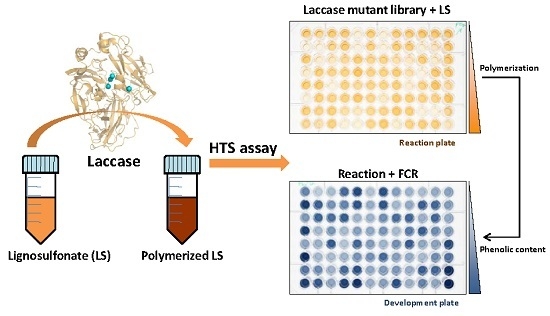
High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization
Abstract
1. Introduction
2. Results
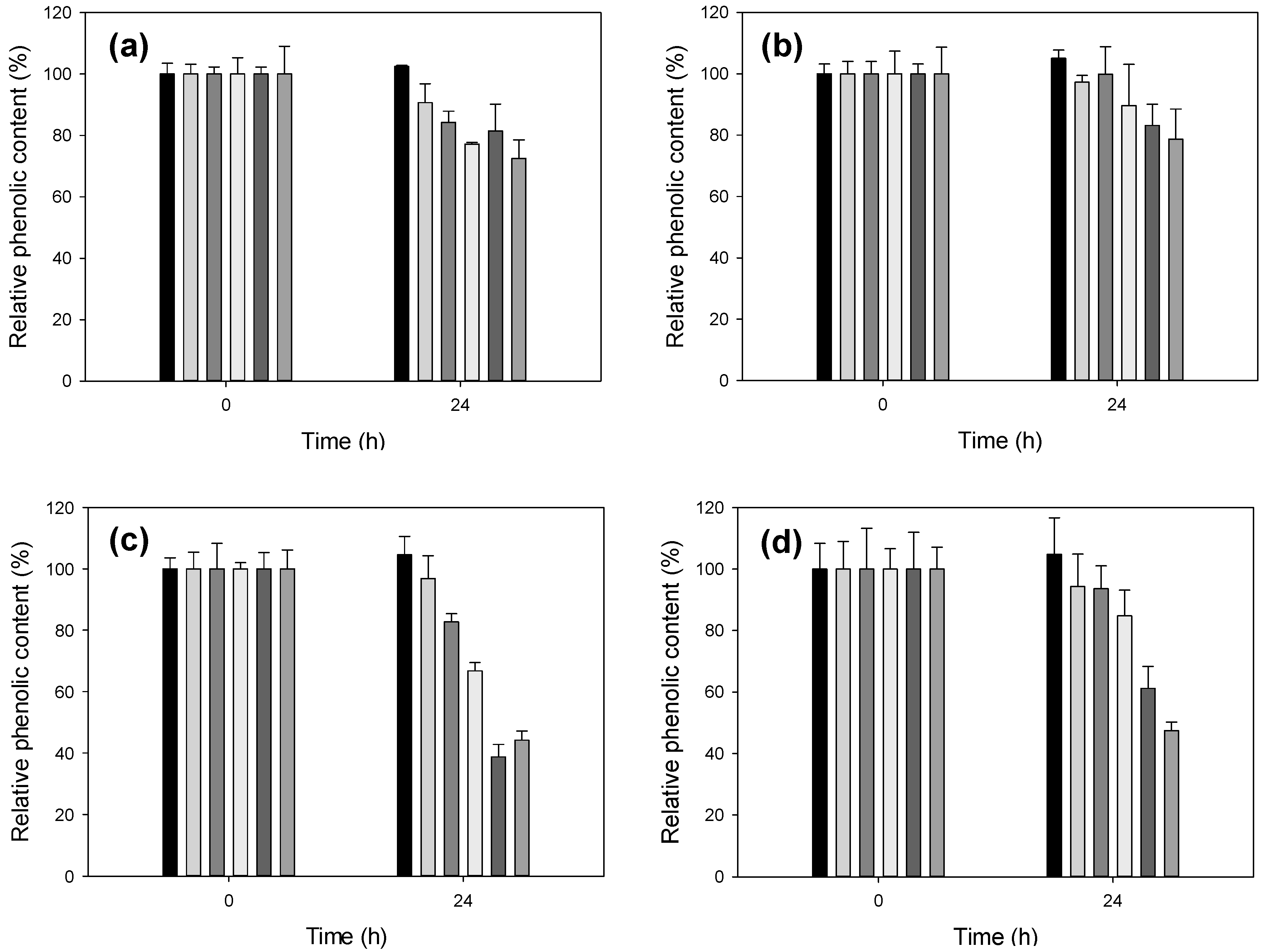
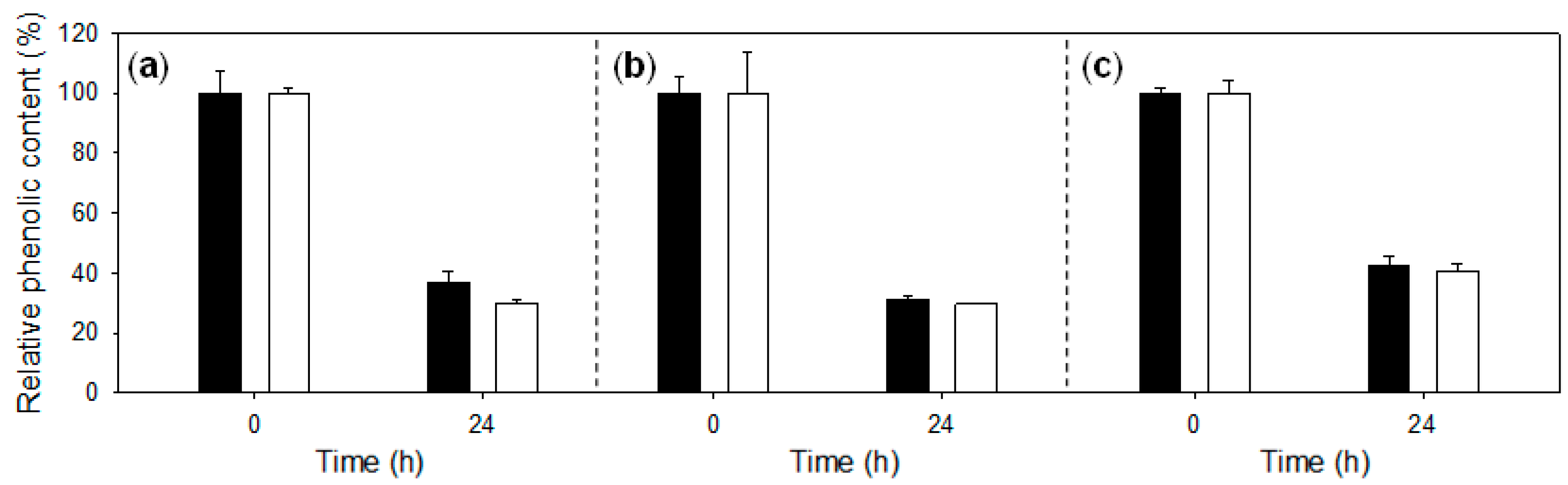
2.1. Treatment of Lignosulfonates with Laccase: Decrease of Phenolic Content
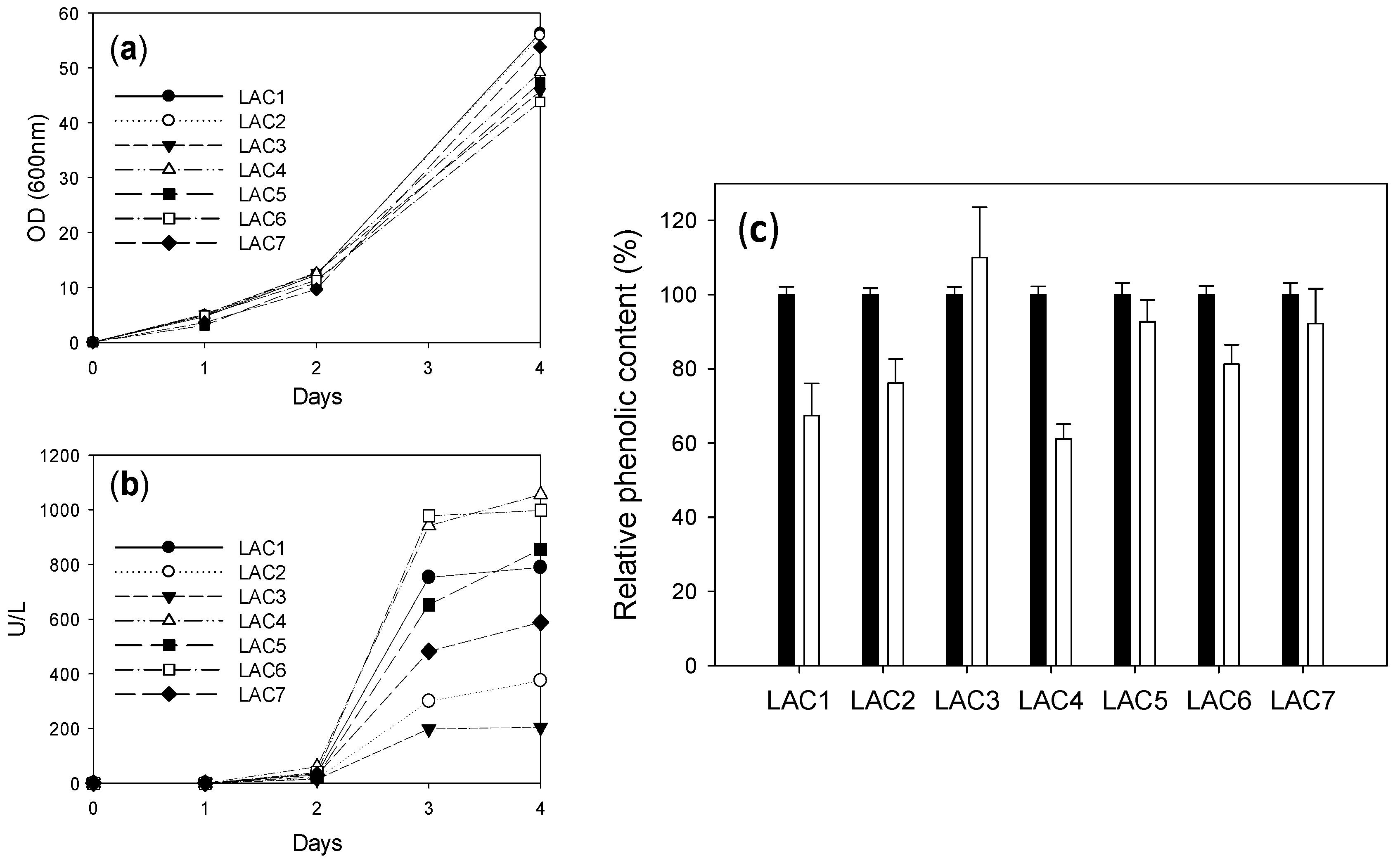
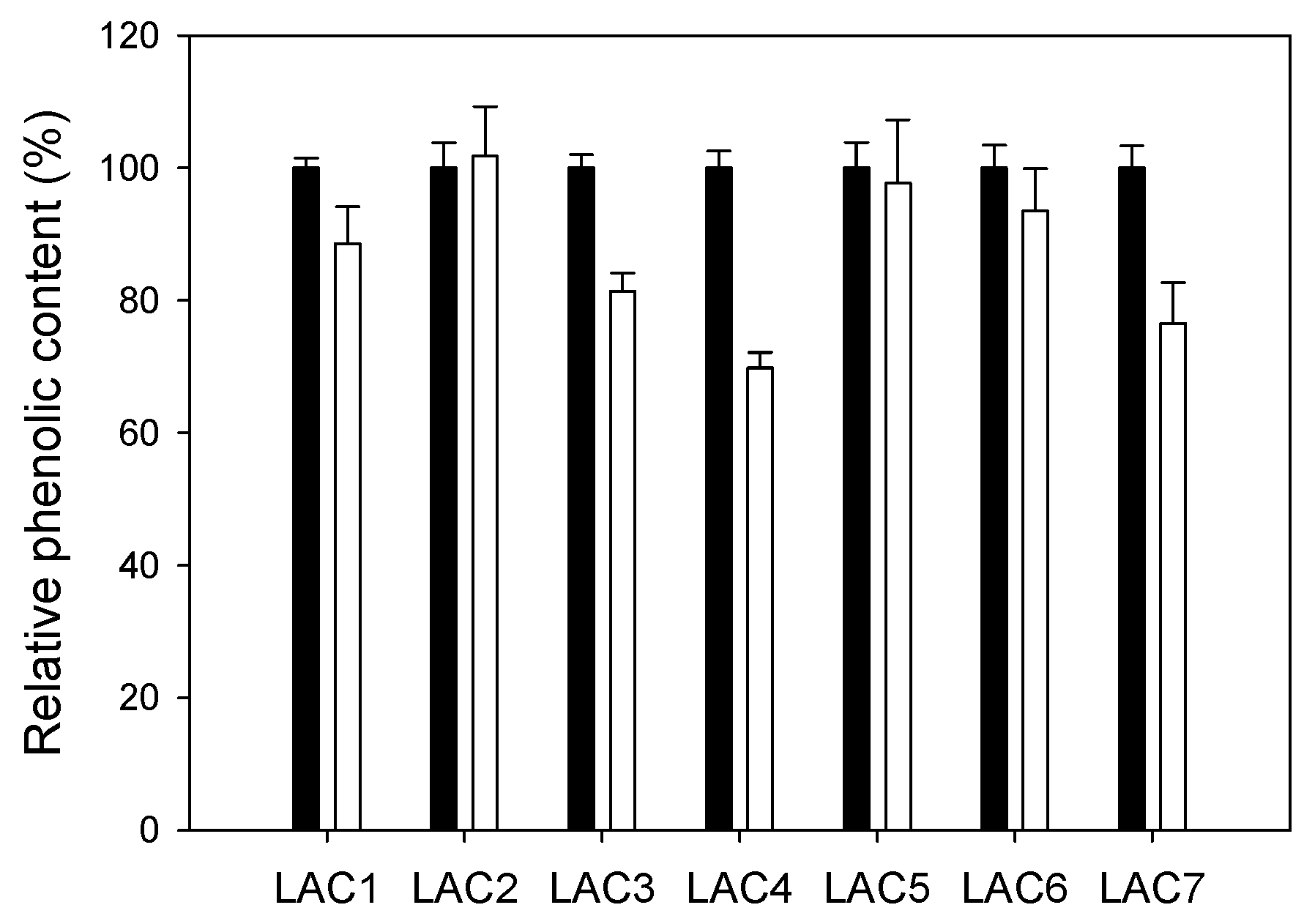
2.2. Comparison of Different Recombinant Laccases
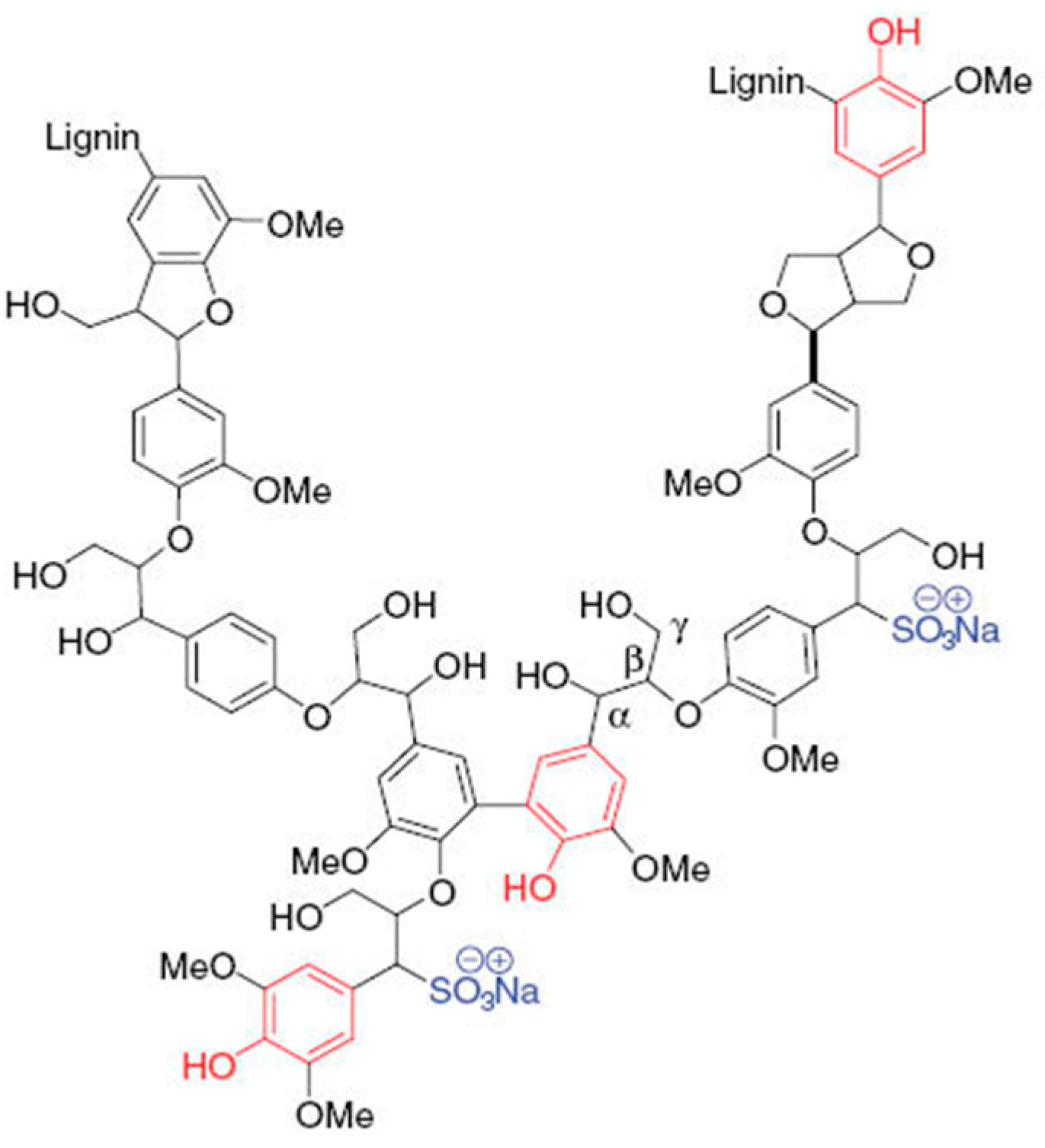
2.3. Polymerization of Lignosulfonate by Laccase with or without Redox Mediators
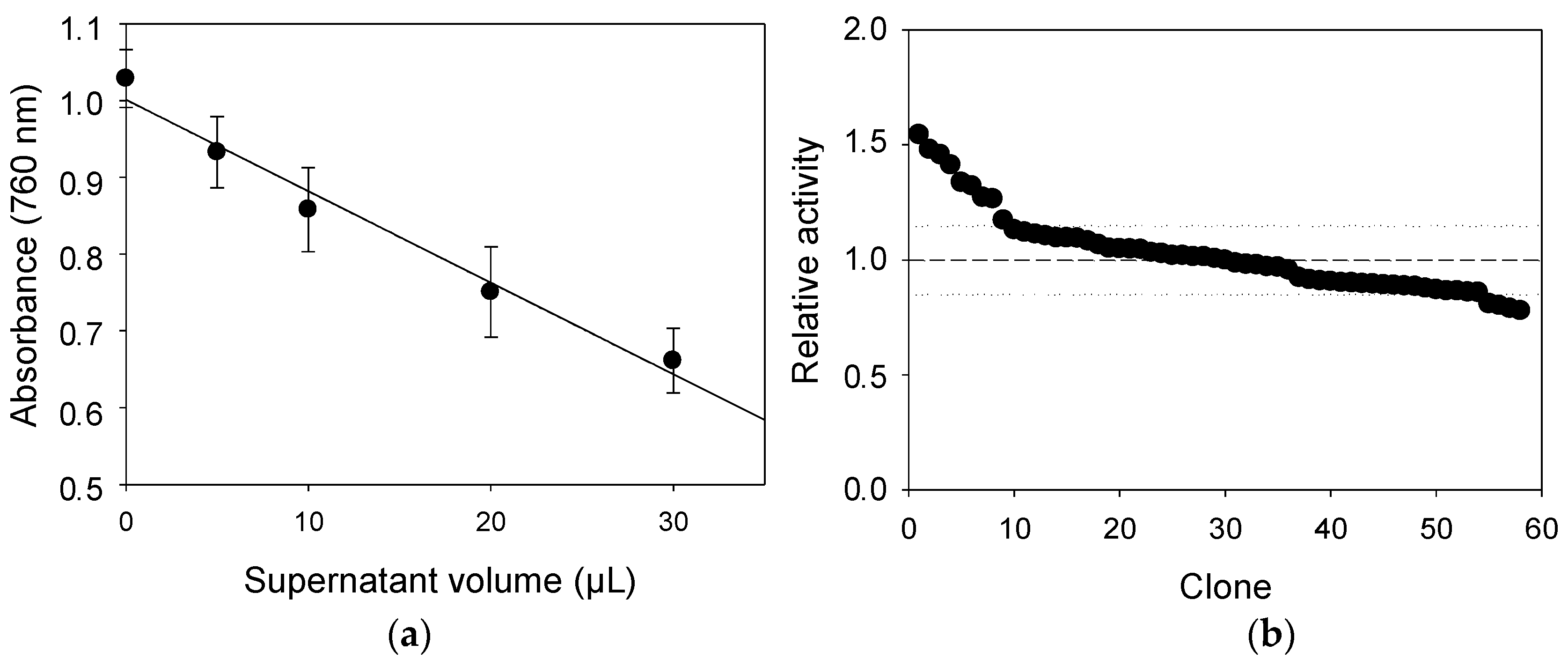
2.4. Comparison of Laccase Variants Produced in S. cerevisiae Microcultures for LS Polymerization
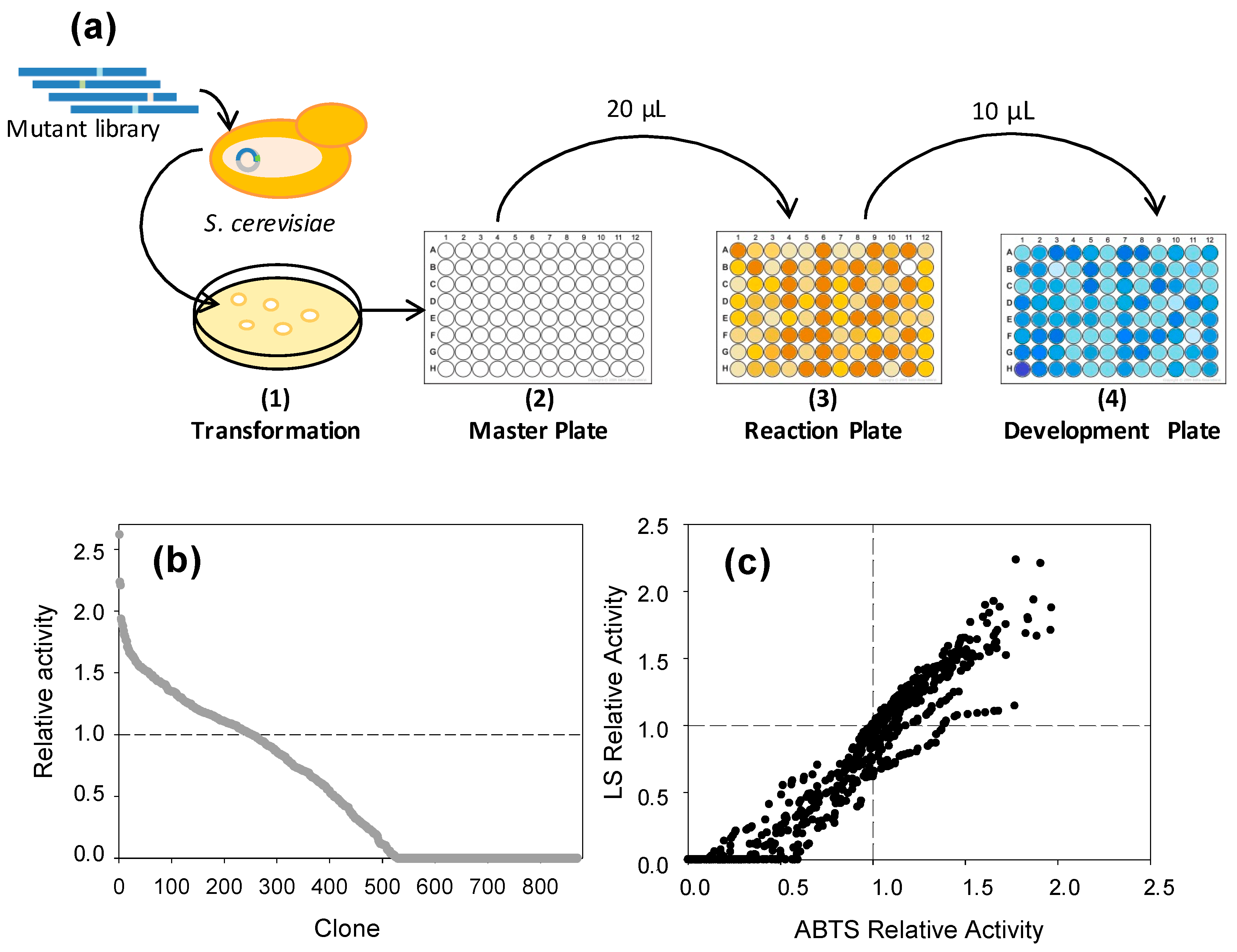
2.5. LS-FCR High-Throughput Screening Assay
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CV | Coefficient of variation |
| DP399 | Lignosulfonate from softwood (Piceaabies) |
| DP401 | Lignosulfonate from hardwood (Eucalyptus grandis) |
| epPCR | Error-prone PCR |
| FCR | Folin–Ciocalteau reagent |
| HRPL | High-redox-potential laccase |
| HTS | High-throughput screening |
| LAC | Laccase |
| LS | Lignosulfonates |
| Mr | Molecular weight |
References
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value Added Chemicals from Biomass-Volume II, Results of Screening for Potential Candidates from Biorefinery Lignin; No. PNNL-16983; Pacific Northwest National Lab. (PNNL): Richland, WA, USA; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2007. [Google Scholar]
- Strassberger, Z.; Tanase, S.; Rothenberg, G. The pros and cons of lignin valorization in an integrated biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Jamrozik, A.; Strzemiecka, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Activation of magnesium lignosulfonate and kraft lignin: Influence on the properties of phenolic resin-based composites for potential applications in abrasive materials. Int. J. Mol. Sci. 2017, 18, 1224. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, H.; Qin, Y.; Zhong, R.; Bai, M.; Qiu, X. Structure and properties of sodium lignosulfonate with different molecular weight used as dye dispersant. J. Dispersion Sci. Technol. 2014, 36, 532–539. [Google Scholar] [CrossRef]
- Areskogh, D.; Li, J.; Gellerstedt, G.; Henriksson, G. Investigation of the molecular weight increase of commercial lignosulfonates by laccase catalysis. Biomacromolecules 2010, 11, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Madad, N.; Chebil, L.; Charbonnel, C.; Ioannou, I.; Ghoul, M. Enzymatic polymerization of sodium lignosulfonates: Effect of catalysts, initial molecular weight, and mediators. Can. J. Chem. 2013, 91, 220–225. [Google Scholar] [CrossRef]
- Prasetyo, N.E.; Kudanga, T.; Østergaard, L.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Ignacio Santos, J.; Nieto, L.; Jiménez-Barbero, J.; Martínez, A.T.; et al. Polymerization of lignosulfonates by the laccase-HBT (1-hydroxybenzotriazole) system improves dispersibility. Biores. Technol. 2010, 101, 5054–5062. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Silva, C.; Zille, A.; Lopez, C.; Evtuguin, D.V.; Cavaco-Paulo, A. Characterisation of enzymatically oxidised lignosulfonates and their application on lignocellulosic fabrics. Polym. Int. 2009, 58, 863–868. [Google Scholar] [CrossRef]
- Sáez-Jiménez, V.; Baratto, M.; Pogni, R.; Rencoret, J.; Gutiérrez, A.; Santos, J.I.; Martínez, A.T.; Ruiz-Dueñas, F.J. Demonstration of lignin-to-peroxidase direct electron transfer. J. Biol. Chem. 2015, 290, 23201–23213. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Umezawa, T.; Higuchi, T. Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch. Biochem. Biophys. 1988, 262, 99–110. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G.; Reid, I.D.; Lanthier, P.; Yaguchi, M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61, 1876–1880. [Google Scholar] [PubMed]
- Pardo, I.; Vicente, A.I.; Maté, D.M.; Alcalde, M.; Camarero, S. Development of chimeric laccases by directed evolution. Biotech. Bioeng. 2012, 109, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Camarero, S. Exploring the oxidation of lignin-derived phenols by a library of laccase mutants. Molecules 2015, 20, 15929–15943. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.; de Salas, F.; Lucas, F.; Monza, E.; Acebes, S.; Martinez, A.T.; Camarero, S.; Guallar, V. Computer-aided laccase engineering: Toward biological oxidation of arylamines. ACS Cat. 2016, 6, 5415–5423. [Google Scholar] [CrossRef]
- Salazar, O.; Sun, L. Evaluating a screen and analysis of mutant libraries. In Directed Enzyme Evolution Screening and Selection Methods; Arnold, F.H., Georgiou, G., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 85–98. [Google Scholar]
- Barreca, A.; Fabbrini, M.; Galli, C.; Gentili, P.; Ljunggren, S. Laccase/mediated oxidation of a lignin model for improved delignification procedures. J. Mol. Catal. B Enzym. 2003, 26, 105–110. [Google Scholar] [CrossRef]
- Huber, D.; Ortner, A.; Daxbacher, A.; Nyanhongo, G.S.; Bauer, W.; Guebitz, G.M. Influence of oxygen and mediators on laccase-catalyzed polymerization of lignosulfonate. ACS Sustain. Chem. Engin. 2016, 4, 5303–5310. [Google Scholar] [CrossRef]
- Zhou, H.; Chang, Y.; Wu, X.; Yang, D.; Qiu, X. Horseradish peroxidase modification of sulfomethylated wheat straw alkali lignin to improve its dispersion performance. ACS Sustain. Chem. Engin. 2015, 3, 518–523. [Google Scholar] [CrossRef]
- West, M.; Hickson, A.; Mattinen, M.; Lloyd-Jones, G. Evaluating lignins as enzyme substrates: Insights and methodological recommendations from a study of laccase-catalyzed lignin polymerization. Bioresources 2014, 9, 2782–2796. [Google Scholar] [CrossRef]
- Cho, N.-S.; Shin, W.; Jeong, S.-W.; Leonowicz, A. Degradation of lignosulfonate by fungal laccase with low molecular mediators. Bull. Korean Chem. Soc. 2004, 25, 1551–1554. [Google Scholar]
- Huber, D.; Pellis, A.; Daxbacher, A.; Nyanhongo, G.S.; Guebitz, G.M. Polymerization of various lignins via immobilized myceliophthorathermophila laccase (MtL). Polymer 2016, 8, 280. [Google Scholar] [CrossRef]
- Camarero, S.; Pardo, I.; Cañas, A.; Molina, P.; Molina, P.; Record, E.; Martínez, A.T.; Martínez, M.J.; Alcalde, M. Engineering platforms for directed evolution of laccase from pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2011, 78, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Escribano, D.; De Salas, F.; Pardo, I.; Camarero, S. High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization. Int. J. Mol. Sci. 2017, 18, 1793. https://doi.org/10.3390/ijms18081793
Rodríguez-Escribano D, De Salas F, Pardo I, Camarero S. High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization. International Journal of Molecular Sciences. 2017; 18(8):1793. https://doi.org/10.3390/ijms18081793
Chicago/Turabian StyleRodríguez-Escribano, David, Felipe De Salas, Isabel Pardo, and Susana Camarero. 2017. "High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization" International Journal of Molecular Sciences 18, no. 8: 1793. https://doi.org/10.3390/ijms18081793
APA StyleRodríguez-Escribano, D., De Salas, F., Pardo, I., & Camarero, S. (2017). High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization. International Journal of Molecular Sciences, 18(8), 1793. https://doi.org/10.3390/ijms18081793