The Role of Ghrelin and Ghrelin Signaling in Aging
Abstract
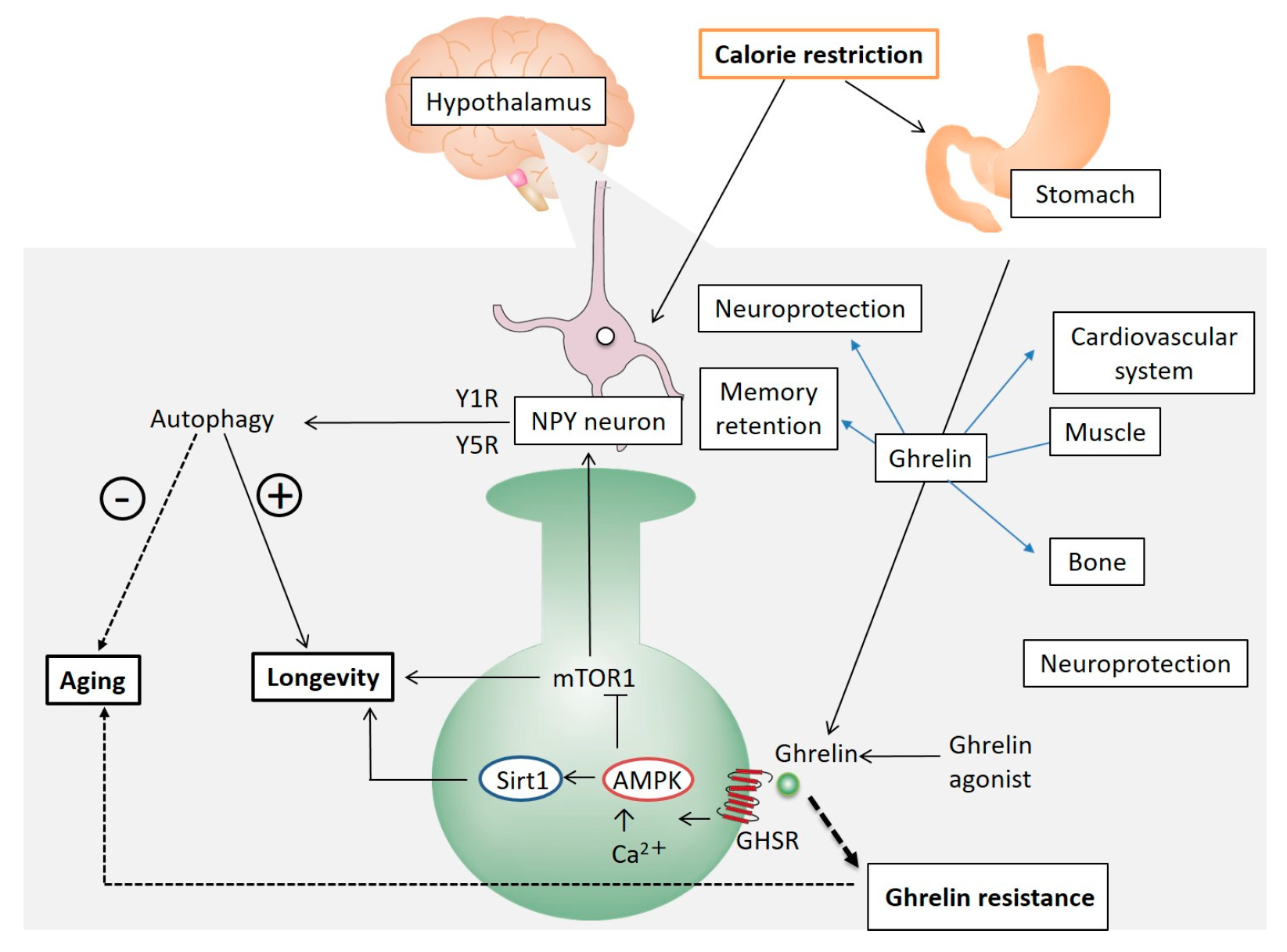
:1. Introduction
2. Aging and Longevity
2.1. Evolution and Aging
2.2. Calories and Longevity
2.3. IGF-1 and Other Age-Related Factors
2.3.1. GH and IGF-1
2.3.2. Sirtuin
2.3.3. Klotho
2.3.4. TOR
3. Ghrelin and Aging
3.1. Ghrelin and NPY
3.2. Sarcopenia and Frailty
3.3. Ghrelin and Memory
4. Effect of CR Mimetics and Ghrelin Agonists on Longevity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgRP | Agouti-related protein |
| AMPK | Adenosine monophosphate-activated protein kinase |
| cAMP | Cyclic adenosine monophosphate |
| CR | Calorie restriction |
| FFA | Free fatty acid |
| FOXO | Forkhead box O |
| GHSR | Growth hormone secretagogue receptor |
| GHSR-1a | Growth hormone secretagogue receptor-1a |
| GLP-1 | Glucagon-like peptide 1 |
| HFD | High fat diet |
| IGF-1 | Insulin-like growth factor 1 |
| Irs2 | Insulin receptor substrate 2 |
| NAD | Nicotinamide adenine dinucleotide |
| NPY | Neuropeptide Y |
| MC4R | Melanocortin 4 receptor |
| mTOR1 | Mammalian TOR 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PGC | Peroxisome proliferator-activated receptor-gamma coactivator |
| POMC | Proopiomelanocortin |
| Ppar | Peroxisome proliferator-activated receptor |
| SAMP8 | Senescence-accelerated mouse prone/8 |
| S6K1 | Ribosomal protein S6 kinase 1 |
| TOR | Target of rapamycin |
| TORC1 | TOR complex 1 |
| TORC2 | TOR complex 2 |
References
- Heemels, M.T. Ageing. Nature 2010, 464, 503. [Google Scholar] [CrossRef] [PubMed]
- Herskind, A.M.; McGue, M.; Holm, N.V.; Sorensen, T.I.; Harvald, B.; Vaupel, J.W. The heritability of human longevity: A population-based study of 2872 danish twin pairs born 1870–1900. Hum. Genet. 1996, 97, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhao, J.H.; Zhang, D.; Kruse, T.A.; Christensen, K. Power for genetic association study of human longevity using the case-control design. Am. J. Epidemiol. 2008, 168, 890–896. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Ageing 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Cheng, K.C.; Li, Y.X.; Asakawa, A.; Inui, A. The role of ghrelin in energy homeostasis and its potential clinical relevance (review). Int. J. Mol. Med. 2010, 26, 771–778. [Google Scholar] [PubMed]
- Kaplan, R.C.; Strizich, G.; Aneke-Nash, C.; Dominguez-Islas, C.; Buzkova, P.; Strickler, H.; Rohan, T.; Pollak, M.; Kuller, L.; Kizer, J.R.; et al. Insulinlike growth factor binding protein-1 and ghrelin predict health outcomes among older adults: Cardiovascular health study cohort. J. Clin. Endocrinol. Metab. 2017, 102, 267–278. [Google Scholar] [PubMed]
- Friedman, D.B.; Johnson, T.E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 1988, 118, 75–86. [Google Scholar] [PubMed]
- Morris, J.Z.; Tissenbaum, H.A.; Ruvkun, G. A phosphatidylinositol-3-oh kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 1996, 382, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Bartke, A.; Antebi, A. The endocrine regulation of aging by insulin-like signals. Science 2003, 299, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Muller, F. Genetics: Influence of tor kinase on lifespan in C. Elegans. Nature 2003, 426, 620. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Guarente, L. Calorie restriction, sirt1 and metabolism: Understanding longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935, 10, 63–79. [Google Scholar]
- Mair, W.; Dillin, A. Aging and survival: The genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008, 77, 727–754. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the nia study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.E.; Merry, B.J. The molecular basis by which dietary restricted feeding reduces mitochondrial reactive oxygen species generation. Mech. Ageing Dev. 2011, 132, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Xu, X.; Ingram, R.L.; D’Costa, A. Moderate caloric restriction alters the subcellular distribution of somatostatin mrna and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology 1995, 61, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Wilcox, B.J.; Wilcox, C.D. Implications from and for food cultures for cardiovascular disease: Longevity. Asia Pac. J. Clin. Nutr. 2001, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005, 293, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Bouret, S.G.; Andrews, Z.B. Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol. Metab. 2016, 27, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: The calerie 2 randomized clinical trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Leese, P.T.; Trang, J.M.; Blum, R.A.; de Groot, E. An open-label clinical trial of the effects of age and gender on the pharmacodynamics, pharmacokinetics and safety of the ghrelin receptor agonist anamorelin. Clin. Pharmacol. Drug Dev. 2015, 4, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Lakowski, B.; Hekimi, S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science 1996, 272, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Defossez, P.A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of chico, a drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, A.P.; Lenham, J.E.; Ingram, R.L.; Sonntag, W.E. Moderate caloric restriction increases type 1 IGF receptors and protein synthesis in aging rats. Mech. Ageing Dev. 1993, 71, 59–71. [Google Scholar] [CrossRef]
- Rudman, D.; Feller, A.G.; Nagraj, H.S.; Gergans, G.A.; Lalitha, P.Y.; Goldberg, A.F.; Schlenker, R.A.; Cohn, L.; Rudman, I.W.; Mattson, D.E. Effects of human growth hormone in men over 60 years old. N. Engl. J. Med. 1990, 323, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, W.L.; Aziz, M.; Ma, G.; Wang, P. Therapeutic effect of human ghrelin and growth hormone: Attenuation of immunosuppression in septic aged rats. Biochim. Biophys. Acta 2017. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Bonelli, L.; Marinazzo, E.; Ghigo, E.; Arvat, E. Growth hormone treatment in human ageing: Benefits and risks. Hormones 2008, 7, 133–139. [Google Scholar] [PubMed]
- Liu, H.; Bravata, D.M.; Olkin, I.; Nayak, S.; Roberts, B.; Garber, A.M.; Hoffman, A.R. Systematic review: The safety and efficacy of growth hormone in the healthy elderly. Ann. Intern. Med. 2007, 146, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Thorner, M.O. Statement by the growth hormone research society on the GH/IGF-I axis in extending health span. J. Gerontol. Biol. Sci. Med. Sci. 2009, 64, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Rogina, B.; Helfand, S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tissenbaum, H.A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006, 127, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Verdin, E. Sirtuins: Critical regulators at the crossroads between cancer and aging. Oncogene 2007, 26, 5489–5504. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J.; et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Imai, S. SIRT1 and caloric restriction: An insight into possible trade-offs between robustness and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Ungvari, Z.; Minor, R.K.; Le Couteur, D.G.; de Cabo, R. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 2012, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Toorie, A.M.; Nillni, E.A. Minireview: Central sirt1 regulates energy balance via the melanocortin system and alternate pathways. Mol. Endocrinol. 2014, 28, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Imai, S. Hypothalamic sirt1 in aging. Aging 2014, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Antunes, C.; Geliang, G.; Liu, Z.W.; Borok, E.; Nie, Y.; Xu, A.W.; Souza, D.O.; Gao, Q.; Diano, S.; et al. Agrp neurons mediate sirt1’s action on the melanocortin system and energy balance: Roles for sirt1 in neuronal firing and synaptic plasticity. J. Neurosci. 2010, 30, 11815–11825. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, K.; Fujimori, T.; Kurotaki, Y.; Honjo, H.; Tsujikawa, H.; Yasui, K.; Lee, J.K.; Kamiya, K.; Kitaichi, K.; Yamamoto, K.; et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 2004, 109, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Manabe, N.; Miyaura, C.; Chikuda, H.; Nakamura, K.; Kuro-o, M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J. Clin. Investig. 1999, 104, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamada, K.; Kim, H.C.; Kim, Y.S.; Noda, Y.; Imura, A.; Nabeshima, Y.; Nabeshima, T. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J. 2003, 17, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Kurabayashi, M.; Sando, Y.; Ohyama, Y.; Maeno, T.; Maeno, Y.; Aizawa, H.; Matsumura, Y.; Kuwaki, T.; Kuro, O.M.; et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 2000, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Asakawa, A.; Amitani, H.; Kaimoto, K.; Sameshima, N.; Koyama, K.I.; Haruta, I.; Tsai, M.; Nakahara, T.; Ushikai, M.; et al. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition 2013, 29, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. Mtor signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mtor. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Regulation of the aging process by autophagy. Trends Mol. Med. 2009, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hands, S.L.; Proud, C.G.; Wyttenbach, A. Mtor’s role in ageing: Protein synthesis or autophagy? Aging 2009, 1, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Chen, D.; Riddle, D.L. The TOR pathway interacts with the insulin signaling pathway to regulate C. Elegans larval development, metabolism and life span. Development 2004, 131, 3897–3906. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.Z.; Palter, J.E.; Rogers, A.N.; Olsen, A.; Chen, D.; Lithgow, G.J.; Kapahi, P. Inhibition of mrna translation extends lifespan in Caenorhabditis elegans. Aging Cell 2007, 6, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Guarente, L. Mtorc1 and sirt1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Maurer, A.D.; Lau, D.C.; Auer, R.N. Long-term dietary restriction influences plasma ghrelin and goat mrna level in rats. Physiol. Behav. 2010, 99, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.C.; Wilson, P.W.; Smulders, T.V.; Sandilands, V.; D’Eath, R.B.; Boswell, T. Hypothalamic agouti-related protein expression is affected by both acute and chronic experience of food restriction and re-feeding in chickens. J. Neuroendocrinol. 2013, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Marques, M.; Aveleira, C.A.; Carmo-Silva, S.; Botelho, M.; Pereira de Almeida, L.; Cavadas, C. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging 2016, 8, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.A.; Botelho, M.; Carmo-Silva, S.; Pascoal, J.F.; Ferreira-Marques, M.; Nobrega, C.; Cortes, L.; Valero, J.; Sousa-Ferreira, L.; Alvaro, A.R.; et al. Neuropeptide y stimulates autophagy in hypothalamic neurons. Proc. Natl. Acad. Sci. USA 2015, 112, E1642–E1651. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.A.; Botelho, M.; Cavadas, C. NPY/neuropeptide Y enhances autophagy in the hypothalamus: A mechanism to delay aging? Autophagy 2015, 11, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Veyrat-Durebex, C.; Quirion, R.; Ferland, G.; Dumont, Y.; Gaudreau, P. Aging and long-term caloric restriction regulate neuropeptide Y receptor subtype densities in the rat brain. Neuropeptides 2013, 47, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Tamashiro, Y.; Park, D.; Kusudo, T.; Fujie, R.; Komatsu, T.; Kim, S.E.; Park, S.; Hayashi, H.; Mori, R.; et al. A key role for neuropeptide y in lifespan extension and cancer suppression via dietary restriction. Sci. Rep. 2014, 4, 4517. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2001, 2, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Inui, A.; Asakawa, A.; Kihara, N.; Fujimura, M.; Fujimiya, M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J. Physiol. 2003, 550, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Tokudome, T.; Hosoda, H.; Miyazato, M.; Kangawa, K. Ghrelin and cardiovascular diseases. J. Cardiol. 2012, 59, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K.; Hosoda, H.; Kakei, M.; Hashiguchi, S.; Watanabe, M.; Kangawa, K.; Yada, T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: Implication in the glycemic control in rodents. Diabetes 2004, 53, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nishi, M.; Doi, A.; Shono, T.; Furukawa, Y.; Shimada, T.; Furuta, H.; Sasaki, H.; Nanjo, K. Ghrelin inhibits insulin secretion through the AMPK-UCP2 pathway in β cells. FEBS Lett. 2010, 584, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Priego, T.; Martin, A.I.; Villanua, M.A.; Lopez-Calderon, A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E486–E492. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, W. The role of ghrelin in senescence: A mini-review. Gerontology 2016, 62, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Molfino, A.; Rianda, S.; Rossi Fanelli, F. The growth hormone secretagogue receptor (Ghs-R). Curr. Pharm. Des. 2012, 18, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Andrews, Z.B. Ghrelin is the metabolic link connecting calorie restriction to neuroprotection. Neural Regen. Res. 2016, 11, 1228–1229. [Google Scholar] [PubMed]
- Rogers, N.H.; Walsh, H.; Alvarez-Garcia, O.; Park, S.; Gaylinn, B.; Thorner, M.O.; Smith, R.G. Metabolic benefit of chronic caloric restriction and activation of hypothalamic AGRP/NPY neurons in male mice is independent of ghrelin. Endocrinology 2016, 157, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Mavrikaki, M.; Southern, M.R.; Smith, R.G.; Butler, A.A. Assessing interactions between ghsr and Mc3r reveals a role for AgRP in the expression of food anticipatory activity in male mice. Endocrinology 2014, 155, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lee, J.H.; Bongmba, O.Y.; Ma, X.; Zhu, X.; Sheikh-Hamad, D.; Sun, Y. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging 2014, 6, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Bongmba, O.Y.N.; Yue, J.; Lee, J.H.; Lin, L.; Saito, K.; Pradhan, G.; Li, D.P.; Pan, H.L.; Xu, A.; et al. Suppression of GHS-R in AgRP neurons mitigates diet-induced obesity by activating thermogenesis. Int. J. Mol. Sci. 2017, 18, 832. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Li, S.M.; Du, F.M.; Zhu, Z.C.; Zhang, J.C.; Li, Y.X. Ghrelin and obestatin levels in hypertensive obese patients. J. Int. Med. Res. 2014, 42, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Dieguez, C.; Vazquez, M.J.; Romero, A.; Lopez, M.; Nogueiras, R. Hypothalamic control of lipid metabolism: Focus on leptin, ghrelin and melanocortins. Neuroendocrinology 2011, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, S.; Ueno, N.; Dube, M.G.; Kalra, P.; Kalra, S. Leptin gene transfer in the hypothalamus enhances longevity in adult monogenic mutant mice in the absence of circulating leptin. Neurobiol. Aging 2007, 28, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.I.; Lockie, S.H.; Benzler, J.; Wu, Q.; Stark, R.; Reichenbach, A.; Hoy, A.J.; Lemus, M.B.; Coleman, H.A.; Parkington, H.C.; et al. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology 2014, 155, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Muto, S.; Hattori, T.; Sadakane, C.; Tsuchiya, K.; Katsurada, T.; Ohkawara, T.; Oridate, N.; Asaka, M. Rikkunshito ameliorates the aging-associated decrease in ghrelin receptor reactivity via phosphodiesterase III inhibition. Endocrinology 2010, 151, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Metter, E.J.; Roth, G.S.; Ingram, D.K.; Mattison, J.A.; Taub, D.D.; Ferrucci, L. Relationship between plasma ghrelin, insulin, leptin, interleukin 6, adiponectin, testosterone and longevity in the baltimore longitudinal study of aging. Aging Clin. Exp. Res. 2011, 23, 153–158. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, C.; Gregoire, F.; Lema-Kisoka, R.; Waelbroeck, M.; Robberecht, P.; Delporte, C. Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 2004, 145, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Haschemi, A.; Marculescu, R.; Endler, G.; Maurer, G.; Wagner, O.; Huber, K.; Mannhalter, C.; Niessner, A. Butyrylcholinesterase activity predicts long-term survival in patients with coronary artery disease. Clin. Chem. 2012, 58, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Brimijoin, S.; Chen, V.P.; Pang, Y.P.; Geng, L.; Gao, Y. Physiological roles for butyrylcholinesterase: A BChE-ghrelin axis. Chem.-Biol. Interact. 2016, 259, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Andersson, M.; Iresjo, B.M.; Lonnroth, C.; Lundholm, K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int. J. Oncol. 2006, 28, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Splenser, A.; Guillory, B.; Luo, J.; Mendiratta, M.; Belinova, B.; Halder, T.; Zhang, G.; Li, Y.P.; Garcia, J.M. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved. J. Cachexia Sarcopenia Muscle 2015, 6, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Trombetta, A.; Dentelli, P.; Cotogni, P.; Rosso, A.; Tschop, M.H.; Granata, R.; Ghigo, E.; Brizzi, M.F. Unacylated ghrelin promotes skeletal muscle regeneration following hindlimb ischemia via SOD-2-mediated miR-221/222 expression. J. Am. Heart Assoc. 2013, 2, e000376. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Pei, X.M.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Siu, P.M. Unacylated ghrelin restores insulin and autophagic signaling in skeletal muscle of diabetic mice. Pflug. Arch. 2015, 467, 2555–2569. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lee, J.H.; Buras, E.D.; Yu, K.; Wang, R.; Smith, C.W.; Wu, H.; Sheikh-Hamad, D.; Sun, Y. Ghrelin receptor regulates adipose tissue inflammation in aging. Aging 2016, 8, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Guillory, B.; Chen, J.A.; Patel, S.; Luo, J.; Splenser, A.; Mody, A.; Ding, M.; Baghaie, S.; Anderson, B.; Iankova, B.; et al. Deletion of ghrelin prevents aging-associated obesity and muscle dysfunction without affecting longevity. Aging Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Papiol, M.; Monteis, R.; Palomera, E.; Cabre, M. Relationship between plasma ghrelin levels and sarcopenia in elderly subjects: A cross-sectional study. J. Nutr. Health Aging 2015, 19, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Pezzoli, S.S.; Oliveri, M.C.; Patrie, J.T.; Harrell, F.E., Jr.; Clasey, J.L.; Heymsfield, S.B.; Bach, M.A.; Vance, M.L.; Thorner, M.O. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: A randomized trial. Ann. Intern. Med. 2008, 149, 601–611. [Google Scholar] [CrossRef] [PubMed]
- White, H.K.; Petrie, C.D.; Landschulz, W.; MacLean, D.; Taylor, A.; Lyles, K.; Wei, J.Y.; Hoffman, A.R.; Salvatori, R.; Ettinger, M.P.; et al. Effects of an oral growth hormone secretagogue in older adults. J. Clin. Endocrinol. Metab. 2009, 94, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Varadhan, R.; Weiss, C.O.; Fried, L.P.; Cappola, A.R. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J. Nutr. Health Aging 2012, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.; van der Velde, M.; van der Eerden, B.C.; Sun, Y.; Geminn, J.M.; van der Lely, A.J.; Smith, R.G.; van Leeuwen, J.P. Genetic manipulation of the ghrelin signaling system in male mice reveals bone compartment specificity of acylated and unacylated ghrelin in the regulation of bone remodeling. Endocrinology 2014, 155, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, M.; van der Eerden, B.C.; Sun, Y.; Almering, J.M.; van der Lely, A.J.; Delhanty, P.J.; Smith, R.G.; van Leeuwen, J.P. An age-dependent interaction with leptin unmasks ghrelin’s bone-protective effects. Endocrinology 2012, 153, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Sun, Y.; Jiang, H.; Albarran-Zeckler, R.; Timchenko, N. Ghrelin receptor (GHS-R1A) agonists show potential as interventive agents during aging. Ann. N. Y. Acad. Sci. 2007, 1119, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Monzon, M.E.; Varas, M.M.; Cragnolini, A.B.; Schioth, H.B.; Scimonelli, T.N.; de Barioglio, S.R. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem. Biophys. Res. Commun. 2002, 299, 739–743. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Varas, M.M.; Cragnolini, A.B.; Schioth, H.B.; Scimonelli, T.N.; de Barioglio, S.R. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem. Biophys. Res. Commun. 2004, 313, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Gaydou, R.C.; Schioth, H.B.; de Barioglio, S.R. Selective serotonin reuptake inhibitor (fluoxetine) decreases the effects of ghrelin on memory retention and food intake. Regul. Pept. 2007, 140, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Patterson, Z.R.; Ducharme, R.; Anisman, H.; Abizaid, A. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor-deficient mice. Eur. J. Neurosci. 2010, 32, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Diano, S.; Farr, S.A.; Benoit, S.C.; McNay, E.C.; da Silva, I.; Horvath, B.; Gaskin, F.S.; Nonaka, N.; Jaeger, L.B.; Banks, W.A.; et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, P.; Carlini, V.P.; Schioth, H.B.; de Barioglio, S.R.; Salvatierra, N.A. Central ghrelin increases anxiety in the open field test and impairs retention memory in a passive avoidance task in neonatal chicks. Neurobiol. Learn. Mem. 2009, 91, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Martini, A.C.; Schioth, H.B.; Ruiz, R.D.; Fiol de Cuneo, M.; de Barioglio, S.R. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience 2008, 153, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C.; Alvarez-Crespo, M.; Taube, M.; Skibicka, K.P.; Schmidt, L.; Karlsson-Lindahl, L.; Egecioglu, E.; Nissbrandt, H.; Dickson, S.L. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology 2014, 79, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.A. Synchronizing an aging brain: Can entraining circadian clocks by food slow alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Fortin, S.M.; Ricks, K.M.; Grill, H.J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 2013, 73, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, I.I.; le Feber, J. Ghrelin accelerates synapse formation and activity development in cultured cortical networks. BMC Neurosci. 2014, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.S.; Xie, D.; Liu, K.; Chen, L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/reperfusion. Chin. J. Physiol. 2006, 49, 244–250. [Google Scholar] [PubMed]
- Chen, L.; Xing, T.; Wang, M.; Miao, Y.; Tang, M.; Chen, J.; Li, G.; Ruan, D.Y. Local infusion of ghrelin enhanced hippocampal synaptic plasticity and spatial memory through activation of phosphoinositide 3-kinase in the dentate gyrus of adult rats. Eur. J. Neurosci. 2011, 33, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Berrout, L.; Isokawa, M. Ghrelin promotes reorganization of dendritic spines in cultured rat hippocampal slices. Neurosci. Lett. 2012, 516, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Laszlo, K.; Lenard, L. Role of intraamygdaloid acylated-ghrelin in spatial learning. Brain Res. Bull. 2010, 81, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Albarran-Zeckler, R.G.; Brantley, A.F.; Smith, R.G. Growth hormone secretagogue receptor (GHS-R1A) knockout mice exhibit improved spatial memory and deficits in contextual memory. Behav. Brain Res. 2012, 232, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Imai, S. A possibility of nutriceuticals as an anti-aging intervention: Activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol. Res. 2010, 62, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Sadakane, C.; Saegusa, Y.; Nahata, M.; Hattori, T.; Asaka, M. Rikkunshito as a ghrelin enhancer. Methods Enzymol. 2012, 514, 333–351. [Google Scholar] [PubMed]
- Takeda, H.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Asaka, M. Rikkunshito and ghrelin secretion. Curr. Pharm. Des. 2012, 18, 4827–4838. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the kampo medicine rikkunshito on the model. PLoS ONE 2017, 12, e0173113. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Hayashi, M.; Sameshima, M.; Amitani, H.; Kojima, S.; Fujimiya, M.; Inui, A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol. Psychiatry 2009, 65, 748–759. [Google Scholar] [CrossRef] [PubMed]
| Formula | Model | Reported Outcome | Study | References |
|---|---|---|---|---|
| Ghrelin | Rat cortical neurons | Stimulation of network formation and activation in cortical neuronal networks | In vitro | Veyrat-Durebex C et al., 2013 [64] |
| Ghrelin and Ghrelin antagonist | Rat cortical neurons | Caloric restriction mimetic cell culture medium stimulated autophagy in rat cortical neurons and ghrelin receptor antagonists blocked this effect. On the other hand, exogenous ghrelin stimulated autophagy in rat cortical neurons. | In vitro | Ferreira-Marques M et al., 2016 [61] |
| Ghrelin | Normal rats | Increase in memory retention | In vivo | Carlini VP et al., 2002 [103] |
| Ghrelin | SAMP8 (Alzheimer’s disease model) | Improvement of retention on the T-maze foot shock avoidance task | In vivo | Diano et al., 2006 [108] |
| Ghrelin | Cerebral ischemia/reperfusion rat model | Increase in survival and reduce cell death of hippocampal neurons following ischemia/reperfusion injury | In vivo | Liu Y et al., 2006 [115] |
| Ghrelin | Normal rats | SSRI decreased the effects of ghrelin on memory retention | In vivo | Carlini VP et al., 2007 [106] |
| Ghrelin | Normal mice | Increase in the impaired memory of mice with 50% food restriction | In vivo | Carlini VP et al., 2008 [110] |
| Ghrelin mimetic | 6- and 75-week-old C57BL/6J mice | Amelioration of aging-associated anorexia in mice via inhibition of PDE3 | In vivo | Takeda et al., 2010 [84] |
| Ghrelin agonist and mimetic | Klotho-deficient, SAMP8 and ICR mice | Decrease in microglial activation in the brain and prolongation of survival in klotho-deficient, SAMP8 and aged ICR mice | In vitro and vivo | Fujitsuka et al., 2016 [58] |
| Ghrelin and GH | Septic aged rats | Prevention of the loss of splenic T cells and improvement of sepsis-induced immunosuppression | In vivo | Zhou et al., 2017 [30] |
| Ghrelin | Obese women | Obesity-linked reductions in ghrelin were reversed by weight loss achieved through caloric restriction | Clinical | Bayliss JA et al., 2016 [75] |
| Ghrelin mimetic | Healthy older adults, randomized, double-blind, placebo-controlled study | Increase in total body weight and lean body mass. However, no significant difference in muscle strength, function and quality of life | Clinical | Nass R et al., 2008 [97] |
| Ghrelin agonist | Healthy older adults, randomized, double-blind, placebo-controlled study | Increase in lean mass, tandem walk and stair climb | Clinical | White HK et al., 2009 [98] |
| BChE | Patients with coronary artery disease | Presentation of CAD affected the effect of BChE on mortality | Clinical | Goliasch G et al., 2012 [87] |
| Ghrelin | Healthy older adults, cohort study | Ghrelin measured during an OGTT predicted major health events and death in older adults | Clinical | Kaplan RC et al., 2017 [6] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amitani, M.; Amitani, H.; Cheng, K.-C.; Kairupan, T.S.; Sameshima, N.; Shimoshikiryo, I.; Mizuma, K.; Rokot, N.T.; Nerome, Y.; Owaki, T.; et al. The Role of Ghrelin and Ghrelin Signaling in Aging. Int. J. Mol. Sci. 2017, 18, 1511. https://doi.org/10.3390/ijms18071511
Amitani M, Amitani H, Cheng K-C, Kairupan TS, Sameshima N, Shimoshikiryo I, Mizuma K, Rokot NT, Nerome Y, Owaki T, et al. The Role of Ghrelin and Ghrelin Signaling in Aging. International Journal of Molecular Sciences. 2017; 18(7):1511. https://doi.org/10.3390/ijms18071511
Chicago/Turabian StyleAmitani, Marie, Haruka Amitani, Kai-Chun Cheng, Timothy Sean Kairupan, Nanami Sameshima, Ippei Shimoshikiryo, Kimiko Mizuma, Natasya Trivena Rokot, Yasuhito Nerome, Tetsuhiro Owaki, and et al. 2017. "The Role of Ghrelin and Ghrelin Signaling in Aging" International Journal of Molecular Sciences 18, no. 7: 1511. https://doi.org/10.3390/ijms18071511
APA StyleAmitani, M., Amitani, H., Cheng, K.-C., Kairupan, T. S., Sameshima, N., Shimoshikiryo, I., Mizuma, K., Rokot, N. T., Nerome, Y., Owaki, T., Asakawa, A., & Inui, A. (2017). The Role of Ghrelin and Ghrelin Signaling in Aging. International Journal of Molecular Sciences, 18(7), 1511. https://doi.org/10.3390/ijms18071511





