrossRef]
- Choi, J.-S.; Kim, J.W.; Kim, K.Y.; Cho, H.-R.; Choi, I.S.; Ku, S.K. Antiosteoporotic effects of polycan in combination with calcium lactate-gluconate in ovariectomized rats. Exp. Ther. Med. 2014, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Shin, H.-S.; Kim, K.Y.; Ku, S.K.; Choi, I.S.; Kim, J.W. Effect of polycalcium, a mixture of polycan and calcium lactate-gluconate in a 1:9 weight ratio, on rats with surgery-induced osteoarthritis. Exp. Ther. Med. 2015, 9, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Kang, S.-J.; Han, C.-H.; Kim, J.-W.; Song, C.-H.; Lee, S.-N.; Ku, S.-K.; Lee, Y.-J. The effects of topical application of polycal (a 2:98 (g/g) mixture of polycan and calcium gluconate) on experimental periodontitis and alveolar bone loss in rats. Molecules 2016, 21, 527. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, L.; Pawłowska, M.; Radzki, R.P.; Bieńko, M.; Polkowska, I.; Belcarz, A.; Karpiński, M.; Słowik, T.; Matuszewski, L.; Ślósarczyk, A.; et al. Effect of a carbonated HAP/β-glucan composite bone substitute on healing of drilled bone voids in the proximal tibial metaphysis of rabbits. Mater. Sci. Eng. C 2015. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Park, M.Y.; Kim, J.D.; Cho, H.R.; Choi, I.S.; Kim, J.W. Safety and efficacy of polycalcium for improving biomarkers of bone metabolism: A 4-week open-label clinical study. J. Med. Food 2013, 16, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Park, M.Y.; Kim, J.W.; Kim, K.Y.; Cho, H.R.; Choi, I.S.; Choi, J.-S.; Ku, S.K.; Park, S.-J. Randomized, double-blind, placebo-controlled trial of the effects of polycan, β-glucan originating from Aureobasidium pullulans, on bone biomarkers in healthy women. J. Physiol. Pathol. Korean Med. 2015, 29, 330–336. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nonaka, Y.; Minato, K.-Y.; Kawakami, S.; Mizuno, M.; Fukuda, I. Suppressive effect of polysaccharides from the edible and medicinal mushrooms, Lentinus edodes and Agaricus blazei, on the expression of cytochrome p450s in mice. Biosci. Biotechnol. Biochem. 2002, 66, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.-H.; Kim, J.W.; Jung, G.-W.; Park, D.-C.; Moon, S.-B.; Cho, H.-R.; Ku, S.K.; Choi, J.-S. Effects of β-glucan and Folium mori extract combinations in STZ-induced diabetic rats: Effectiveness of various BGFM complex compositions in treating diabetes. Curr. Nutr. Food Sci. 2017. [Google Scholar] [CrossRef]
- Tappy, L.; Gugolz, E.; Wursch, P. Effects of breakfast cereals containing various amounts of β-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care 1996, 19, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, I.; Yokoyama, W.; Davis, P.; Hudson, C.; Backus, R.; Richter, D.; Knuckles, B.; Schneeman, B.O. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with β-glucan. Am. J. Clin. Nutr. 1999, 69, 55–63. [Google Scholar] [PubMed]
- Cavallero, A.; Empilli, S.; Brighenti, F.; Stanca, A.M. High (1→3,1→4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002, 36, 59–66. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Jenkins, D.J.A.; Zdravkovic, U.; Wursch, P.; Vuksan, V. Depression of the glycemic index by high levels of β-glucan fiber in two functional foods tested in type 2 diabetes. Eur. J. Clin. Nutr. 2002, 56, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Tapola, N.; Karvonen, H.; Niskanen, L.; Mikola, M.; Sarkkinen, E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Biorklund, M.; Rees, A.V.; Mensink, R.P.; Onning, G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β-glucans from oats or barley: A randomised dose-controlled trial. Eur. J. Clin. Nutr. 2005, 59, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Mäkeläinen, H.; Anttila, H.; Sihvonen, J.; Hietanen, R.M.; Tahvonen, R.; Salminen, E.; Mikola, M.; Sontag-Strohm, T. The effect of β-glucan on the glycemic and insulin index. Eur. J. Clin. Nutr. 2007, 61, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Nyberg, L.; Björck, I. Muesli with 4 g oat β-glucans lowers glucose and insulin responses after a bread meal in healthy subjects. Eur. J. Clin. Nutr. 2008, 62, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Thondre, P.S.; Henry, C.J.K. High-molecular-weight barley β-glucan in chapatis (unleavened Indian flat bread) lowers glycemic index. Nutr. Res. 2009, 29, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Cugnet-Anceau, C.; Nazare, J.A.; Biorklund, M.; le Coquil, E.; Sassolas, A.; Sothier, M.; Holm, J.; Landin-Olsson, M.; Önning, G.; Laville, M.; et al. A controlled study of consumption of β-glucan-enriched soups for 2 months by type 2 diabetic free-living subjects. Br. J. Nutr. 2010, 103, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality Glucans, blood cholesterol, and macrophage function. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kusmiati; Dhewantata, F.X.R. Cholesterol-lowering effect of β-glucan extracted from Saccharomyces cerevisiae in rats. Sci. Pharm. 2016, 84, 153–165. [Google Scholar]
- Davidson, M.H.; Dugan, L.D.; Burns, J.H.; Bova, J.; Story, K.; Drennan, K.B. The hypocholesterolemic effects of β-glucan in oatmeal and oat bran. A dose-controlled study. J. Am. Med. Assoc. 1991, 265, 1833–1839. [Google Scholar] [CrossRef]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R.; Greenberg, I.; Forse, R.A.; Blackburn, G.L. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am. J. Clin. Nutr. 1999, 70, 208–212. [Google Scholar] [PubMed]
- Lovegrove, J.A.; Clohessy, A.; Milon, H.; Williams, C.M. Modest doses of β-glucan do not reduce concentrations of potentially atherogenic lipoproteins. Am. J. Clin. Nutr. 2000, 72, 49–55. [Google Scholar] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Vidgen, E.; Parker, T.; Faulkner, D.; Mehling, C.C.; Garsetti, M.; Testolin, G.; Cunnane, S.C.; et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 2002, 75, 834–839. [Google Scholar] [PubMed]
- Keogh, G.F.; Cooper, G.J.S.; Mulvey, T.B.; McArdle, B.H.; Coles, G.D.; Monro, J.A.; Poppitt, S.D. Randomized controlled crossover study of the effect of a highly β-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2003, 78, 711–718. [Google Scholar] [PubMed]
- Kerckhoffs, D.A.J.M.; Hornstra, G.; Mensink, R.P. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am. J. Clin. Nutr. 2003, 78, 221–227. [Google Scholar] [PubMed]
- He, J.; Streiffer, R.H.; Muntner, P.; Krousel-Wood, M.A.; Whelton, P.K. Effect of dietary fiber intake on blood pressure: A randomized, double-blind, placebo-controlled trial. J. Hypertens. 2004, 22, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Galant, R.; Samuel, P.; Tesser, J.; Witchger, M.S.; Ribaya-Mercado, J.D.; Blumberg, J.B.; Geohas, J. Effects of consuming foods containing oat β-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur. J. Clin. Nutr. 2007, 61, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Kihara, M.; Aoe, S.; Araki, S.; Ito, K.; Hayashi, K.; Watari, J.; Sakata, Y.; Ikegami, S. Effect of high β-glucan barley on serum cholesterol concentrations and visceral fat area in Japanese men—A randomized, double-blinded, placebo-controlled trial. Plant Foods Hum. Nutr. 2008, 63, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Slamenova, D.; Labaj, J.; Krizkova, L.; Kogan, G.; Sandula, J.; Bresgen, N.; Eckl, P. Protective effects of fungal β-d-glucan derivatives against oxidative DNA lesions in V79 hamster lung cells. Cancer Lett. 2003, 198, 153–160. [Google Scholar] [CrossRef]
- Krizkova, L.; Zitnanova, I.; Mislovicova, D.; Masarova, J.; Sasinkova, V.; Durackova, Z.; Krajcovica, J. Antioxidant and antimutagenic activity of mannan neoglycoconjugates: Mannan-human serum albumin and mannan-penicillin G acylase. Mutat. Res. 2006, 606, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.J.; Ribeiro, L.R.; Silva, A.F.; Matuo, R.; Mantovani, M.S. Evaluation of antimutagenic activity and mechanisms of action of β-glucan from barley, in CHO-K1 and HTC cell lines using the micronucleus test. Toxicol. In Vitro 2006, 20, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Ribeiro, L.R.; Gonzaga, M.L.C.; Soares, S.A.; Ricardo, M.P.S.N.; Tsuboy, M.S.; Stidl, R.; Knasmuller, S.; Linhares, R.E.; Mantovani, M.S. Protective effects of β-glucan extracted from Agaricus brasiliensis against chemically induced DNA damage in human lymphocytes. Cell Biol. Toxicol. 2006, 22, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Ribeiro, L.R.; Bellini, M.F.; Mantovani, M.S. Anticlastogenic effect of β-glucan extracted from barley towards chemically induced DNA damage in rodent cells. Hum. Exp. Toxicol. 2006, 25, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.P.F.; Ribeiro, L.R.; Bellini, M.F.; Mantovani, M.S. β-Glucan extracted from the medicinal mushroom Agaricus blazei prevents the genotoxic effects of benzo[a]pyrene in the human hepatoma cell line HepG2. Arch. Toxicol. 2009, 83, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Erkol, H.; Kahramansoy, N.; Kordon, Ö.; Büyükaşık, O.; Serin, E.; Ulaş, N. Effects of β-glucan on hepatic damage caused by obstructive jaundice. Ulus Travma Acil Cerrahi Derg 2011, 17, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, A.M.; Akkaya, V.B.; Gulecol, S.C.; Ceyhan, B.M.; Ozguner, F.; Chen, W. Protective effects of β-glucan against oxidative injury induced by 2.45-GHz electromagnetic radiation in the skin tissue of rats. Arch. Dermatol. Res. 2012, 304, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Pillai, T.G.; Devi, P.U. Mushroom β-glucan: Potential candidate for post irradiation protection. Mutat. Res. 2013, 751, 109–115. [Google Scholar] [CrossRef] [PubMed]
| β-Glucan | Abbreviation | Source | Structure | Reference |
|---|---|---|---|---|
| Fungal β-Glucan | ||||
| Schizophyllan/Sizofiran/Sonifilan | SPG | Scizophyllum commune | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [39,40] |
| Sclerotinan/Sclerotan | SSG | Sclerotinia sclerotiorum, Sparassis crispus | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [39,41,42] |
| Scleroglucan/Sclero-β-glucan | SR-glucan | Sclerotium rolfsii, Sclerotium glucanicum | Linear (1,3;1,6) β-glucan | [43,44] |
| Pestalotan | - | Pestalotia sp. | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [45] |
| Epiglucan | - | Epicoccum nigrum | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [46] |
| Pachymaran/Pachyman | - | Poria cocos | Linear (1,3) β-glucan | [47,48] |
| T-4-N, T-5-N | - | Dictyophora indusiata Fisch, Phallus indusiata | Branched (1,3;1,6) β-glucan | [49] |
| β-glucan | - | Glomerella cingulata | Branched (1,3;1,6) β-glucan | [50,51] |
| Grifolan | GRN | Grifola frondosa | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [52,53,54] |
| Lentinan | LNT | Lentinula edodes | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [55,56,57] |
| LC11 | - | Lentinus edodes | Branched (1,3;1,4) β-glucan | [57] |
| Coriolan | - | Coriolus versicolor | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [58] |
| Krestin | PSK | Trametes versicolor | Protein-bound linear (1,3) β-glucan | [59] |
| Pleuran | HA-glucan | Pleurotus tuber-regium, Pleurotus ostreatus | Branched (1,3;1,6) β-glucan | [60] |
| β-glucan | MFL-glucan | Monilinia fructicola | Branched (1,3;1,6) β-glucan | [61] |
| β-glucan | MFN-glucan | Monilinia fructigena | Branched (1,3;1,6) β-glucan | [61] |
| β-glucan | AM-ASN | Amanita muscaria | Branched (1,3;1,6) β-glucan | [61] |
| β-glucan | AAG | Auricularia auricular-judae | Branched (1,3;1,6) β-glucan | [62] |
| Tylopilan | - | Tylopilus felleus | Branched (1,3;1,6) β-glucan | [63,64] |
| β-glucan | - | Cryptoporus volvatus | Branched (1,3;1,6) β-glucan | [65] |
| β-glucan | - | Pythium aphanidermatum | Branched (1,3;1,6) β-glucan | [66] |
| Polysaccharide-glucan | PS-G | Ganoderma lucidum | Branched (1,3;1,6) β-glucan | [67,68] |
| β-glucan | - | Agaricus blazei | Branched (1,3;1,6) β-glucan | [69,70] |
| β-glucan | - | Cordyceps sinensis | Branched (1,3;1,6) β-glucan | [71,72] |
| β-glucan | HEP3 | Hericium erinaceus | Branched (1,3;1,6) β-glucan | [73] |
| β-glucan | SBG | Sparassis crispa | Branched (1,3;1,6) β-glucan | [74] |
| Polycan | - | Aureobasidium pullulans | Branched (1,3;1,6) β-glucan | [75,76,77] |
| β-glucan | BG-PN | Pholiota nameko | Branched (1,3;1,6) β-glucan | [78] |
| Pendulan | - | Porodisulus pendulus | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [79] |
| Lichen β-Glucan | ||||
| Pustulan | - | Gyrophera esculenta, Umbiliaria papulosa | Linear (1,3) β-glucan | [80] |
| Lichenan/Lichenin | - | Cetraria islandica | Linear (1,3;1,4) β-glucan | [80] |
| Yeast β-Glucan | ||||
| Zymosan | - | Saccharomyces cerevisiae | Branched (1,3;1,6) β-glucan | [81,82,83] |
| Βetafectin/TH-glucan | PGG | Saccharomyces cerevisiae | Branched (1,3;1,6) β-glucan | [84,85] |
| Yeast whole β-glucan particles | WPG, WGPs | Saccharomyces cerevisiae | Yeast whole β-glucan particles | [86,87] |
| β-glucan | MG | Saccharomyces cerevisiae | Linear (1,3) β-glucan | [81] |
| β-glucan | IS-2 | S. cerevisiae (Mutated) | - | [88] |
| Yestimun | - | Saccharomyces cerevisiae | Branched (1,3;1,6) β-glucan | [89] |
| Cerevan | - | Saccharomyces cerevisiae | Branched (1,3;1,6) β-glucan | [90] |
| Bacterial β-Glucan | ||||
| Curdlan | - | Alcaligenes faecalis, Agrobacterium rhizogenes, Agrobacterium radiobacter | Linear (1,3) β-glucan | [91,92,93,94] |
| β-glucan | DMJ-E | Agrobacterium sp. R259 | Linear (1,3) β-glucan | [95] |
| Seaweed/Algal β-Glucan | ||||
| Laminaran/Laminarin | - | Laminaria sp. (brown algae), Laminaria cichorioides | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [96,97] |
| Mycolaminarin | - | Phytophthora sp. | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [98,99] |
| Chrysolaminarin | CL-2 | Ochromonas malhamensis, Odontella aurita, Chaetoceros muelleri | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [100,101,102] |
| Phycarine | - | Laminaria digitata | Linear (1,3) β-glucan | [103] |
| Paramylon | - | Euglena gracilis, Pavlova mesolychnon | Linear (1,3) β-glucan | [104,105] |
| Leucosin | - | Phaeodactylum tricornutum | Linear (1,3) β-glucan with (1,6)-linked-β-glucosyl or β-oligoglucosyl side chain | [106] |
| Cereal β-Glucan | ||||
| Barley β-glucan | - | Hordeum vulgare L. | Linear (1,3;1,4) β-glucan | [107,108] |
| Oat β-glucan | - | Avena sativa L. | Linear (1,3;1,4) β-glucan | [109,110] |
| Wheat β-glucan | - | Triticum vulgare | Linear (1,3;1,4) β-glucan | [111,112] |
| β-Glucan | Cell Line | Analysis | Results | Reference |
|---|---|---|---|---|
| Fungal β-glucan | Human PBMC cell line | Cytokine inducing activity, TNF-α activity | Increased TNF-α activity. | [113] |
| Barley β-glucan | CHO-k1 cell line, and HTC cell line from Ratus novergicus | Micronucleus test in bi-nucleated cells to check mutagenicity | Chemoprotective and antimutagenic activity. | [114] |
| Polysaccharide-glucan from different sources | Human dendritic cells | Cell proliferation assay, FITC-dextran endocytosis assay, and ELISA | Ganoderma lucidum isolated polysaccharide significantly induced human PBMC proliferation and production of IL-10, and IL-12. | [115] |
| Yeast p-β-glucan (WGP, PGG) | BMDC, CD4+ T cells, MUC1-trasfected lymphoma RMA cells, Ovalbumin-transfected mammary adenocarcinoma cell line | T-cell differentiation assay, and Fluorescence-based neutrophil-mediated in vitro killing assay | Activated DCs and macrophages, promoted Th1 and cytotoxic T-lymphocyte priming and differentiation. | [87] |
| Mutated yeast β-glucan | Highly metastatic cell line of colon 26 carcinoma, colon 26-M3.1 and B16-BL6 melanoma cells, L5178Y-ML25 lymphoma cells, and mouse splenocytes | Antitumor and immunostimulating activities, Cytotoxicity analyses, and NK cell activity | Enhanced splenocyte proliferation activity in a dose-dependent manner, Increased NK cytotoxicity against Yac-1 tumor cells but did not affect the growth of colon 26-M3.1 cells. | [88] |
| Curdlan | Mo-DCs from healthy human volunteers and Leukemic cell line (THP-1) | ELISA, and RT-PCR | Th17-inducing activity. | [116] |
| Oat low molecular weight β-glucan (1,3;1,4)-β-d-glucan | Human Me45 cell line, Mouse macrophage cell line (P388/D1), Human HaCaT cell line, Human carcinoma A431 cell line | MTT assay, Cloning efficiency test, and Caspase-12 expression assay | Decreased cell viability of cancer cells while no toxicity to normal cells. | [117] |
| Fungal β-glucan | Sarcoma-180 cell line | Limulus amebocyte lysate coagulation test, Binding of Congo red, Toxicity test by brine shrimp assay, and MTT assay | Not toxic to brine shrimp assay. | [118] |
| Yeast β-glucan (WGP), Soluble β-glucan (NSG), Barley β-glucan | Lewis lung carcinoma cell line transfected with human MUCI (LL/2-MUCI), and Murine macrophage cell line J774 | Analysis of macrophage degradation, and Analysis of bioactivity | Enhanced tumor regression and antitumor activity. | [119] |
| Lentinan | Sarcoma 180 tumor cell line | SEC-LLS measurements, Viscometric analysis, and MTT assay | Maximum inhibition ratio against Sarcoma-180 tumor cell growth. | [120] |
| Phycarine, Lentinan | BALB/c mouse-derived mammary tumor cell line Ptas64, Murine tumor cell line Yac-1, Blood from healthy volunteers | Flow cytometry, Phagocytosis, and Cytokine evaluation | Increased NK cell-mediated killing of tumor cell. | [121] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Fungal β-glucan (OL-2) | Specific pathogen-free male ICR mice | Physiochemical properties, NMR, Congo-red assay, and Antitumor activity assay | Low or no antitumor activity against solid form of Sarcoma-180. However, significant antitumor activity against ascites form of Sarcoma-180 and MH-134. | [122] |
| Fungal β-glucan (OL-2-I, II, III) | Male ICR mice | GLC, GLC-MS, and Antitumor activity assay | Antitumor activity against Sarcoma-180 tumor. | [123] |
| Fungal β-glucan (H-3-B; S-H-3-B) | ICR-JCL female mice | Electron microscopy, NMR spectroscopy, and Antitumor activity assay | Antitumor activity against Sarcoma-180 tumor. | [65] |
| Fungal β-(1,3)-glucan | Male ICR albino mice, transfected with Sarcoma-180 tumor cells | VDH response, and Mitogenic test | Triggered proliferation of splenic lymphocytes, vascular dilation, and VDH response. | [117] |
| Commercial Sonifilan | Male ICR mice | NMR, MALDI-MS, VDH reaction, and Congo Red test | Antitumor activity against solid Sarcoma-180 tumor, strong vascular dilation, and hemorrhage reaction. Enhanced hematopoietic response to cyclophosphamide induced leukopenic mice. | [124] |
| Grifolan LE (GRN), Commercial Sonifilan | 5-weeks old male ICR mice | Antitumor activity assay, NMR, and ELISA | Antitumor activity against the solid form of Sarcoma-180 tumor. | [125] |
| Fungal β-glucan | BALB/c mice | Adoptive transfer test, Chemotactic factor assay, and Antitumor activity assay | Significant macrophage chemotactic factor activity. Increased IAP levels in serum, and inhibited growth of Meth-A tumor. | [126] |
| Yeast β-glucan (WGP), Soluble β-glucan (NSG), Barley β-glucan | Normal C57BL/6 mice deficient in either C3 or CR3 and their wild-type littermates | Analysis of elicited peritoneal granulocytes, peritoneal granulocyte-mediated, and splenic macrophage-mediated cytotoxicity | Barley and yeast β-glucans showed enhanced tumor regression and survival, and killed iC3b-opsonized tumor cells in bone marrow. | [119] |
| Wellmune + anti-tumor mAb therapy | 6-weeks old male C57/B16 mice, transfected with human MUC1 lymphoma, in combination with mAb | Measurement of cytokine secretion in murine peritoneal macrophages, and BMDCs | Increased production of cytokine IL-2 in DCs. | [127] |
| Lentinan | BALB/c and C3H He/N and C3H He/J | Determination of EROD activity, and CYP1As levels, and DNA-binding activities of NF-κB and AhR | Suppression of CYP1As, decrease in EROD and DNA-binding activity of AhR, and decreased production of TNF-α. | [128] |
| Lentinan (L-FV-IB) | 8-weeks old male BALB/c mice | Tumor weights, inhibition ratio, and enhancement ratio of body weight | Maximum inhibition ratio against Sarcoma-180 solid tumor. | [120] |
| Phycarine, Lentinan | 6–10 week old female BALB/c mice | Flow cytometry, Phagocytosis, and Cytokine evaluation | Significantly stimulated phagocytic activity. | [121] |
| Yeast p-β-glucan (WGP, PGG) | Wild type C57B1/6 mice, C57B1/6 C3, and CR3-deficient mice, CD4 and CD8 ovalbumin T-cell receptor transgenic OT-I and OT-II mice, EO771/ovalbumin tumor model, RAM-MUC1 tumor model | Phagocytosis, binding, and staining assay, and qRT-PCR | Potent antitumor immune response, and drastic down-regulation of immunosuppressive cells, leading to the delayed tumor progression. | [87] |
| Mutated yeast β-glucan | 6-week old pathogen free female BALB/C, C57BL/6, and CDF1 mice | Antitumor, immunostimulating, and NK cell activity | Dose-dependent inhibition of lung tumor metastasis via activation of macrophages and NK cells. | [88] |
| β-Glucan | Cell Line | Analysis | Results | Reference |
|---|---|---|---|---|
| Fungal β-glucan | 38–84 years old patients with advanced malignancies receiving chemotherapy | Changes in blood, and neutrophil counts, chemotherapy related symptoms (e.g., nausea and vomiting), and Hematological toxicity assay | Well tolerated in cancer patients receiving chemotherapy. | [129] |
| Yeast β-glucan | 28–56 years old women with breast carcinoma | A randomized, double-blind, placebo-controlled study. Measurement of HRQL | Significant increase in global health status. | [130] |
| β-Glucan | Cell Line | Analysis | Results | Reference |
|---|---|---|---|---|
| Yeast p-β-glucan (Cerevan) | Wistar rat thymocytes | HPGPC, Mitogenic, and co-mitogenic activity assay | Higher stimulation indices of immunomodulatory activity. | [90] |
| PGG-Glucan | Human monocytic cell lines U937, HL-60, THP-1, Murine monocytes J774.1, RAW264.7, P388D(I), Murine B cell line LB27.4, Primary human fibroblasts, Keratinocytes, Bronchial epithelial cells, Murine monocyte line BMC2.3, and T cell line DO11 | Whole blood chemiluminescence assay, Microbicidal assay, Measurement of cytokine secretion from whole blood, 3H-PGG-Glucan binding assay, Flow cytometry, and Electrophoretic mobility shift assay | Induced activation of NF-κB-Like nuclear transcription factor in purified human neutrophils, and enhanced neutrophil anti-microbial function. | [131] |
| PS-G | DC from PBMC, and CD14+ | Determination of cytokine levels, RT-PCR, Flow cytometry analysis, Western blot, Allogeneic MLR, EMS, and IKK activity assay | Increased activation and maturation of immature DC, suggesting a potential regulation of immune response. | [132] |
| Yeast p-β-glucan (synthetic glucan) | Porcine alveolar macrophages and bone hematopoietic cell-derived dendritic cells | MTT assay, ELISA, RACE PCR, and Phagocytic activity | Enhanced cell activity and phagocytosis, and complex collaborating interaction between dectin-1 and TLRs. | [133] |
| Barley β-glucan, Oat β-glucan, Fungal β-glucan | Human monocyte leukemia cell line | Size exclusion chromatography, Cytotoxicity assay, NO assay, H2O2 assay, Phagocytic activity, and qRT-PCR | Up-regulated inflammation related gene expression, and No production of NO, and H2O2. | [134] |
| Algal β-glucan | Murine splenic cells from BALB/c mice | NMR, Immunomodulatory activity assay, Immunofluorescence staining assay, and FACSCanto II flow cytometry | Increased activation of CD19+ B lymphocytes. | [135] |
| Polysaccharide glucan fractions | Spleen cells from female C3H/He mice, and Bone marrow cells from C57BL/6 mice | Mitogenic activity assay, and CSF-inducing activity assay | T-4-N and T-5-N fraction showed mitogenic and CSF-inducing activities. | [49] |
| Yeast β-glucan (WGP) | Mouse intestinal tumor cell line Colon26 produced in BALB/c mice | ELISA, and Tumor-protective effect assay | Stimulation of cytokines such as IL-2, IFN-γ, and TNF-α. | [136] |
| Bacterial β-glucan | Cancer cell lines, Human monocyte cell line, HPV-18-positive cervical cancer cell line, HPV-16-positive cervical cancer cell lines, such as CASki and C3, Hepatoma cancer cell line HepG2 | RT-PCR, IFN-γ assay, NO, and cell viability assay | Synthesis of NO in the monocyte cell lines, enhanced cytotoxic, and antitumor activity. | [95] |
| Phycarine | Lewis lung carcinoma, and YAC-1 cell lines | Cytotoxicity assay, and Phagocytosis activity assay | Stimulation of both humoral and cellular branch of immune reactions could be used to cure gastrointestinal diseases. | [103] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Polysaccharide glucan fractions | 8–10 weeks old female C3H/He, C57BL/6, and ICR mice | Mitogenic activity assay, and CSF-inducing activity assay | T-4-N and T-5-N fractions showed mitogenic and CSF-inducing activities. | [49] |
| Fungal SSG glucan | CDF1 mice | Phagocytosis, H2O2, and CS activity assay | Enhanced colony stimulating activity, and activation of Peyer’s patch cells. | [137] |
| Yeast p-β-glucan | Male A/J, and Melanoma B16 model C57BL/6J mice | Histopathological analysis, and Bacterial susceptibility study | Significant reduction in the growth of a syngeneic anaplastic mammary carcinoma and melanoma B16. Prolonged survival of mice with subcutaneous tumor implants, decreased renal necrosis in S. aureus challenged mice and anti-staphylococcal activity. | [138] |
| Yeast β-glucan | Outbred male mice (CD-1, ICR), Inbred male rats (Fischer-344), Healthy mature and laboratory-conditioned cynomolgus male and female monkeys (Macaca fascicularis), seronegative to VEE virus-neutralizing antibody | Measurement of nonspecific potentiation, and specific enhancement of resistance | Significantly enhanced survival of mice challenged with either VEE virus or Rift Valley fever virus. Significant resistance of Glucan + VEE vaccine to homologous virus challenges. | [139] |
| Fungal Schizophyllan | 3-weeks old specific-pathogen free male ICR/CRJ (CD-1) mice | Determination of protective effects of schizophyllan against primary Sendai virus infection in mice, and virus production in the infected lung and serum | Inhibited spread of virus in the lungs. Augmented protective immune responses induced by low doses of a live Sendai virus vaccine. | [140] |
| PGG + Cefazolin | Low inoculum albino Hartley guinea pigs | Bacterial growth, Prophylaxis studies, and MIC assay | PGG + Cefazolin synergistically prevented staphylococcal wound infection. | [141] |
| Oat β-glucan | 6-weeks old female C57BL/6 mice | ELISA, and ELISPOT assay | Higher levels of total serum immunoglobulins and antigens against Eimeria vermiformis infection. | [142] |
| Yeast β-glucan (WGP) | 6-weeks old female BALB/c mice | Anthrax-protective prophylactic effect and tumor-protective effect assay | Significant effect as a prophylactic treatment to reduce the mortality of anthrax infection. | [136] |
| SSG-glucan | 6-week old, female inbred, specific pathogen-free NIH/OlaHsd mice | Mouse survival rate and the number of bacteria in blood samples | A significant dose-dependent effect of SSG against Streptococcus pneumoniae type 4 and 6B. | [143] |
| Fungal β-glucan | NC/Nga mice | Cell cytotoxicity, Sarcoma-180 tumor size, Blood IgE levels, Scratching index, and Human NK cell activity | Prolonged survival, reduction in tumor size, blood IgE levels, scratching index of NC/Nga mice, and enhanced cell cytotoxicity of human NK cells. | [144] |
| Yeast β-glucan | Male and female Wistar albino rats | Biochemical analysis, Apoptosis, Cell death, and Histopathological analysis | Reduced tissue damage. Inhibited the decrease in the stimulation index caused by methotrexate. | [145] |
| Bacterial β-glucan | 4-weeks old male BALB/c and ICR mice | IFN-γ assay of PBMCs, and Antitumor activity assay | Induced IFN-γ and cytokines in spleens and thymus of mice. Enhanced cytotoxic and antitumor activity. | [95] |
| β-glucan from different sources | 8-week old female BALB/c mice | Changes in blood glucose and blood cholesterol levels, and Phagocytosis of HEMA particles | Significant stimulation of IL-2 production and phagocytosis of peripheral blood leukocytes. Lowered blood sugar and cholesterol levels. | [6] |
| Phycarine | 6–10 weeks old female BALB/c and C57B1/6J mice, and male and female pups | Apoptosis, Absorption, and Phagocytosis activity assay | Significant stimulation of phagocytosis, Strong influence on experimentally induced leucopenia, could be used to cure gastrointestinal diseases. | [103] |
| β-glucan from different sources | 3-, and 8-weeks old BALB/c female mice | Phagocytosis, Cytokine assay, Tumor inhibition assay, and RT-PCR | Significant stimulation of phagocyte activity. Increase synthesis and release of ILs, and TNF-α. Inhibited growth of tumor cells in breast cancer cells. | [146] |
| Yeast insoluble-β-glucan | 8-weeks old female BALB/c mice | Phagocytosis, Cold stress response, Changes in serum corticosterone and cytokine production levels | Inhibition of stress related suppression, normal phagocytosis activity. Inhibition of corticosterone, above normal levels of IL-6 and IL-12 secretion. | [147] |
| Lentinan | Male BN/RijHsd rats | Hematopoiesis, Flow cytometry, and Serum cytokine analysis | Significant increase in weight gains, monocytes, blood cells, circulatory cytotoxic T-cells and a reduction in anti-inflammatory cytokines IL-4, IL-6, and IL-10. Increased in cage-side health of acute myeloid leukemia. | [148] |
| Polysaccharide β-glucan | 6–8 weeks old male Swiss albino mice | Macrophage activity assay, Flow cytometry, In vitro NK cell assay, Serum biochemistry and Histological analysis | Significant increase in IL-1 and NO production and increased phagocytic potential. Increased activation of NK cells and proliferation of splenocytes. | [149] |
| Paramylon | 5-week old NC/Nga mice | Histopathological, and Macroscopic analysis | Significantly inhibited the development of atopic dermatitis-like skin lesions with no adverse effect on weight loss. | [150] |
| β-glucan | 5–6 week old Sprague-Dawley male and female rats | Subacute toxicological study, Clinical examination, Pathological analysis, and Flow cytometry | Significant increase in red blood cell, white blood cell, hemoglobin, and thrombocytes. No adverse effect on general condition, growth, behavior, and feed consumption. | [151] |
| Commercial β-1,3;1,6-glucan | Private owned dogs with signs of atopic dermatitis, the dog breeds include: West highland white terriers, Staffordshire bull terriers, German shepherds, Heidewachtels small Munsterlander pointers, Crossbreeds and others | Signs of itching, How many times dog scratches, and Changes in skin color, and thickness | Canine atopic dermatitis diminished. | [152] |
| β-glucan | Adult male Sprague Dawley rats | Physical exercise, Determination of exhaustive time, and Immunohistochemical analysis of oncogenes (c-Jun and c-Fos) | An alleviating effect on the exercise-induced stress through the suppression of oncogenes expression in the brains of exhausted rats. | [153] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| PGG-glucan | More than 18 years old patients who underwent a major abdominal or non-cardiac thoracic surgery | Postoperative infection response | A dose-dependent protective response against the postoperative infection. | [154] |
| β-1,3-polyglucose (β-glucan) | Paracoccidiodes brasiliensis infected patients | Erythrocyte sedimentation rate, and Phytohemagglutinin skin test | Increase in number of CD4+ T lymphocytes, higher serum level of TNF-α. Stronger and more favorable response to therapy. | [155] |
| Commercial Curdlan, Paramylon, Laminarin, Scleroglucan, Pustulan | 28–56 years old, healthy as well as volunteer patients allergic to house dust mites | Histamine release test from blood leukocytes | Enhanced IgE-mediated histamine release. | [156] |
| Yeast β-glucan | 6–12 years old children with mild to moderate persistent asthma | Calculation of serum IL-10, and Asthmatic symptoms | Significant increase in serum IL-10 levels and a significant reduction in asthma. | [157] |
| Oat β-glucan | Healthy, normal female and male volunteers, with mean age: 22.6 ± 0.7 years | Changes in blood plasma glucose, insulin, ghrelin, CCK, PYY, and GLP-1 levels. Subjective appetite measurements, and Biochemical analysis | Postprandial increase in satiety, plasma glucose, insulin, CCK, GLA-1, and PYY and a greater decrease in postprandial ghrelin. | [158] |
| WGP-glucan | Male and female volunteers | Flow cytometry, and Separate multiplex assay | A significantly enhanced CD14+, and CD14+/CD16+. LPS-stimulated production of IFN-γ and IL-2, IL-4, and IL-5. | [159] |
| Fungal β-glucan | Clinical pulmonary disease and trauma, suffering patients | Serum lipid profile analysis, Serum hs-CRP, cytokine, and NK cell activity assay | Increased NK cell activities, and serum pre-albumin, and decreased hs-CRP. | [160] |
| Yeast β-glucan (Wellmune, WGP) | 18–53 years old, male and female marathon runners | A randomized, double-blind, placebo-controlled trial. Profile of mood state assessment | Decreased URTI symptoms, fatigue and anger. An increase in overall health and vigor. | [161] |
| Yeast β-glucan (Wellmune, WGP) | 18–65 years old, moderate to high-stressed male and female adults | A randomized, double-blind, placebo-controlled trial. Respiratory tract infection analysis | Decreased URTI symptoms, fatigue and tension. Improved overall health and vigor. | [162] |
| Yeast β-glucan (Wellmune, WGP) | 26–50 years old healthy women with moderate levels of psychological stress | A randomized, double-blind, placebo-controlled trial. Changes in mental/physical energy levels and mood states. | Decreased URTI symptoms, and increased mental/physical energy levels. | [163] |
| Yeast β-Glucan (Glucan #300) | 8–12 years old, male and female children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Changes in levels of lysozyme, albumin, and CRP in saliva | Increased changes in production of lysozyme and CRP. Improvement in the general condition and stimulated mucosal immunity. | [164] |
| Yeast β-glucan (Glucan #300) | 8–12 years old children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Measurement of levels of IgA, IgG, and IgM | A significant increase in production of salivary immunoglobulins, and improvement in the mucosal immunity. | [165] |
| Yeast β-glucan (Glucan #300) | 8–12 years old children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Physical endurance test and estimation of eNO levels | A significant improvement in physical endurance, eNO levels, and general conditions. | [166] |
| Yeast β-glucan (Glucan #300) | 8–12 years old children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Measurement of levels of lysozyme, albumin, CRP, and calprotectin in saliva | A significant increase in production of salivary CRP, lysozyme, and calprotectin. | [167] |
| Yeast β-glucan (Glucan #300) | 7–14 years old children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Measurement of levels of cortisol, salivary IgE, and cotinine | Decreased salivary cortisol and cotinine levels. An increase in physical endurance and improvement of affected children. | [168] |
| Yeast β-glucan (Glucan #300) | 8.2–12.4 years old children with chronic respiratory problems | A randomized, double-blind, placebo-controlled trial. Measurement of levels of eNO, salivary IgA, and physical activity (6MWT test) | A significant decrease in eNO levels. Physical endurance and stabilization of the salivary IgA levels. | [169] |
| Imunoglukan P4H (a syrup containing Pleuran) | 3–7 years old children with RRTIs | Open-label trial. Monitoring the occurrence of RRTIs | A 50% reduction in frequency of RRTIs. | [170] |
| Imunoglukan P4H (a syrup containing Pleuran) | 3–8 years old children with RRTIs | A randomized, double-blind, placebo-controlled trial. Blood sample analysis for immune parameters | Significant reduction in frequency of RRTIs, number of flu-like diseases, respiratory tract infections, and an increase in number of healthy children. | [171] |
| Imunoglukan P4H (a syrup containing Pleuran) | 2–5, and 6–10 years old children with RRTIs | A randomized, double-blind, placebo-controlled trial. Measurement of total IgE, specific IgE levels, and BECs | Significant reduction of peripheral blood eosinophilia as well as stabilized levels of total IgE in serum. | [172] |
| The effect of Imunoglukan P4H (a cream containing Pleuran) | Male and female patients with atopic dermatitis, with mean age of 20.4 years | Objective and subjective symptoms of AD, including visual analysis, EASI | Significant decline in the number of days with AD exacerbation and its severity. Decline of pruritus by visual analog scale. Significant decline of EASI on the site of β-glucan application. | [173] |
| Imunoglukan P4H (a syrup containing Pleuran) | 3 years old children with RRTIs | A multi-center, open-label trail. Monitoring the occurrence of RRTIs | A significant reduction in RRTIs frequency, and the occurrence of respiratory diseases, such as common cold, laryngitis, tonsillpharyngitis, pneumonia, and bronchitis. | [174] |
| Imunoglukan P4H (a syrup containing Pleuran) | 3.7 years old children with RRTIs | Open-label trail. Monitoring the occurrence of RRTIs | A significant reduction in RRTIs frequency, and the occurrence of respiratory diseases, such as laryngitis, common cold, and bronchitis. | [175] |
| β-Glucan | Cell line | Analysis | Results | Reference |
|---|---|---|---|---|
| PGG-glucan | Human BMMC, and isolated bone marrow CD34+ cells | BMMC myeloid colony formation assay, Human hematopoietic activity, and ELISA | Increased BMMC myeloid colony formation, and enhanced human hematopoietic activity. | [176] |
| Polycalcium [Polycan and calcium lactate-gluconate (1:9)] | Human hOBs, and murine osteoclast progenitor (RAW264.7) cells | Cell proliferation and alkaline phosphatase activities of osteoblasts and osteoclast differentiation | Stimulation of osteoblast proliferation and prevented RANKL-induced osteoclast differentiation. Accelerated bone formation and inhibited bone resorption activity. | [177] |
| Fungal β-glucan | Normal diploid human fetal dermal fibroblast cell line (FW20-2), and primary human dermal fibroblasts | Cell proliferation assay, RP-HPLC, Fibroblast-populated collagen lattice, and wounding | Reduction in fibroblast proliferation and migration were significantly and dose-dependently inhibited. | [178] |
| Chitosan/β-1,3-glucan/hydroxyapatite complex (Chit/glu/HA) | Human fetal osteoblast cell line (hFOB 1.19) | Biocompatibility of scaffolds, cytotoxicity, and osteoblast proliferation rate, Porosity using computed microtomography analysis and mechanical properties by compression test | Improved flexibility and porosity, significant higher water uptake capability, favorable osteoblast survival, proliferation, and spreading, but poor mechanical properties. | [179] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| β-glucan | 2–3 months old CD-1 male mice | Chromosomal aberrations and mitotic activity | Reduced total number of cells with structural chromosomal aberrations in bone marrow and spermatogonial cells. Markedly restored mitotic activity of bone marrow cells, suppressed by anti-neoplastic drugs. | [180] |
| Polycalcium [Polycan and calcium lactate-gluconate (1:9)] | 6-weeks old, Sprague-Dawley specific pathogen-free female ovariectomy-induced osteoporotic rats | Changes in body and bone weight, serum osteocalcium and bone-specific alkaline phosphatase levels, Urine Dpd/creatinine ratio, and Histological analysis | Markedly decreased OVX-induced osteoporotic changes. Preserved bone mass and strength. | [181] |
| Polycalcium [Polycan and calcium lactate-gluconate (1:9)] | 6-weeks old Sprague-Dawley specific pathogen-free male rats | Changes in body weight, knee thinness, cartilage glycosaminoglycan content, and Histopathological assay | Inhibited osteoarthritis related changes and induction of chondrocyte proliferation. | [182] |
| Polycal [Polycan and calcium-gluconate (2:98)] | 6-weeks old male SD (Crl:CD1) rats | Changes in body weight, alveolar bone loss index, total number of buccal gingival aerobic bacterial cells, IL-1, TNF-α levels, and myeloperoxidase activity | Bacterial proliferation, periodontitis, and alveolar bone loss induced by ligature placement were significantly inhibited. | [183] |
| CHAP + β-glucan composite material | 6-months old New Zealand male white rabbits | Radiological imaging and Histological analysis. Peripheral quantitative computed tomography, Densitometry and SEM analysis | No sign of graft rejection, stimulating effect of biomaterial on bone formation and mineralization. Enabled regeneration of bone tissue. | [184] |
| Polycan | An oestrogen-deficient ovariectomy model and a hypocalcemic and hypoparathyroid thyroparathyroidectomy model | Changes in bone mineral density in the femur, tibia, and lumber (L6) vertebrate using dual-energy X-ray absorptiometry, and changes in Ca bioavailability | Marked increase in the BMD of femur, tibia, and L6. Enhanced absorption and bioavailability of Ca and improved Ca balance. | [76] |
| Polycan | 6-weeks old virgin Sprague-Dawley pathogen free female rats as an oestrogen-deficient ovariectomy model | Changes in body weight, bone mineral content, density, failure load, Histological profile, and Histomorphometric indices | Inhibited OVX-induced alterations in bone resorption. Increased serum expression levels of BLAP and all histomorphometrical indices for bone formation. | [77] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Polycalcium (Polycan + calcium lactate-gluconate) | 40–60 years old healthy women | Anti-osteoporotic effect, Measurement of changes in DPYR, OSC, BALP, CTx, and P levels | Improved bone metabolism and well tolerated polycalcium effect. | [185] |
| Polycan | 40–70 years old, healthy premenopausal women | Anti-osteoporotic effect, Measurement of changes in OCS, BALP, Ca, and P levels | Increased changes in OSC, and BALP, Ca, P, CTx, NTx, and DPYR. Increase in CTx was modestly inhibited. | [186] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Lentinan | Female BALB/c mice | Spectrophotometric analysis of the total CYP contents, Western blot analysis, ECOD, EROD, and EMSA activities | Suppression of constitutive and 3-methylcholanthrene-induced CYP expression and EROD activity in liver. | [187] |
| Chitin-glucan | 9-weeks old, male C57BL6/J mice | Oral glucose tolerance test, Microbial analysis of the cecal contents, ELISA, and Histochemical analysis | Decreased mouse gut microbiota, body weight gains, fat mass development, glucose intolerance, hepatic triglyceride accumulation and hypercholesterolemia. | [188] |
| Polycan | 7-weeks old male hamsters | Changes in body weight, food consumption, liver weight, Serum biochemistry, Histopathological, and Histomorphometric analysis | No significant change in body weight and food consumption, serum levels of AST, ALT, triglyceride, LDL- and total-cholesterol levels. Dose-dependent reduction of atherosclerosis with relatively good protective effects on liver damage. | [75] |
| Yeast β-glucan + Folium mori extract (BG-FM) | STZ-induced diabetic rats | Changes in blood glucose levels, body weight, liver, and kidney weight, and Serum BUN, AST, ALT levels | Reduced hyperglycemic changes in the F. mori extract. Dose-dependent increase in anti-diabetic and hypoglycemic effect. | [189] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Oat β-glucan | 49–57 years old NIDDM men and women, with a BMI range of 22.6–38.9 kg/m2 | Plasma glucose and glycemic response | Increased plasma glucose, postprandial insulin, and 50% decrease in glycemic response. | [190] |
| Barley β-glucan | 26–30 years old, healthy men with mildly higher fasting total cholesterol concentration, with a BMI range of 22–25 or 27–29 kg/m2 | Insulin, glucose, cholecystokinin, and lipid response | Increased plasma glucose and insulin concentrations, stimulation of reverse cholesterol transport contributing to the cholesterol lowering ability. | [191] |
| Barley β-glucan | 20–27 years old, healthy, non-diabetic men and women | Sensory properties, proximate composition, and glycemic indices | Dose-dependent decrease in glycemic content, and decreased postprandial glycemic index. | [192] |
| Oat β-glucan | 59–63 years old, type 2 diabetic men and women, with a BMI range of 27–31 kg/m2 | Changes in blood glucose, total-, HDL-, and LDL-cholesterol, and triglyceride levels | Reduced blood glucose levels, glycemic indices, and postprandial glycemia. | [193] |
| Oat β-glucan | 61–73 years old, type 2 diabetic men and women, with a BMI range of 25.4–32.4 kg/m2 | Glucose tolerance test, and Finger-prick capillary blood analysis | Decreased glycemic, and postprandial glycemic response. | [194] |
| Oat β-glucan, Barley β-glucan | 18–70 years old, healthy men and women with mildly elevated serum cholesterol concentration and a BMI range of 20–30 kg/m2 | Changes in plasma glucose, serum total-, HDL-, and LDL-cholesterol, triacylglycerol, apolipoproteins A1, and postprandial changes in serum | Oat β-glucan showed reduced total-cholesterol, postprandial glucose, and insulin concentrations as well as improved lipid and glucose metabolism. | [195] |
| Oat β-glucan | Healthy volunteer men and women | Changes in insulin and glycemic response index, and Glucose tolerance test | Reduced insulin and glycemic index. | [196] |
| Oat β-glucan | Healthy volunteer men and women | Blood insulin and glucose response | Significant reduction in insulin and glucose responses in healthy people. | [197] |
| Barley β-glucan | 26–50 years old, healthy men and women, with a BMI range of <30 kg/m2 | Changes in blood glucose contents, and GR, and GI response | Significantly reduced postprandial blood glucose, and glycemic index. | [198] |
| Oat β-glucan | 30–75 years old, diabetic men and women, with a BMI range of 20–35 kg/m2 | Changes in lipid profile, apo B, TAG, HbA1c, and fasting glucose concentrations | A single daily ingestion of 3.5 g oat β-glucan showed no significant changes in lipid profile and HbA1c in type 2 diabetic subjects whereas, TAG significantly decreased. | [199] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Yeast-WGP | 8-week old hypercholesterolemic BALB/c mice | Phagocytosis, and Biochemical analysis | A dose-dependent decrease in plasma cholesterol and triglyceride levels. | [200] |
| Yeast β-glucan | Sprague-Dawley rats | Serum total cholesterol, triglyceride, and malondialdehyde analysis | Significantly reduced and maintained cholesterol levels in blood plasma and liver. Triglyceride and MDA levels significantly reduced. | [201] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Oat β-glucan | 30–65 years old men and women with LDL-cholesterol levels of >3.37 mmol/L | Changes in total-, LDL-, and HDL-cholesterol levels | Significantly decreased total-, and LDL-cholesterol concentrations. | [202] |
| Barley β-glucan | 21–42 years old healthy men with total-cholesterol levels (between 4.1 and 6.2 mmol/L), triacylglycerol levels (<2.26 mmol/L), and a BMI range of 22–25 or 27–29 kg/m2 | Changes in plasma glucose, insulin, triacylglycerol, cholesterol concentrations, and Radioimmunoassay | Increased plasma glucose, insulin, triacylglycerol and cholecystokinin levels. Stimulation of reverse cholesterol transport mechanism. | [191] |
| Yeast β-glucan | 20–60 years old hypercholesterolemic obese male patients with serum total cholesterol concentrations of >6.21 mmol/L | Changes in plasma total-, LDL-, and HDL- cholesterol and triacylglycerol levels | Reduced plasma total-, HDL- and LDL-cholesterol concentrations. Triacylglycerol concentrations did not change significantly. | [203] |
| Oat β-glucan | 30–70 years old mild-to-moderate hyperlipidemic healthy men and women, with a BMI range of 20–32 kg/m2 | Changes in total-, LDL-, and HDL-cholesterol, triacylglycerol, glucose, insulin, postprandial triacylglycerol, glucose, and insulin concentrations | No significant difference in total-, or LDL-cholesterol at a low dosage of β-glucan (3 g/d). | [204] |
| Oat β-glucan | 33–82 years old hyperlipidemic men and women | Changes in blood lipids, apolipoproteins, cardiovascular risk factor, blood pressure, and gastrointestinal symptoms | Reduced total-, total- to HDL-cholesterol ratio, LDL- to HDL-cholesterol ratio. Apolipoprotein (B:A-I) reduction in CVD risk, and small reduction in blood pressure. | [205] |
| Barley β-glucan | 18–65 years old mildly hyperlipidemic men, with a BMI range of 22–32 kg/m2 | Changes in total-, LDL-, and HDL-cholesterol, triacylglycerol, fasting plasma glucose, and postprandial plasma glucose levels | No significant change in total-, LDL- or HDL-cholesterol, triacylglycerol, fasting glucose, or postprandial glucose. | [206] |
| Oat β-glucan | 18–65 years old mildly hypercholesterolemic men and women, with a BMI range of >30 kg/m2 | Changes in total-, HDL-, LDL- cholesterol and triacylglycerol levels. High performance size-exclusion chromatography | Decreased LDL-, and ratio of total- to HDL-cholesterol concentrations. No significant change in HDL-cholesterol and triacylglycerol levels. | [207] |
| Barley β-glucan | 28–62 years old moderately hypercholesterolemic men | Changes in total-, HDL-, and LDL-cholesterol, and triacylglycerol concentrations, and NMR | Significantly lowered triacylglycerols, total-, and LDL-cholesterol, but higher HDL-cholesterol concentrations. | [25] |
| Barley β-glucan | 38–53 years old mildly hypercholesterolemic men and women with a BMI range of 25–37 kg/m2 | Changes in cholesterol, and triacylglycerol levels, and NMR | Lowered total-, and HDL-cholesterol concentrations. Triacylglycerol concentration did not differ. | [26] |
| Oat β-glucan | 30–65 years old men and women with elevated blood pressure or stage-1 hypertension | Changes in plasma glucose, insulin levels, and blood pressure | Lowered systolic and diastolic blood pressure. | [208] |
| Oat β-glucan | More than 40 years old men and women with elevated blood pressure (between 130 and 179 mm Hg), controlled with anti-hypertensive medications | Clinical laboratory measurements of plasma glucose and insulin levels, Oxidative stress, and Blood pressure | Lowered insulin levels, and systolic and diastolic blood pressures. Biomarkers of oxidative stress did not show significant differences. | [209] |
| Oat β-glucan | 22–65 years old hypercholesterolemic men and women at a risk for CVD | Changes in total-, HDL-, and LDL-cholesterol, triglycerides, glucose, insulin, homocysteine, and CRP levels, and blood pressure | Significant reduction in total-, LDL-cholesterol in subjects with elevated cholesterol levels, and a significant reduction of lipids. | [109] |
| Barley β-glucan | 30–60 years old hypercholesterolemic Japanese men with a BMI range of >22 kg/m2 | CT-scan, Blood analysis for serum TG, TC, LDL-, and HDL-cholesterol levels | Significant reduction in serum concentration of LDL-C, TC, and visceral fat area. | [210] |
| Oat β-glucan | 50–75 years old patients (both men and women) with T2D, LDL-cholesterol concentration (>3.37 mmol/L), and a BMI range of 23–35 kg/m2 | Changes in BMI, waist circumference, LDL-, Total-, HDL-, and non-HDL-cholesterol concentrations, HbA, and systolic BP | Significant reduction in LDL-, total-cholesterol concentrations, FPI, and Homa-IR. Improvement in lipid profile and insulin resistance in patients with T2D. | [27] |
| β-Glucan | Cell line | Analysis | Results | Reference |
|---|---|---|---|---|
| Yeast β-glucan, Fungal β-glucan + chitin complex from Aspergillus niger | Chinese hamster lung fibroblasts V79 | HPLC, H2O2 assay, and Comet assay | Increased comet activity, and protective effect against oxidative DNA damage. | [211] |
| Yeats cell wall mannan, and mannan conjugates | Unicellular flagellate Euglena gracilis cells exposed to the genotoxic agents ofloxacin and acridine orange | HPLC, FT-IR spectroscopy, Antioxidant assay (ABTS-radical scavenging activity), and Euglena gracilis mutagenicity assay | Protective antigenotoxic activity, and inhibited AO-induced chloroplast DNA damage. | [212] |
| β-glucan | Chinese hamster ovary cell line, and the hepatoma cell lines from Ratus vovergicus | Micronucleus assay | Increased chemoprotective, and anti-mutagenic activity. | [213] |
| Fungal β-glucan | Human peripheral lymphocytes | Binding, Comet assay, and H2O2 assay | Dose-dependent protective effect against damage induced by H2O2 and Trp-P-2. | [214] |
| Barley β-glucan | Chinese hamster ovary cell line, and the hepatoma cell line | Chromosomal aberration assay, and Anti-clastogenic activity | Protective effect in the presence of a DNA polymerase-β inhibitor. | [215] |
| Fungal β-glucan | Human hepatoma cell line | FT-IR, NMR, Comet assay, and Cytokinesis-block micronucleus assay | Does not exert a genotoxic or mutagenic effect, but protected effect against DNA damage caused by bezo[a]pyrene (B[a]P). | [216] |
| Chrysolaminarin | - | FT-IT, NMR, H2O2, and DPPH-radical scavenging activity | Significant hydroxyl radical scavenging activity. | [101] |
| Fungal β-glucan (SBG) | Human umbilical vein endothelial cells, highly metastatic B16-F10 and B16-BL6 cells | Dorsal air sac assay, Matrigel plug assay, and Methylation analysis | Suppression of growth and number of metastatic tumor foci in lung, and improved anti-angiogenic and anti-metastatic effect. | [74] |
| β-Glucan | Organism | Analysis | Results | Reference |
|---|---|---|---|---|
| Fungal β-glucan (SBG) | Neoplasm, Female ICR, and C57BL/6J mice | Dorsal air sac assay, Matrigel plug assay, and Methylation analysis | Suppression of growth and number of metastatic tumor foci in lung. Anti-angiogenic and anti-metastatic effect. | [74] |
| β-glucan | Wistar albino rats | SOD, MPO, MDA, LPO, and GSH activity analyses | A significant reduction in AST, ALT, LDH, GGT, MPO, LPO, and MDA levels and greater levels of GSH and SOD. | [217] |
| Yeast β-glucan | Male Wistar albino young, healthy rats | Antioxidant activities (SOD, GSH-Px, CAT, MDA) | Significantly reversed elevation of MDA levels and reduction in SOD activities. Slightly enhanced activity of CAT and prevented depletion of GSH-Px activity caused by EMR, and higher antioxidant activities. | [218] |
| Fungal β-glucan | 6–8 weeks old swiss albino mice | Antioxidant activities (H2O2, Ferric reducing power assay), lipid peroxidation assay, Biochemical and Hematological analyses | Increased post-irradiation survival of mouse, significant reduction in number of aberrant cells. | [219] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, K.M.I.; Choi, J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. https://doi.org/10.3390/ijms18091906
Bashir KMI, Choi J-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. International Journal of Molecular Sciences. 2017; 18(9):1906. https://doi.org/10.3390/ijms18091906
Chicago/Turabian StyleBashir, Khawaja Muhammad Imran, and Jae-Suk Choi. 2017. "Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future" International Journal of Molecular Sciences 18, no. 9: 1906. https://doi.org/10.3390/ijms18091906
APA StyleBashir, K. M. I., & Choi, J.-S. (2017). Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. International Journal of Molecular Sciences, 18(9), 1906. https://doi.org/10.3390/ijms18091906