Transgenic Cotton Plants Expressing the HaHR3 Gene Conferred Enhanced Resistance to Helicoverpa armigera and Improved Cotton Yield
Abstract
:1. Introduction
2. Results
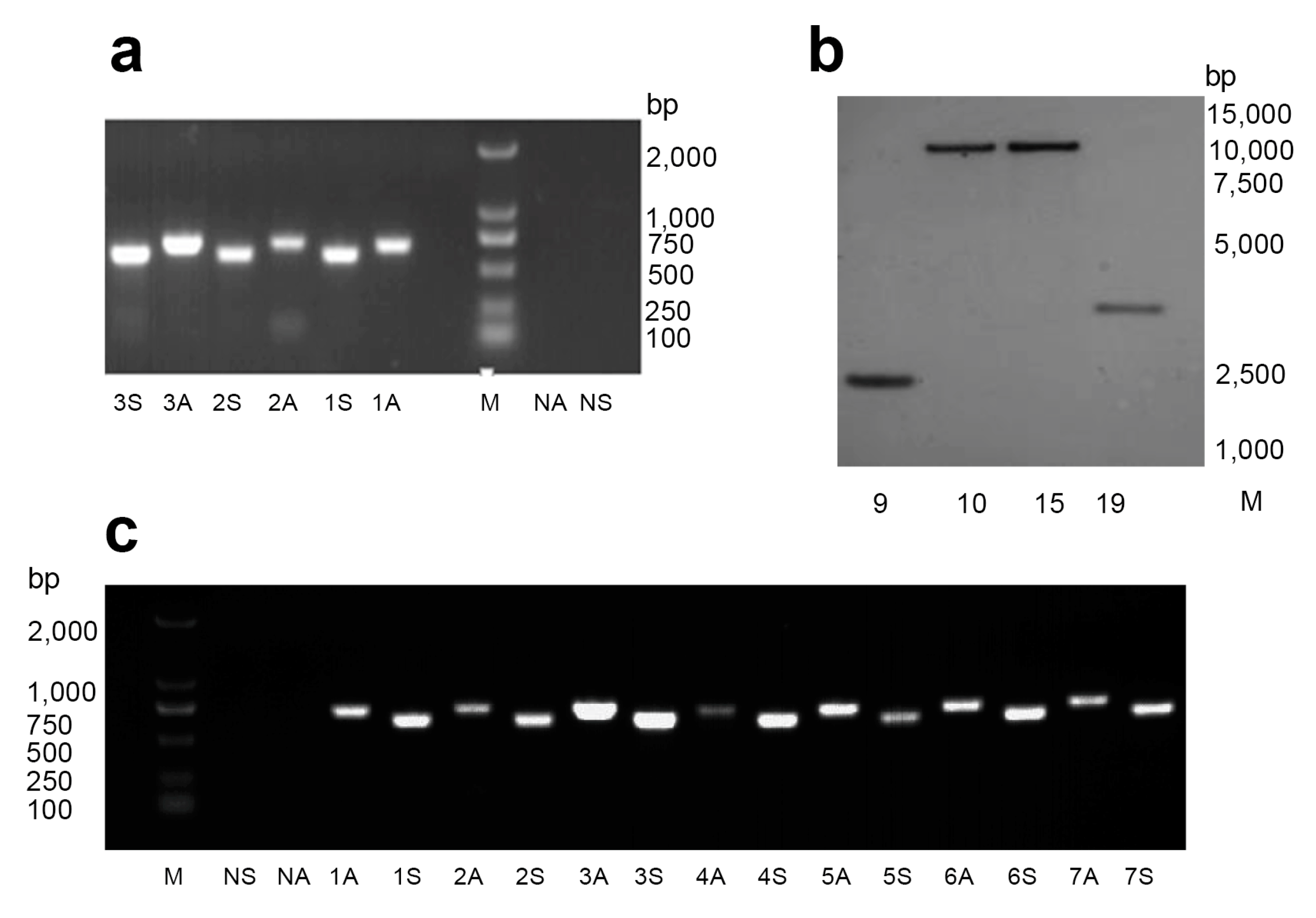
2.1. Generation of Transgenic Cotton Plants Expressing dsHaHR3
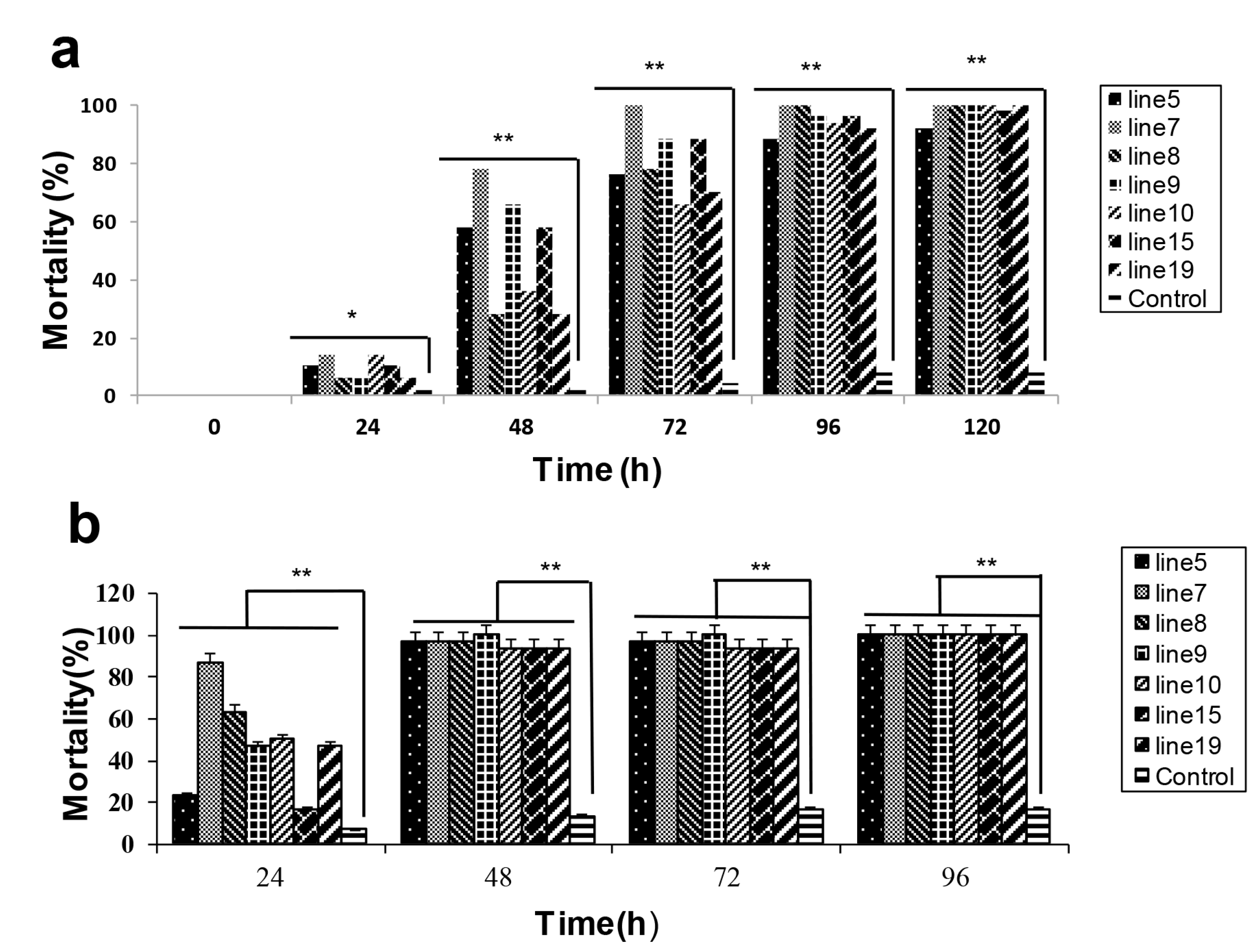
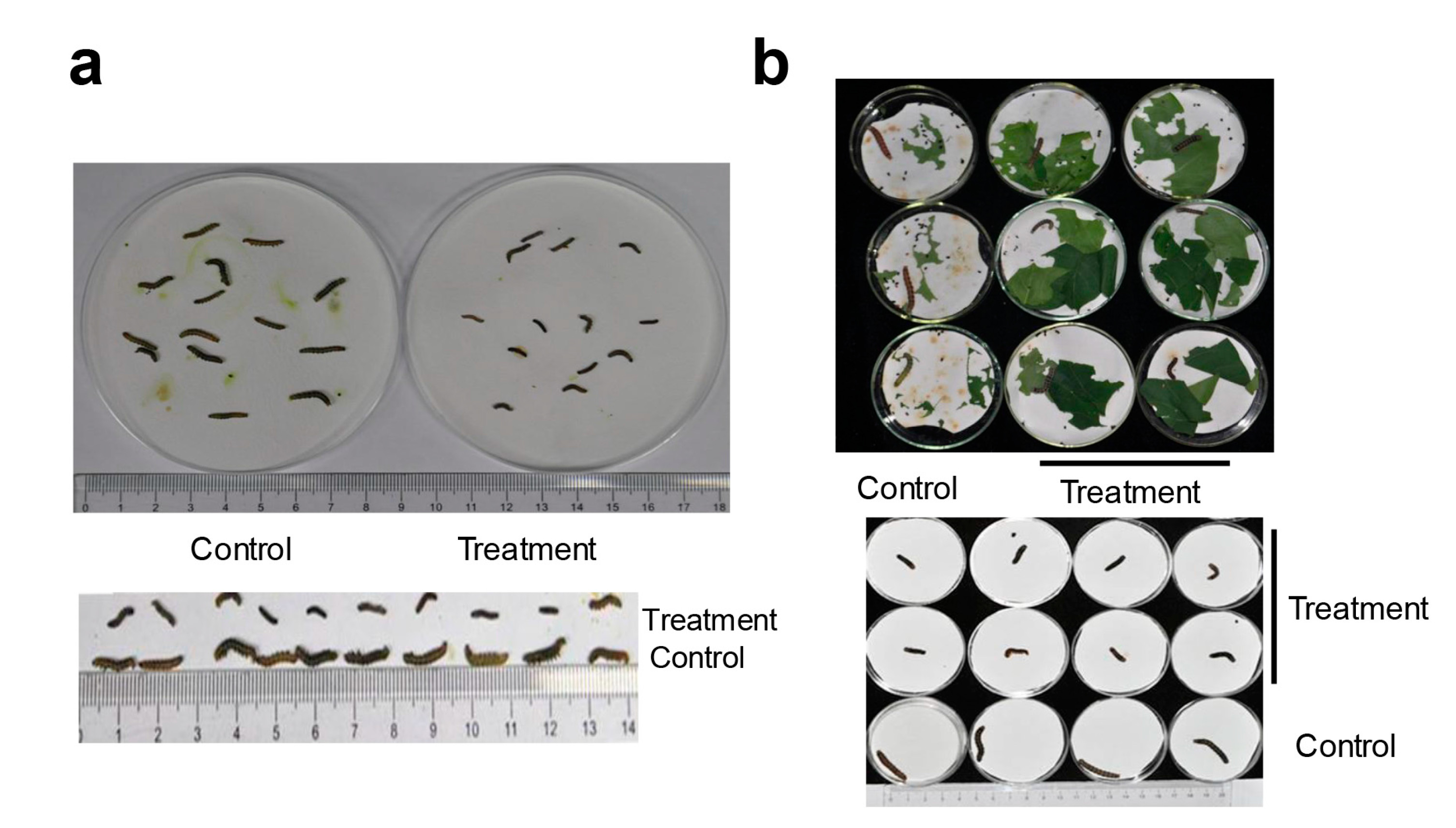
2.2. HaHR3 Transgenic Cotton Showed Enhanced Protection from Bollworms
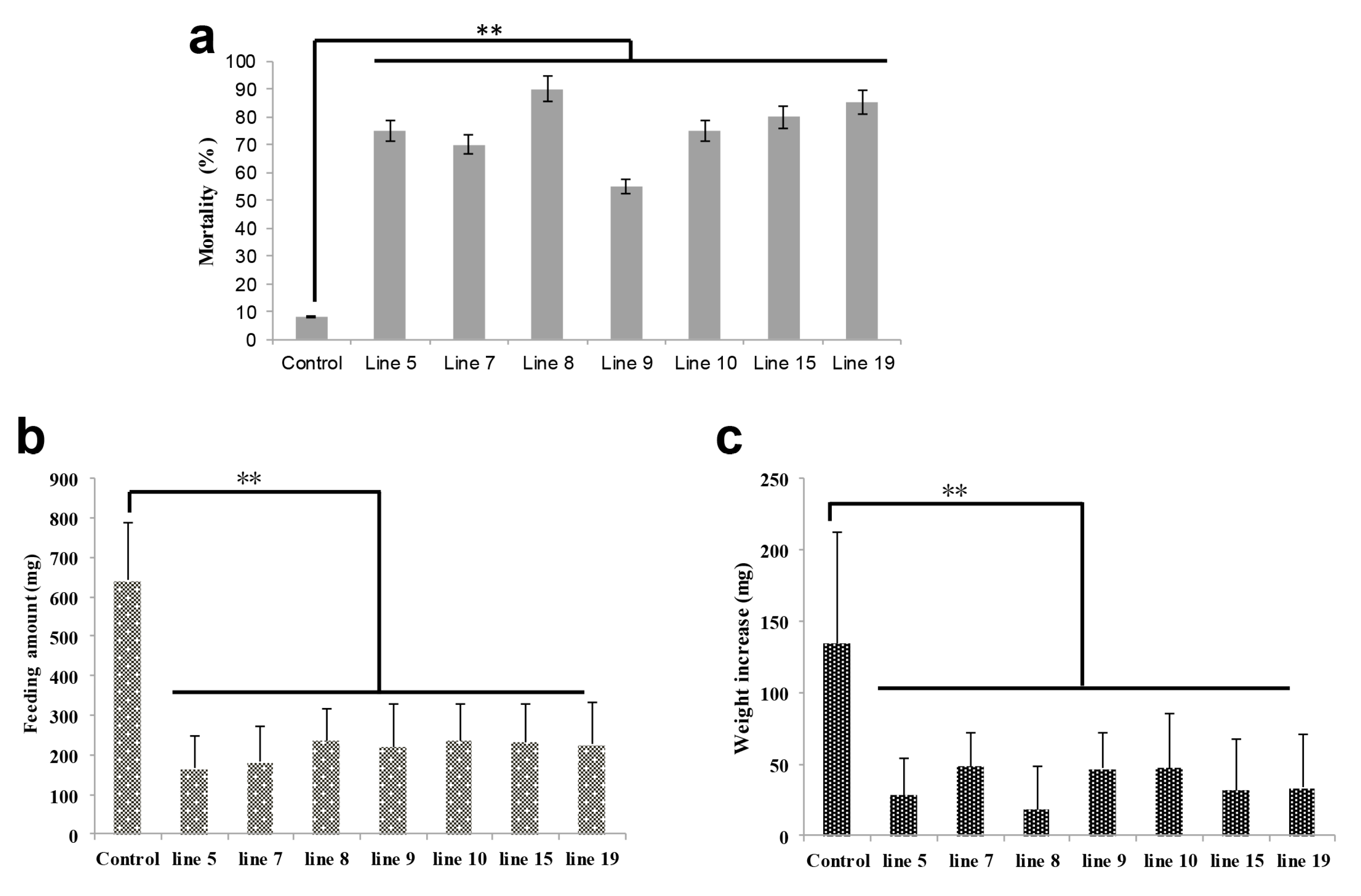
2.3. HaHR3 Transgenic Cotton Affected the Development and Propagation of H. armigera
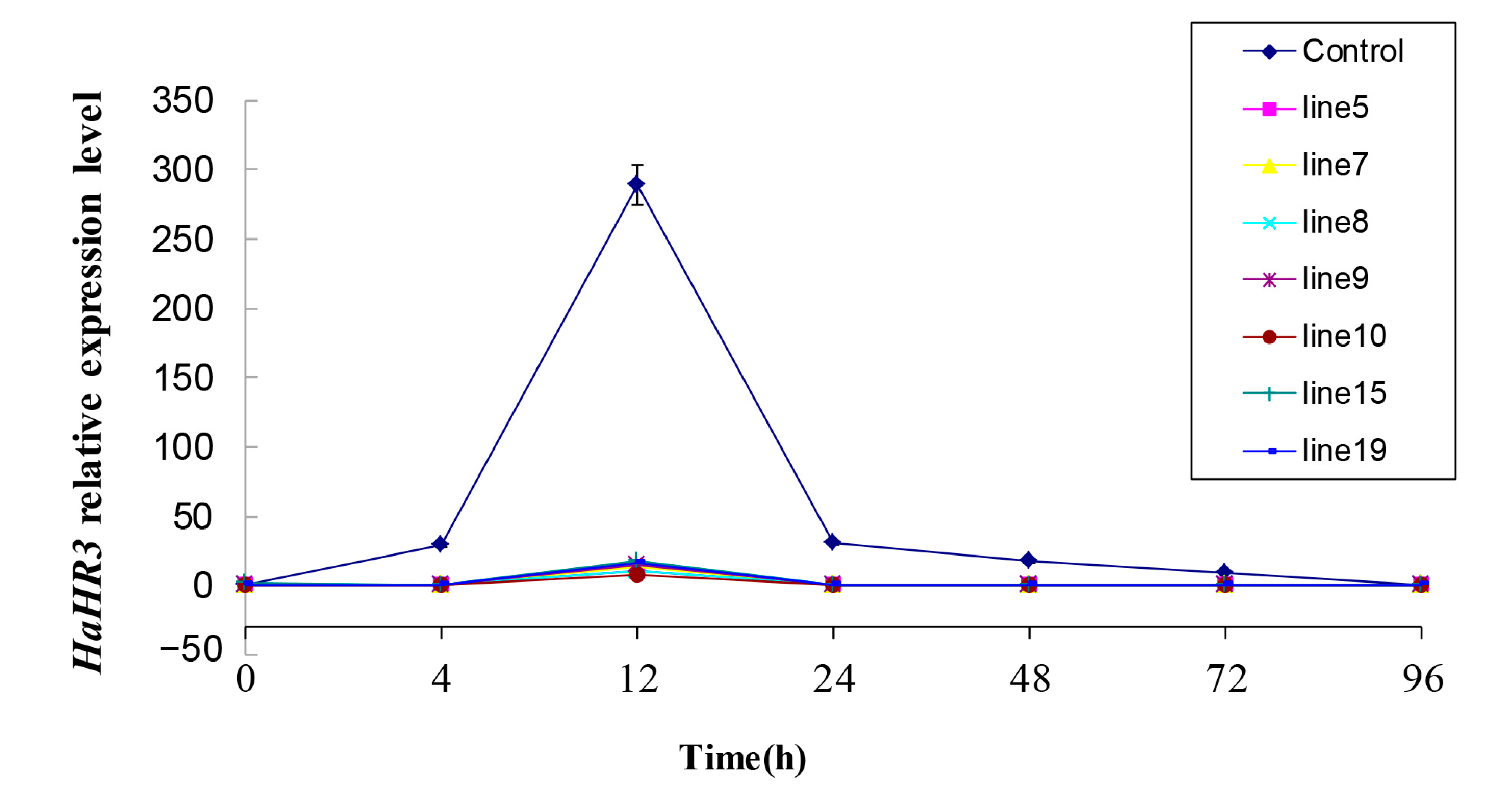
2.4. HaHR3 Expression in H. armigera after Feeding on Transgenic Cotton Plants
2.5. HaHR3 Transgenic Cotton Showed an Improved Cotton Yield
3. Discussion
4. Materials and Methods
4.1. Plant Expression Vector Construction and Cotton Plant Transformation
4.2. Insect and Plant Culture
4.3. Transgenic Cotton Molecular Analysis
4.4. Quantitative Real-Time (qRT)-PCR Analysis
4.5. Feeding Bioassays with Cotton Expressing HaHR3
4.6. Cotton Yield and Fiber Quality Measurement
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, M.; Zheng, X.; Song, S.; Zeng, Q.; Hou, L.; Li, D.; Zhao, J.; Wei, Y.; Li, X.; Luo, M. Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat. Biotechnol. 2011, 29, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bellaloui, N.; Stetina, S.R.; Turley, R.B. Cottonseed protein, oil, and mineral status in near-isogenic Gossypium hirsutum cotton lines expressing fuzzy/linted and fuzzless/linted seed phenotypes under field conditions. Front. Plant Sci. 2015, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Klasson, K.T.; Olanya, O.M.; Uknalis, J. Application of tung oil to improve adhesion strength and water resistance of cottonseed meal and protein adhesives on maple veneer. Ind. Crops Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, H.; Lu, Y.; Yang, Y.; Wu, K.; Tabashnik, B.E.; Wu, Y. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 2015, 33, 169. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Huby, R.D.; Dearman, R.J.; Kimber, I. Why are some proteins allergens? Toxicol. Sci. Off. J. Soc. Toxicol. 2000, 55, 235–246. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Figureira, A. Management of Insect Pest by RNAi—A New Tool for Crop Protection; InTechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Bate, M.; Martinez Arias, A. Fly Assembly. (Book Reviews: The Development of Drosophila melanogaster.). Science 1994, 265, 819–820. [Google Scholar]
- Hiruma, K.; Riddiford, L.M. Regulation of transcription factors MHR4 and betaFTZ-F1 by 20-hydroxyecdysone during a larval molt in the tobacco hornworm, Manduca sexta. Dev. Biol. 2001, 232, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. Hormone Receptors and the Regulation of Insect Metamorphosis. Am. Zool. 1993, 3, 203–209. [Google Scholar] [CrossRef]
- Huet, F.; Ruiz, C.; Richards, G. Sequential gene activation by ecdysone in Drosophila melanogaster: The hierarchical equivalence of early and early late genes. Development 1995, 121, 1195–1204. [Google Scholar] [PubMed]
- Thummel, C.S.; Chory, J. Steroid signaling in plants and insects—Common themes, different pathways. Genes Dev. 2002, 16, 3113–3129. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Henrich, V.C. Arthropod nuclear receptors and their role in molting. FEBS J. 2009, 276, 6128–6157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, X.; Xu, Y.; Liu, Y.; Wang, J. Recombinant expression and purification of inclusion bodies of the molt—Regulating transcription factor HHR3 from Helicoverpa armigera (Lepidoptera: Noctuidae). Kun Chong Xue Bao 2004, 47, 281–286. [Google Scholar]
- Yin, F.S.; Zeng, H.M.; Zhang, Y.L.; Qiu, D.W.; Yang, X.F.; Wang, L.M. Cloning of Helicoverpa armigera Gene HaHR3 and Construction of Its RNA Interference Vector. Cotton Sci. 2010, 22, 157–162. [Google Scholar]
- Xiong, Y.; Zeng, H.; Zhang, Y.; Xu, D.; Qiu, D. Silencing the HaHR3 Gene by Transgenic Plant-mediated RNAi to Disrupt Helicoverpa armigera Development. Int. J. Biol. Sci. 2013, 9, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Martinezcampos, M.; Zipperlen, P.; Fraser, A.G.; Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001, 2, RESEARCH0002.1. [Google Scholar] [PubMed]
- Kostrouchova, M.; Krause, M.; Kostrouch, Z.; Rall, J.E. Nuclear hormone receptor CHR3 is a critical regulator of all four larval molts of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2001, 98, 7360–7365. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.; Hall, B.L.; Bender, M.; Thummel, C.S. DHR3 is required for the prepupal-pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev. Biol. 1999, 212, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Ma, J.; Zhao, J. Silencing of cytochrome P450 CYP6B6 gene of cotton bollworm (Helicoverpa armigera) by RNAi. Bull. Entomol. Res. 2013, 103, 584. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Tao, X.Y.; Xue, X.Y.; Wang, L.J.; Chen, X.Y. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res. 2011, 20, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Cheng, L.; Qi, X.; Ge, Z.; Niu, C.; Zhang, X.; Jin, S. Transgenic Cotton Plants Expressing Double-stranded RNAs Target HMG-CoA Reductase (HMGR) Gene Inhibits the Growth, Development and Survival of Cotton Bollworms. Int. J. Biol. Sci. 2015, 11, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Robinson, K.E.; Jain, R.G.; Chandra, G.S.; Asokan, R.; Asgari, S.; Mitter, N. Diet-delivered RNAi in Helicoverpa armigera—Progresses and challenges. J. Insect Physiol. 2016, 85, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, L.; Du, M.; Evers, J.B.; Werf, W.V. D.; Tian, X.; Li, Z. Managing mepiquat chloride and plant density for optimal yield and quality of cotton. Field Crops Res. 2013, 149, 1–10. [Google Scholar] [CrossRef]
- Long, R.; Michaelp, B.; Stuartg, G.; Marinushjvander, S.; Geoffreyrs, N.; Grega, C. Fiber quality and textile performance of some Australian cotton genotypes. Crop Sci. 2010, 50, 1509–1518. [Google Scholar] [CrossRef]
- Zhou, G.; Weng, J.; Zeng, Y.; Huang, J.; Qian, S.; Liu, G. Introduction of exogenous DNA into cotton embryos. Methods Enzymol. 1983, 101, 433–481. [Google Scholar] [PubMed]
- Cho, Y.S.; Huang, Y.L.; Yang, C.J. Studies on the artificial diets of the cotton bollworm Heliothis armigera (Hubner). Acta Entomologica Sinica 1981, 24, 108–110. [Google Scholar]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Southern, E.M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 1975, 98, 503IN3509–508IN5517. [Google Scholar] [CrossRef]
- Ru, Z.L.; Wang, G.X.; He, S.P.; Du, X.M. The difference of fiber quality and fiber ultrastructure in different natural colored cotton. Cotton Sci. 2013, 25, 184–188. [Google Scholar]
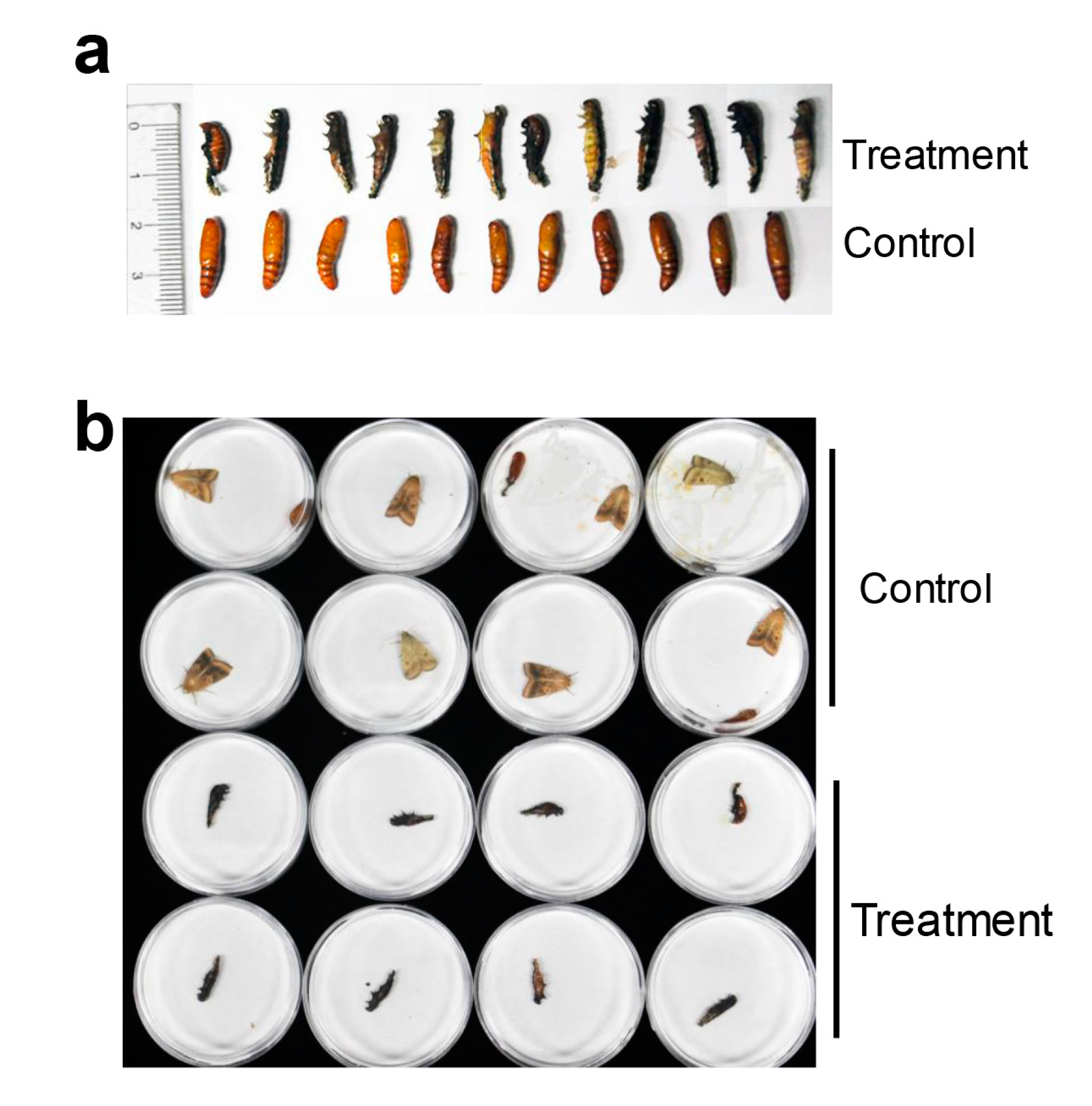
| Line | Pupation Deformity Rate/% | Eclosion Rate/% |
|---|---|---|
| Control | 0 | 100 |
| Line 5 | 100 | 0 |
| Line 7 | 83.3 | 16.7 |
| Line 8 | 100 | 0 |
| Line 9 | 78.8 | 21.2 |
| Line 10 | 80 | 20 |
| Line 15 | 100 | 0 |
| Line 19 | 100 | 0 |
| Line | Boll Number | Poll Weight (g) | Lint (%) | Fiber Quality | |||
|---|---|---|---|---|---|---|---|
| Upper-Half Mean Fiber Length (mm) | Bundle Strength (cN/tex) | Micronaire | Uniformity Index | ||||
| F15 | 18.5 ± 2.6 A | 6.3 ± 0.30 A | 41.5 ± 0.6 A | 31.13 ± 0.32 A | 30.7 ± 0.8 A | 5.34 ± 0.15 A | 145 ± 1.5 A |
| Line5 | 29.5 ± 2.7 B | 5.8 ± 0.54 A | 41.2 ± 0.4 A | 31.43 ± 0.63 A | 31.4 ± 0.92 A | 4.64 ± 0.56 B | 150 ± 2.6 B |
| Line7 | 28.4 ± 3.6 B | 6.2 ± 0.15 A | 38.9 ± 0.7 A | 28.79 ± 0.65 A | 32.4 ± 1.22 A | 4.65 ± 0.43 B | 147 ± 2.8 A |
| Line8 | 26.5 ± 3.2 B | 5.9 ± 0.24 A | 39.8 ± 0.9 A | 31.22 ± 0.82 A | 31.5 ± 0.72 A | 4.96 ± 0.42 B | 158 ± 3.8 B |
| Line9 | 22.4 ± 2.2 B | 6.3 ± 0.22 A | 40.3 ± 0.7 A | 30.45 ± 0.26 A | 30.8 ± 0.76 A | 4.87 ± 0.88 B | 149 ± 2.3 B |
| Line10 | 26.8 ± 3.4 B | 6.1 ± 0.65 A | 39.5 ± 0.5 A | 30.12 ± 0.23 A | 31.8 ± 1.21 A | 4.92 ± 0.28 B | 157 ± 2.4 B |
| Line15 | 34.2 ± 3.4 B | 5.8 ± 0.18 A | 39.7 ± 0.2 A | 30.13 ± 0.44 A | 32.6 ± 0.95 A | 5.25 ± 0.24 A | 144 ± 3.2 A |
| Line19 | 30.5 ± 2.6 B | 6.0 ± 0.28 A | 39.5 ± 0.7 A | 30.14 ± 0.64 A | 33.4 ± 1.12 A | 4.74 ± 0.16 B | 168 ± 1.9 B |
| Target Gene | Accession No. | Primer Sequence |
|---|---|---|
| HaHR3 | FJ009448 | F:5′-ccCTCGAGATGAACAACAACCAGTTCCACGAT-3′ |
| R:5′-gaAGATCTCACGCAGGCTTTATTCCGTGGACA-3′ | ||
| Sense-Frag. | FJ009448 | F:5′-ccCTCGAGATGAACAACAACCAGTTCCACGAT-3′ |
| R:5′-gaAGATCTCACGCAGGCTTTATTCCGTGGACA-3′ | ||
| Anti-sense Frag. | FJ009448 | F:5′-gcGTCGACATGAACAACAACCAGTTCCACGAT-3′ |
| R:5′-cgGGATCCCACGCAGGCTTTATTCCGTGGACA-3′ | ||
| qRT-HaHR3 | FJ009448 | F:5′-GCCACAGGATGTCTCCAAGC-3′ |
| R:5′-GAGCCAGATTTCAAGAGCAAAA-3′ | ||
| qRT-actA3b | X97615 | F:5′-CCCCGTCCACAATGAAGATC-3′ |
| R:5′-GGCCAGACTCGTCGTACTCCT-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Wang, Z.; He, Y.; Xiong, Y.; Lv, S.; Li, S.; Zhang, Z.; Qiu, D.; Zeng, H. Transgenic Cotton Plants Expressing the HaHR3 Gene Conferred Enhanced Resistance to Helicoverpa armigera and Improved Cotton Yield. Int. J. Mol. Sci. 2017, 18, 1874. https://doi.org/10.3390/ijms18091874
Han Q, Wang Z, He Y, Xiong Y, Lv S, Li S, Zhang Z, Qiu D, Zeng H. Transgenic Cotton Plants Expressing the HaHR3 Gene Conferred Enhanced Resistance to Helicoverpa armigera and Improved Cotton Yield. International Journal of Molecular Sciences. 2017; 18(9):1874. https://doi.org/10.3390/ijms18091874
Chicago/Turabian StyleHan, Qiang, Zhenzhen Wang, Yunxin He, Yehui Xiong, Shun Lv, Shupeng Li, Zhigang Zhang, Dewen Qiu, and Hongmei Zeng. 2017. "Transgenic Cotton Plants Expressing the HaHR3 Gene Conferred Enhanced Resistance to Helicoverpa armigera and Improved Cotton Yield" International Journal of Molecular Sciences 18, no. 9: 1874. https://doi.org/10.3390/ijms18091874
APA StyleHan, Q., Wang, Z., He, Y., Xiong, Y., Lv, S., Li, S., Zhang, Z., Qiu, D., & Zeng, H. (2017). Transgenic Cotton Plants Expressing the HaHR3 Gene Conferred Enhanced Resistance to Helicoverpa armigera and Improved Cotton Yield. International Journal of Molecular Sciences, 18(9), 1874. https://doi.org/10.3390/ijms18091874





