Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Incidence of Acute Kidney Injury
2.2. suPAR and proENK in Patients Developing Acute Kidney Injury after Cardiac Surgery
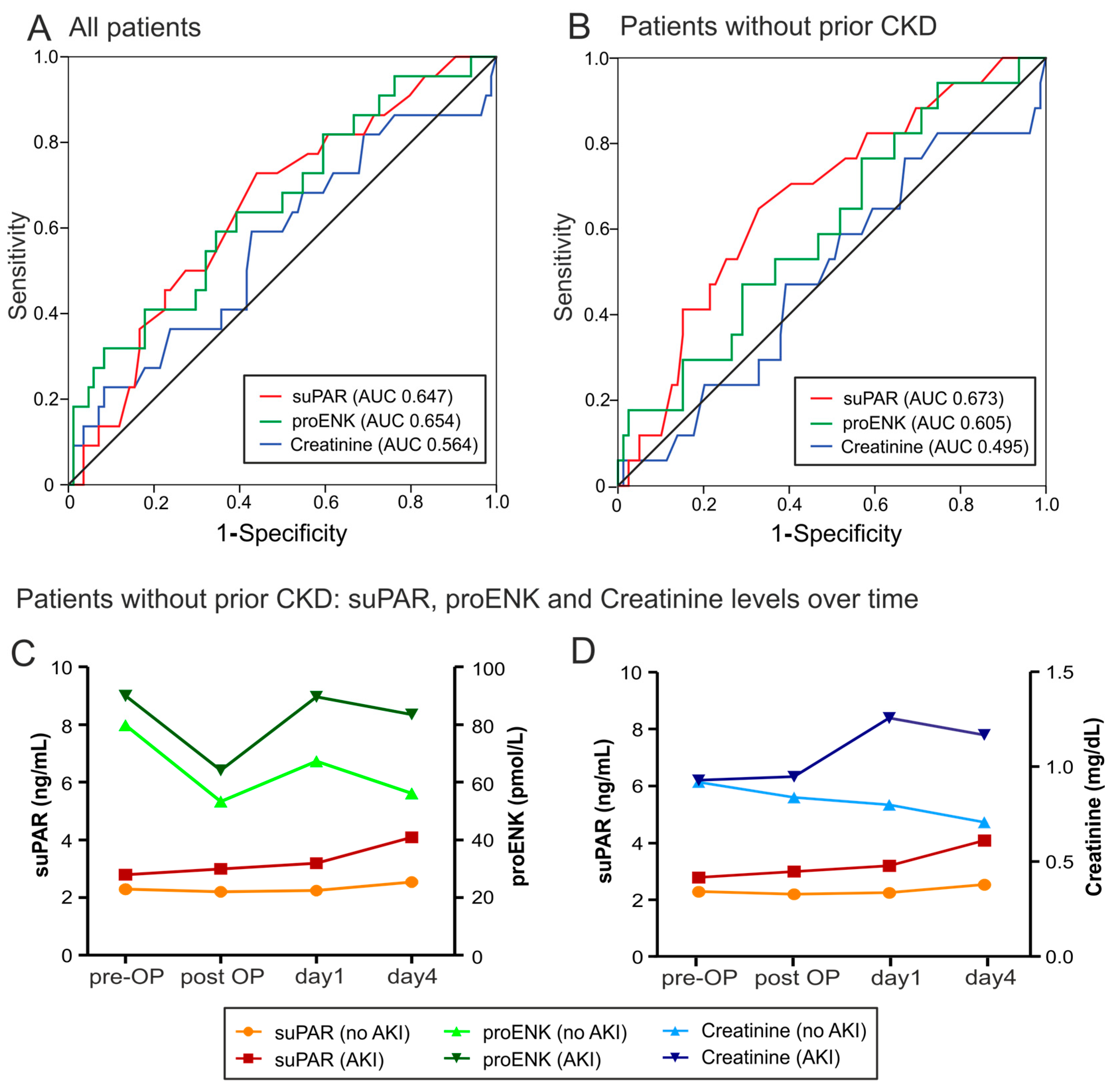
2.3. Risk Prediction for AKI by suPAR and proENK
3. Discussion
Limitations of Our Study
4. Methods
4.1. Study Design and Patient Characteristics
4.2. Management of Anesthesia and Surgical Procedures
4.3. Determination of suPAR, proENK and Creatinine Serum Concentrations
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karkouti, K.; Wijeysundera, D.N.; Yau, T.M.; Callum, J.L.; Cheng, D.C.; Crowther, M.; Dupuis, J.Y.; Fremes, S.E.; Kent, B.; Laflamme, C.; et al. Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 2009, 119, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mariscalco, G.; Lorusso, R.; Dominici, C.; Renzulli, A.; Sala, A. Acute kidney injury: A relevant complication after cardiac surgery. Ann. Thorac. Surg. 2011, 92, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Chando, S.; Crowe, S.; Manns, B.; Winkelmayer, W.C.; Hemmelgarn, B.; Craig, J.C. Research priority setting in kidney disease: A systematic review. Am. J. Kidney Dis. 2015, 65, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Cochran, R.P.; Dacey, L.J.; Ross, C.S.; Kunzelman, K.S.; Dunton, R.F.; Braxton, J.H.; Charlesworth, D.C.; Clough, R.A.; Helm, R.E.; et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation 2006, 114 (Suppl. 1), 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- De Geus, H.R.; Betjes, M.G.; Bakker, J. Biomarkers for the prediction of acute kidney injury: A narrative review on current status and future challenges. Clin. Kidney J. 2012, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Taub, P.; Patel, M.; Rehfeldt, M.; Struck, J.; Clopton, P.; Mehta, R.L.; Maisel, A.S. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin. Nephrol. 2015, 83, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Kohrle, J.; Bergmann, A. Proenkephalin A 119-59, a stable proenkephalin A precursor fragment identified in human circulation. Peptides 2006, 27, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Abas, M.; Elgharably, H.; Tripathi, R.; Theofilos, T.; Bhandary, S.; Sai-Sudhakar, C.; Sen, C.K.; Roy, S. Endogenous opioids in wound-site neutrophils of sternotomy patients. PLoS ONE 2012, 7, e47569. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Lu, C.; Li, W.; Meng, J.; Wang, D.; Plotnikoff, N.P.; Wang, E.; Shan, F. Comparison of stimulating effect on subpopulations of lymphocytes in human peripheral blood by methionine enkephalin with IL-2 and IFN-gamma. Hum. Vaccines Immunother. 2012, 8, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Arbit, B.; Marston, N.; Shah, K.; Lee, E.L.; Aramin, H.; Clopton, P.; Maisel, A.S. Prognostic usefulness of proenkephalin in stable ambulatory patients with heart failure. Am. J. Cardiol. 2016, 117, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.A.; Christensson, A.; Ericson, U.; Almgren, P.; Hindy, G.; Nilsson, P.M.; Struck, J.; Bergmann, A.; Melander, O.; Orho-Melander, M. High level of fasting plasma proenkephalin-A predicts deterioration of kidney function and incidence of CKD. J. Am. Soc. Nephrol. JASN 2016, 28, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.L.; Sandhu, J.K.; Narayan, H.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Bergmann, A.; Maisel, A.; Jones, D.J. Proenkephalin and prognosis after acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Montuori, N.; Visconte, V.; Rossi, G.; Ragno, P. Soluble and cleaved forms of the urokinase-receptor: Degradation products or active molecules? Thromb. Haemost. 2005, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Voigt, S.; Kruschinski, C.; Sanson, E.; Duckers, H.; Horn, A.; Yagmur, E.; Zimmermann, H.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care 2011, 15, R63. [Google Scholar] [CrossRef] [PubMed]
- Lyngbaek, S.; Marott, J.L.; Sehestedt, T.; Hansen, T.W.; Olsen, M.H.; Andersen, O.; Linneberg, A.; Haugaard, S.B.; Eugen-Olsen, J.; Hansen, P.R.; et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int. J. Cardiol. 2013, 167, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Backes, Y.; van der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.W.; Bang, C.N.; Wachtell, K.; Eugen-Olsen, J.; Jeppesen, J.L. suPAR: A new biomarker for cardiovascular disease? Can. J. Cardiol. 2015, 31, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; El Hindi, S.; Li, J.; Fornoni, A.; Goes, N.; Sageshima, J.; Maiguel, D.; Karumanchi, S.A.; Yap, H.K.; Saleem, M.; et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 2011, 17, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.S.; Sever, S.; Ko, Y.A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble urokinase receptor and chronic kidney disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Estrella, M.M.; Coresh, J.; Brower, R.G.; Liu, K.D.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.D.; Thompson, B.T.; Ancukiewicz, M.; Steingrub, J.S.; Douglas, I.S.; Matthay, M.A.; Wright, P.; Peterson, M.W.; Rock, P.; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network; et al. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit. Care Med. 2011, 39, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Van Aken, H.; Martens, S.; Rossaint, J.; Singbartl, K.; Gorlich, D.; Kellum, J.A.; Zarbock, A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 2014, 9, e93460. [Google Scholar] [CrossRef] [PubMed]
- Wetz, A.J.; Richardt, E.M.; Wand, S.; Kunze, N.; Schotola, H.; Quintel, M.; Brauer, A.; Moerer, O. Quantification of urinary TIMP-2 and IGFBP-7: An adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit. Care 2015, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Moledina, D.G.; Coca, S.G.; Thiessen-Philbrook, H.R.; Garg, A.X. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016, 89, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Riisbro, R.; Christensen, I.J.; Hogdall, C.; Brunner, N.; Hogdall, E. Soluble urokinase plasminogen activator receptor measurements: Influence of sample handling. Int. J. Biol. Mark. 2001, 16, 233–239. [Google Scholar]
- Dickson, E.W.; Hogrefe, C.P.; Ludwig, P.S.; Ackermann, L.W.; Stoll, L.L.; Denning, G.M. Exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H402–H408. [Google Scholar] [CrossRef] [PubMed]
- Theilade, S.; Lyngbaek, S.; Hansen, T.W.; Eugen-Olsen, J.; Fenger, M.; Rossing, P.; Jeppesen, J.L. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J. Intern. Med. 2015, 277, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Trachtman, H.; Li, J.; Dong, C.; Friedman, A.L.; Gassman, J.J.; McMahan, J.L.; Radeva, M.; Heil, K.M.; Trautmann, A.; et al. Circulating suPAR in two cohorts of primary FSGS. J. Am. Soc. Nephrol. JASN 2012, 23, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.M.; Sanyal, A.J.; Peixoto, A.J.; Perazella, M.A.; Lim, J.; Thiessen-Philbrook, H.; Ansari, N.; Coca, S.G.; Garcia-Tsao, G.; Parikh, C.R.; et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014, 60, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Kdigo, A. KI Work Group: KDIGO clinical practice guideline for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar]
- Dreymueller, D.; Goetzenich, A.; Emontzpohl, C.; Soppert, J.; Ludwig, A.; Stoppe, C. The perioperative time course and clinical significance of the chemokine CXCL16 in patients undergoing cardiac surgery. J. Cell. Mol. Med. 2016, 20, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Benz, F.; Cardenas, D.V.; Lutz, M.; Hippe, H.J.; Luedde, T.; Trautwein, C.; Frey, N.; Koch, A.; Tacke, F.; et al. Persistently elevated osteopontin serum levels predict mortality in critically ill patients. Crit. Care 2015, 19, 271. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Zimmermann, H.W.; Gassler, N.; Jochum, C.; Weiskirchen, R.; Bruensing, J.; Buendgens, L.; Duckers, H.; Bruns, T.; Gerken, G.; et al. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014, 34, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
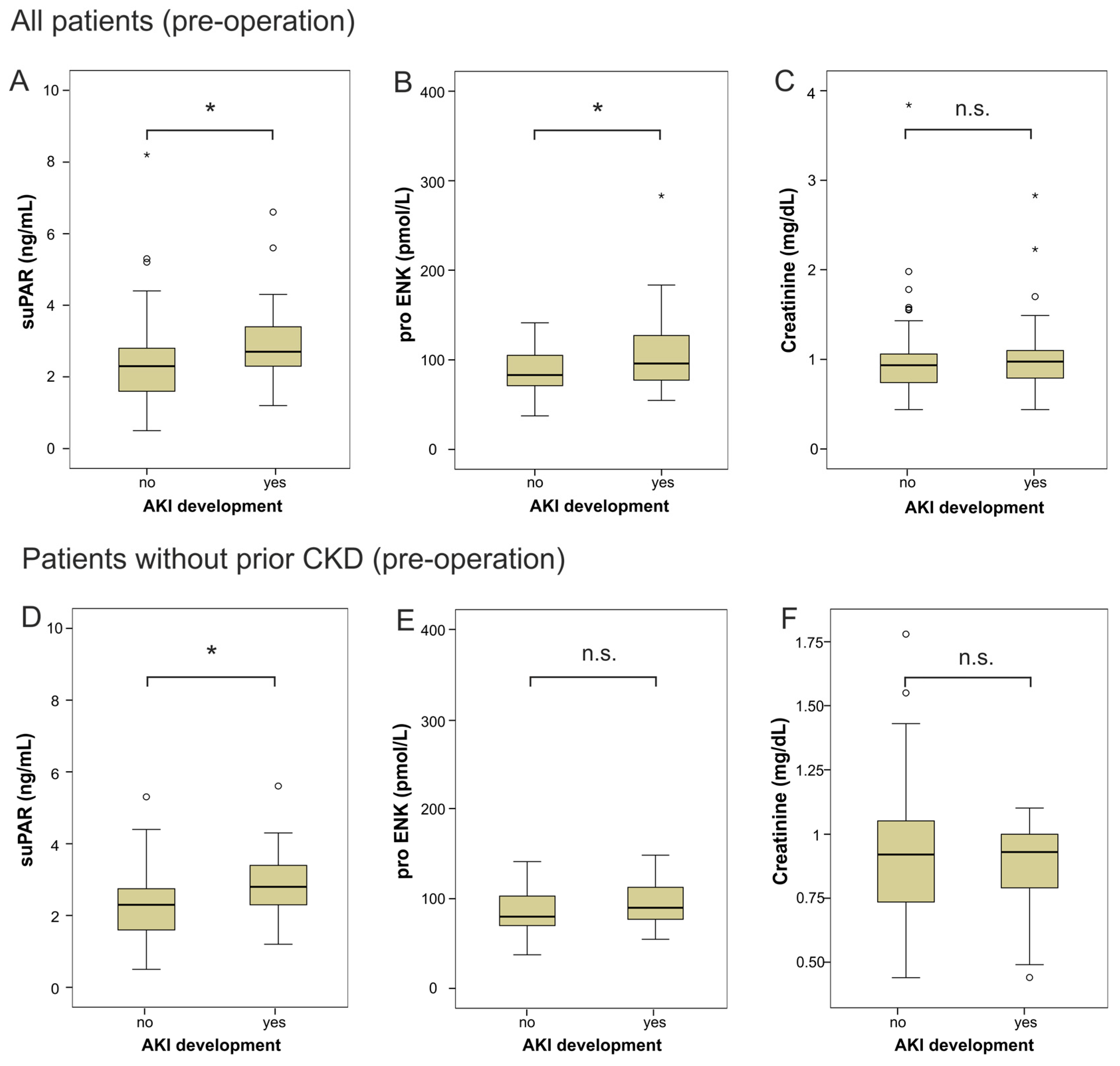
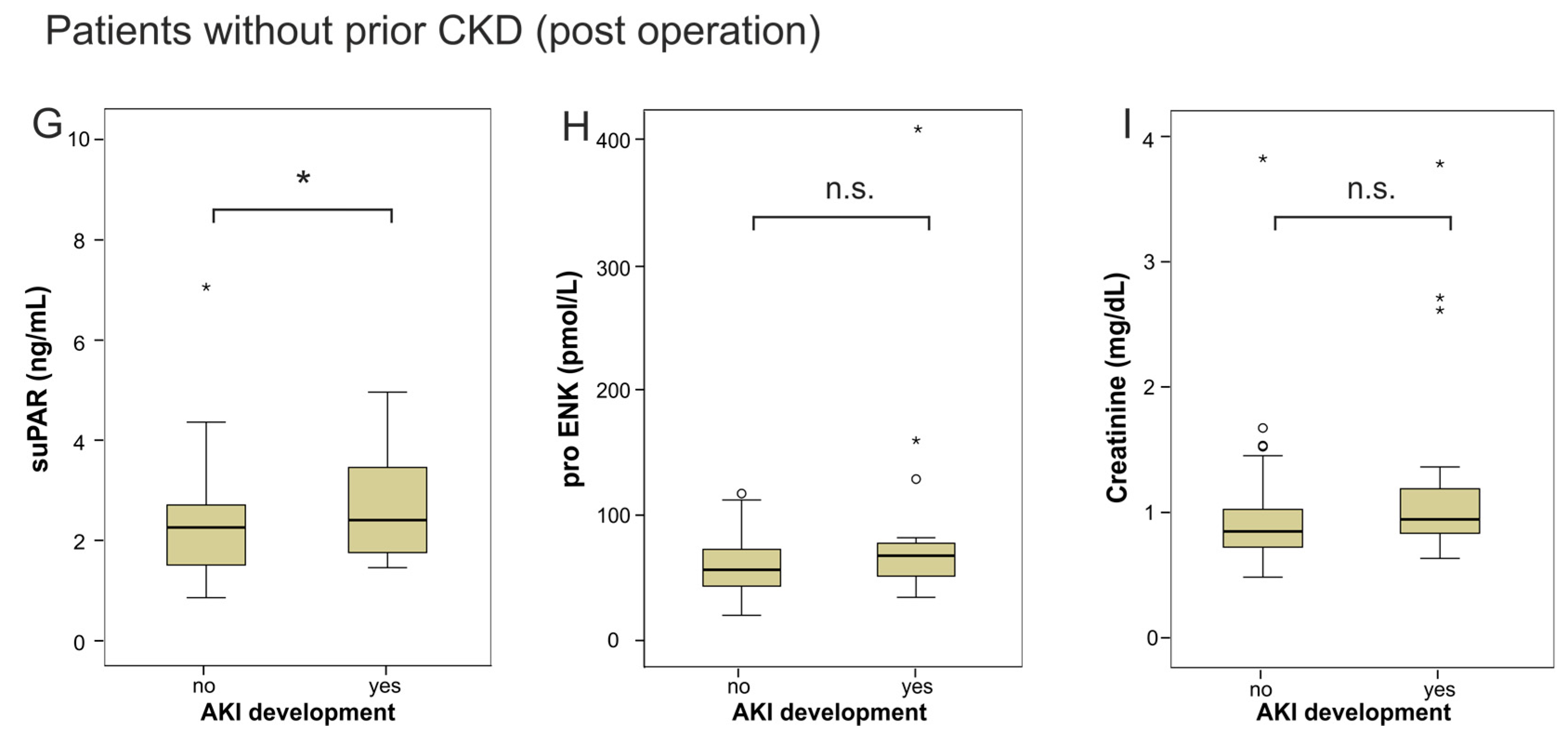
| Parameter | All Patients | Non-AKI | AKI | p-Value |
|---|---|---|---|---|
| n = 107 | n = 86 | n = 21 | ||
| Demographics | ||||
| Sex (male/female) | 77/30 | 62/24 | 15/6 | 0.952 |
| Age median (IQR) (years) | 69 (61–76) | 67 (61–75) | 75 (66–79) | 0.062 |
| Body mass index (IQR) (BMI) | 27 (24–30) | 27 (24–30) | 27 (25–30) | 0.613 |
| 30 days Mortality n (%) | 1 (1) | 0 (0) | 1 (5) | 0.186 |
| 90 days Mortality n (%) | 3 (3) | 1 (1) | 2 (10) | 0.110 |
| Surgery and ICU observation | ||||
| CABG n (%) | 54 (51) | 47 (55) | 7 (32) | 0.080 |
| CABG + AVR n (%) | 17 (16) | 10 (12) | 7 (32) | 0.015 |
| AVR n (%) | 9 (8) | 6 (7) | 3 (14) | 0.279 |
| Bentall, David or Hemashield n (%) | 8 (7) | 7 (8) | 1 (5) | 0.598 |
| Other cardiac surgery n (%) | 28 (26) | 16 (19) | 3 (13) | 0.642 |
| Ischemia time (IQR) (min) | 79 (60–110) | 79 (60–109) | 83 (60–114) | 0.750 |
| Time of CBP (IQR) (min) | 125 (101–156) | 125 (100–160) | 124 (101–156) | 0.729 |
| Total time of surgery (min) | 251 (220–290) | 252 (217–287) | 249 (220–315) | 0.476 |
| Postoperative period | ||||
| ICU days median (IQR) (days) | 3 (1–12) | 3 (1–10) | 5 (2–12) | <0.001 |
| SAPS day1 median (IQR) | 29 (26–35) | 29 (25–33) | 35 (28–39) | 0.027 |
| SAPS day4 median (IQR) | 24 (19–31) | 22 (17–28) | 29 (21–37) | 0.060 |
| SOFA day1 median (IQR) | 5 (3–7) | 5 (3–6) | 6 (5–7) | 0.011 |
| SOFA day4 median (IQR) | 0 (0–3) | 0 (0–2) | 3 (1–5) | <0.001 |
| Nephrotoxic antibiotics n (%) | 3 (3) | 2 (2) | 1 (5) | 0.484 |
| Dialysis n (%) | 3 (3) | 2 (2) | 1 (5) | 0.484 |
| AKI stages | ||||
| Stage I n (%) | 17 (16) | 0 (0) | 17 (81) | |
| Stage II n (%) | 3 (3) | 0 (0) | 3 (14) | |
| Stage III n (%) | 1 (1) | 0 (0) | 1 (5) | |
| Comorbidities | ||||
| Diabetes n (%) | 39 (36) | 32 (37) | 7 (33) | 0.741 |
| Hypertension n (%) | 81 (76) | 64 (74) | 17 (81) | 0.531 |
| Chronic kidney disease n (%) | 10 (9) | 5 (6) | 5 (23) | 0.011 |
| Medication | ||||
| Diuretics use n (%) | 54 (50) | 37 (43) | 17 (81) | 0.002 |
| β–blocker use n (%) | 75 (70) | 61 (71) | 14 (67) | 0.702 |
| AT II receptor antagonist use n (%) | 24 (22) | 16 (19) | 8 (36) | 0.055 |
| ACE inhibitor use n (%) | 52 (49) | 43 (50) | 9 (43) | 0.557 |
| Statin use n (%) | 67 (63) | 53 (62) | 14 (64) | 0.669 |
| Calcium channel blocker use n (%) | 24 (22) | 16 (9) | 8 (36) | 0.055 |
| Laboratory parameters | ||||
| WBC median (IQR) (×103 µL−1) | 7.9 (6.4–10.5) | 7.5 (6.2–9.9) | 9.0 (8.2–13.3) | 0.009 |
| Creatinine pre-OP (IQR) (mg·dL−1) | 1 (0.74–1.1) | 0.9 (0.74–1.1) | 1 (0.7–1.1) | 0.269 |
| eGFR pre-OP (IQR) (mL·min−1) | 75 (63–90) | 77 (65–90) | 70 (48–90) | 0.233 |
| suPAR pre-OP (IQR) (ng·mL−1) | 2.4 (1.7–3.1) | 2.3 (1.6–2.8) | 2.8 (2.3–3.4) | 0.021 |
| proENK pre-OP (IQR) (pmol·L−1) | 85 (72–111) | 84 (71–105) | 96 (77–127) | 0.037 |
| Cut-Off | Sensitivity | Specificity | Youden Index | LHR+ | LHR− | Diagnostic Odds Ratio | |
|---|---|---|---|---|---|---|---|
| suPAR | 2.45 | 0.73 | 0.57 | 0.30 | 1.70 | 0.47 | 3.58 |
| proENK | 93.2 | 0.59 | 0.65 | 0.24 | 1.69 | 0.63 | 2.67 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mossanen, J.C.; Pracht, J.; Jansen, T.U.; Buendgens, L.; Stoppe, C.; Goetzenich, A.; Struck, J.; Autschbach, R.; Marx, G.; Tacke, F. Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery. Int. J. Mol. Sci. 2017, 18, 1662. https://doi.org/10.3390/ijms18081662
Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, Struck J, Autschbach R, Marx G, Tacke F. Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery. International Journal of Molecular Sciences. 2017; 18(8):1662. https://doi.org/10.3390/ijms18081662
Chicago/Turabian StyleMossanen, Jana C., Jessica Pracht, Tobias U. Jansen, Lukas Buendgens, Christian Stoppe, Andreas Goetzenich, Joachim Struck, Rüdiger Autschbach, Gernot Marx, and Frank Tacke. 2017. "Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery" International Journal of Molecular Sciences 18, no. 8: 1662. https://doi.org/10.3390/ijms18081662
APA StyleMossanen, J. C., Pracht, J., Jansen, T. U., Buendgens, L., Stoppe, C., Goetzenich, A., Struck, J., Autschbach, R., Marx, G., & Tacke, F. (2017). Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery. International Journal of Molecular Sciences, 18(8), 1662. https://doi.org/10.3390/ijms18081662







