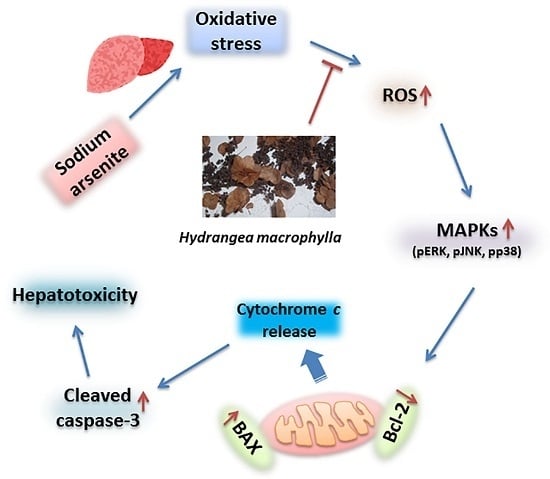
Hepatoprotective Role of Hydrangea macrophylla against Sodium Arsenite-Induced Mitochondrial-Dependent Oxidative Stress via the Inhibition of MAPK/Caspase-3 Pathways
Abstract
:1. Introduction
2. Results
2.1. Analysis of Total Phenolic and Flavonoid Content of HM
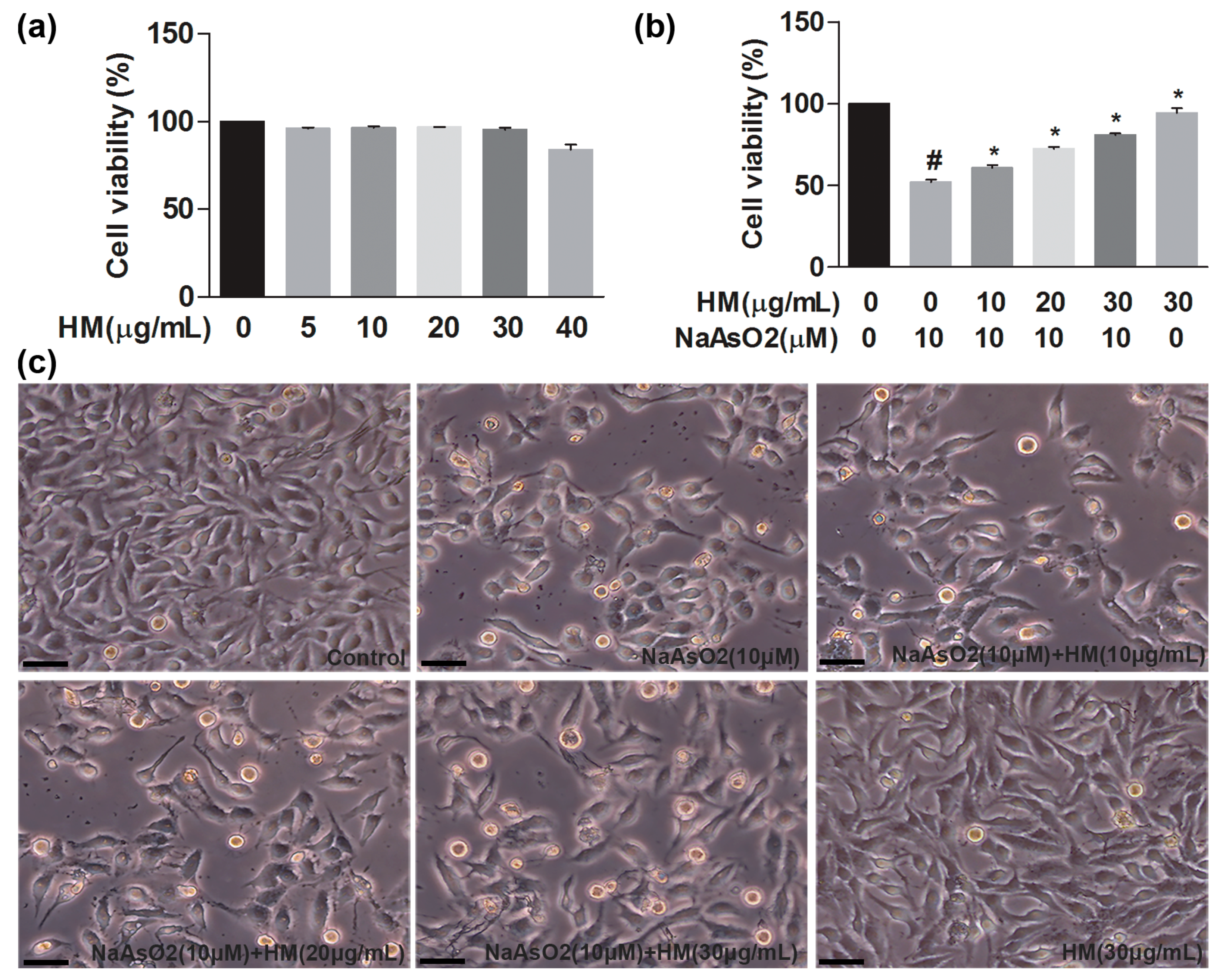
2.2. HM Reduced NaAsO2-Induced Oxidative Stress in Human Liver Cancer (HepG2) Cells
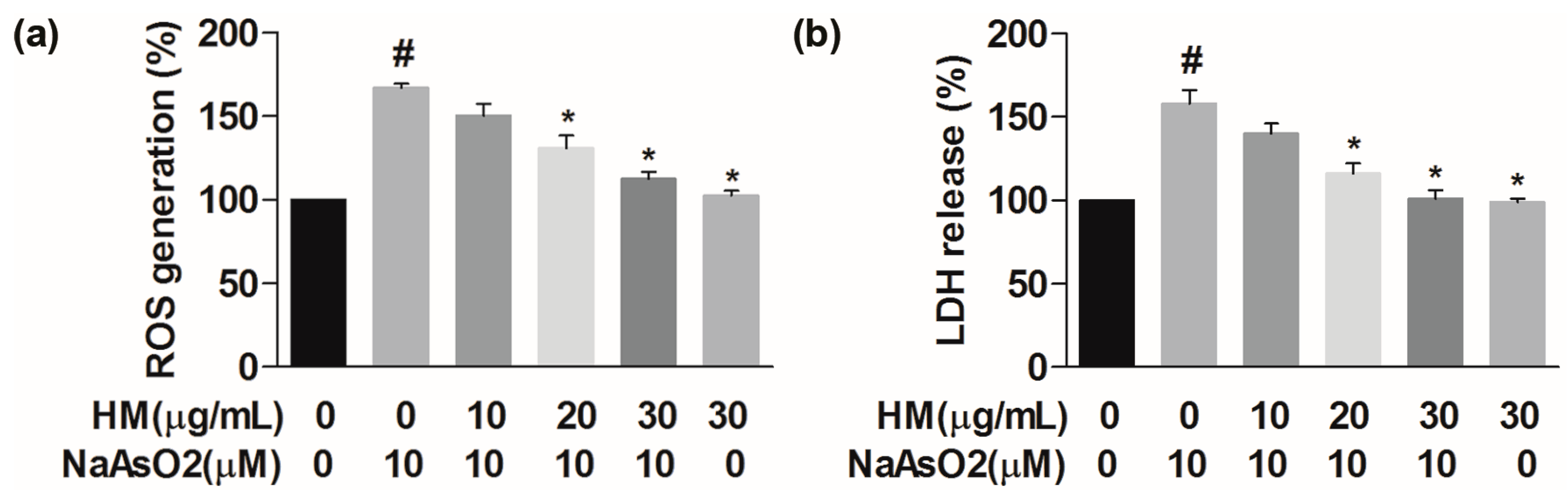
2.3. HM Decreased the Intracellular ROS Generation
2.4. HM Inhibited the Lactate Dehydrogenase (LDH) Release
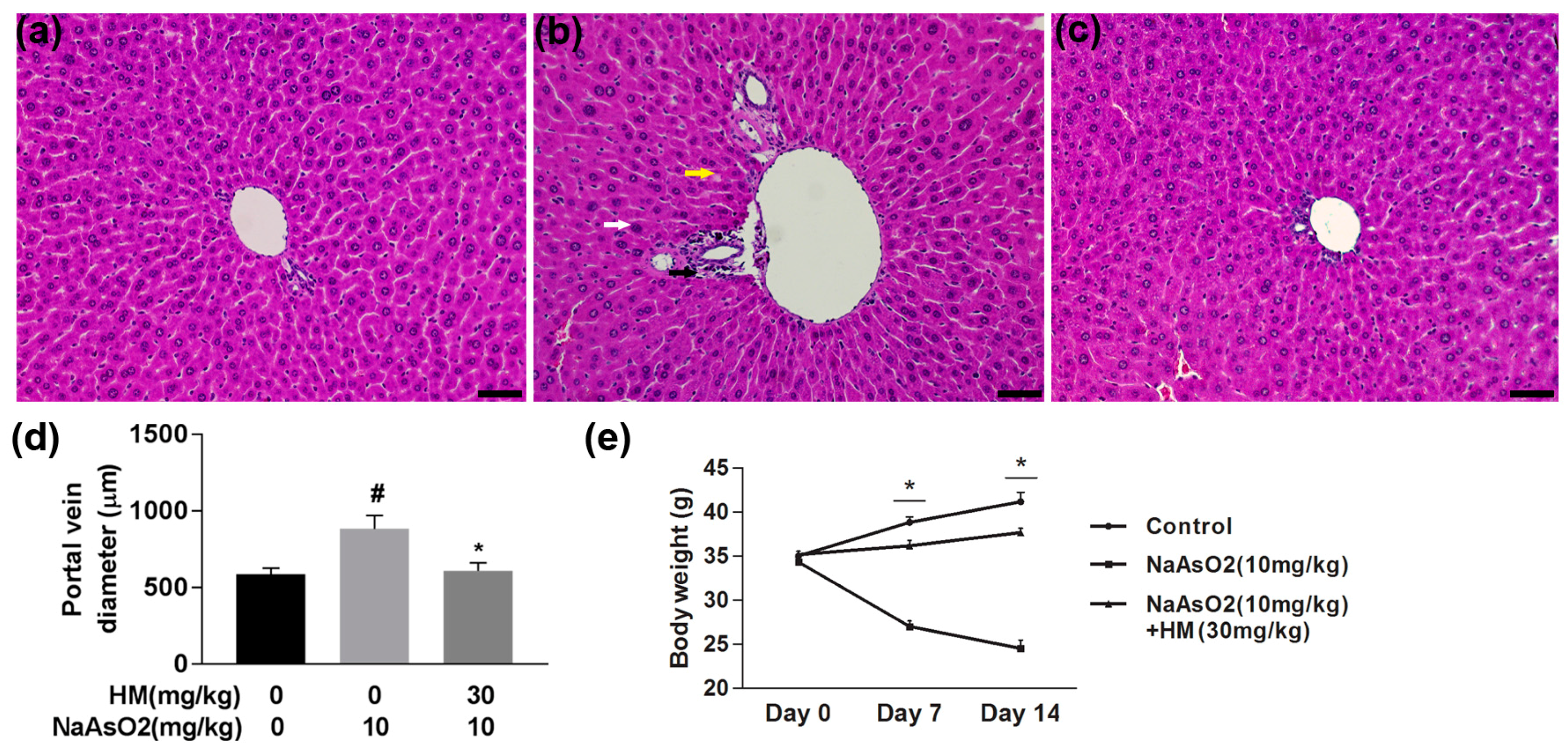
2.5. HM Improved the Liver Histopathology and Body Weight
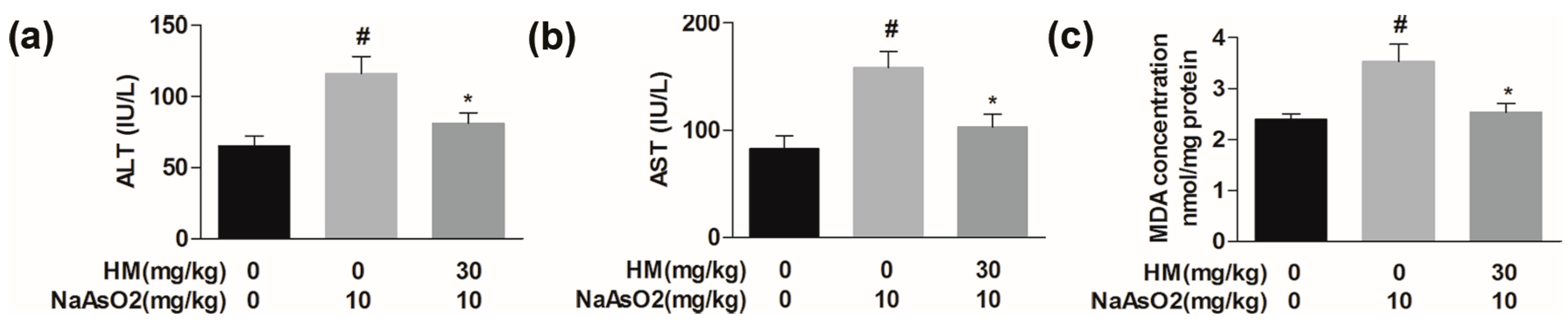
2.6. HM Regulated the Serum Biochemical Parameters
2.7. HM Controlled the Lipid Peroxidation Production
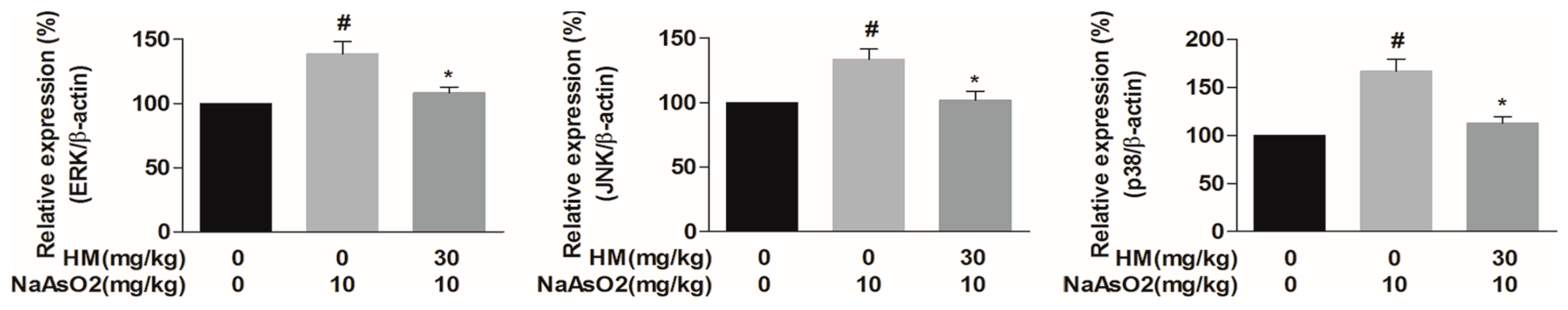
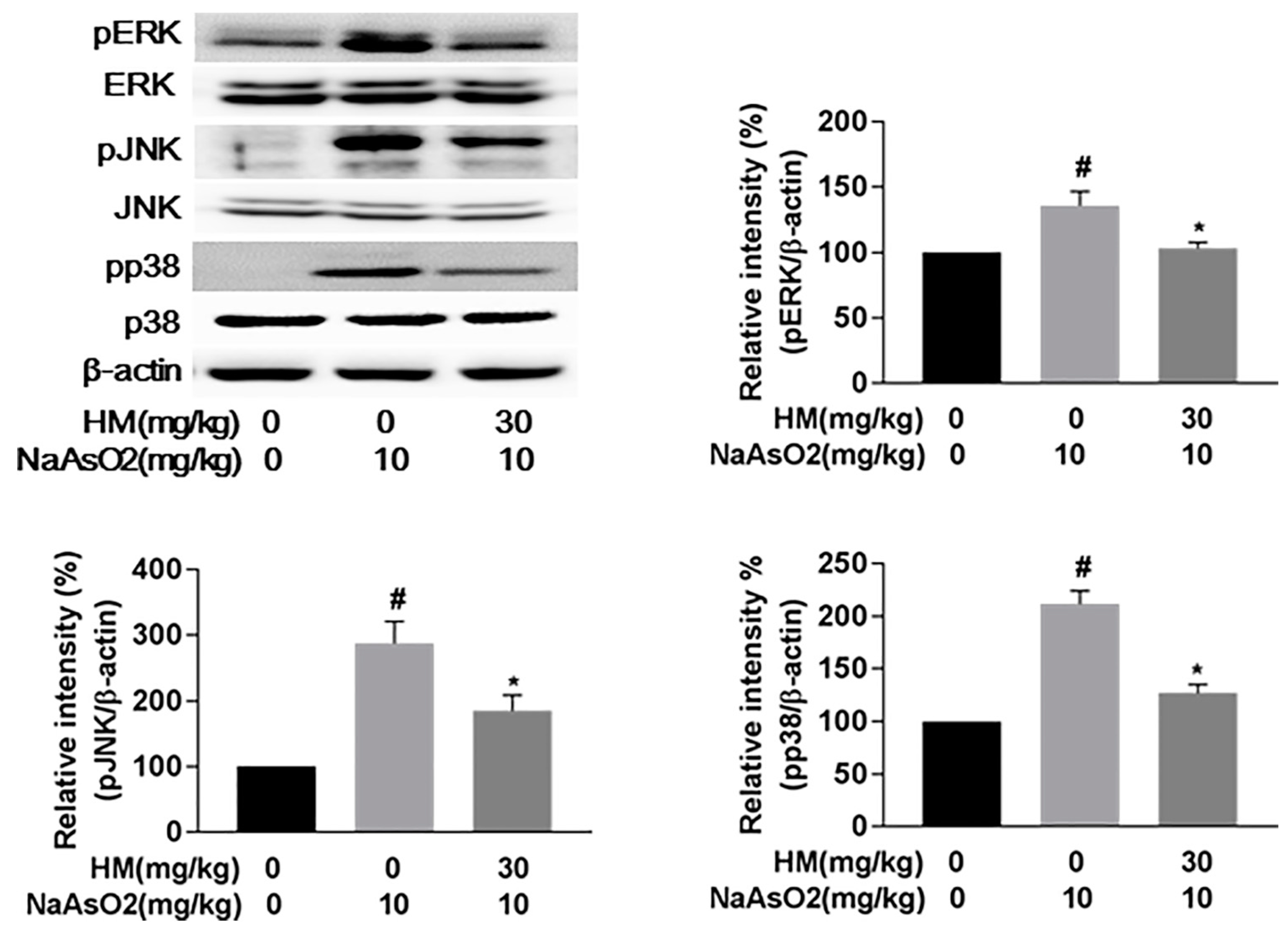
2.8. HM Suppressed the Gene Expression of MAPKs (Extracellular Signal-Regulated Kinases (ERK), C-Jun N-Terminal Kinases (JNK), and p38)
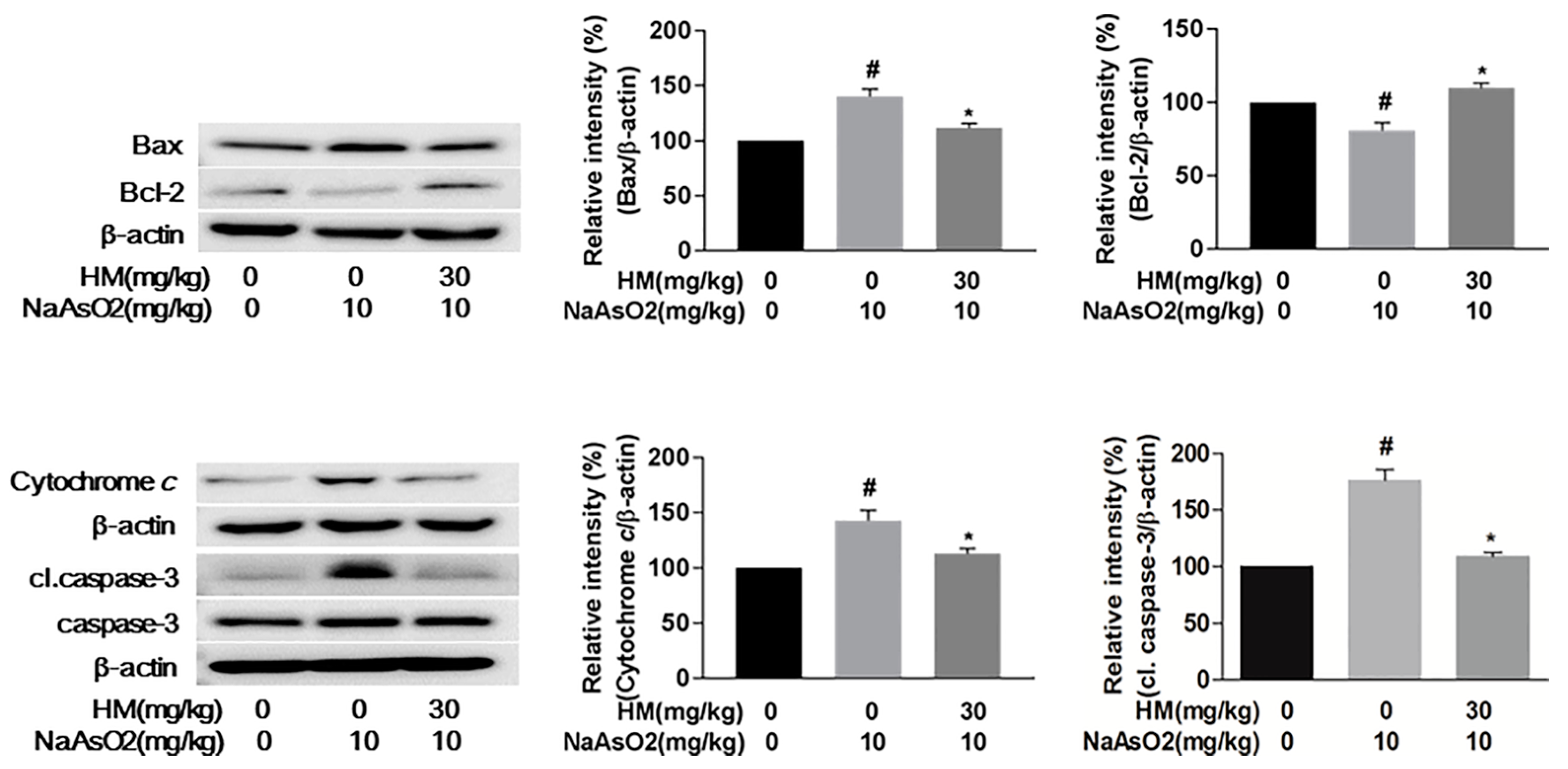
2.9. HM Mitigated NaAsO2-Mediated Hepatotoxicity by Regulating Anti-Apoptotic Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Preparation of Hydrangea Macrophylla Seed Extract
4.3. Determination of Total Phenolic and Flavonoid Content
4.4. Cell Culture
4.5. Assessment of Cell Viability
4.6. Measurement of Reactive Oxygen Species (ROS) Generation
4.7. Determination of Lactate Dehydrogenase (LDH) Release
4.8. Mice Management and Experimental Design
4.9. Histopathological Study of the Liver
4.10. Serum Biochemical Analysis
4.11. Lipid Peroxidation Assay
4.12. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.A.; Molinari, B.L. Effect of sodium arsenite on mouse skin carcinogenesis. Toxicol. Pathol. 2015, 43, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, R.S.; Chandravanshi, L.P.; Shukla, R.K.; Dhuriya, Y.K.; Chauhan, L.K.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol. Appl. Pharmacol. 2014, 279, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rashid, K.; Sadhukhan, P.; Agarwal, N.; Sil, P.C. Attenuative role of mangiferin in oxidative stress-mediated liver dysfunction in arsenic-intoxicated murines. Biofactors 2016, 42, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, W.; Dhibi, M.; Haouas, Z.; Chreif, I.; Neffati, F.; Hammami, M.; Sakly, R. Effects of sodium arsenate exposure on liver fatty acid profiles and oxidative stress in rats. Environ Sci. Pollut. Res. Int. 2014, 21, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- States, J.C.; Srivastava, S.; Chen, Y.; Barchowsky, A. Arsenic and cardiovascular disease. Toxicol. Sci. 2009, 107, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Aminaka, Y.; Yoshida, K.; Sun, G.F.; Pi, J.B.; Waalkes, M.P. Evaluation of DNA damage in patients with arsenic poisoning: Urinary 8-hydroxydeoxyguanine. Toxicol. Appl. Pharmacol. 2004, 198, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Ghosh, J.; Manna, P.; Sinha, M.; Sil, P.C. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol. Lett. 2009, 187, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, K.T.; Conolly, R. Arsenic-induced carcinogenesis−Oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem. Res. Toxicol. 2010, 23, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Biswas, A.; Dhali, G.K.; Chowdhury, A.; Boyer, J.L.; Santra, A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol. Appl. Pharmacol. 2011, 251, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Dai, J.; Chalmers-Redman, R.M.; Tatton, W.G.; Waxman, S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 1999, 94, 2102–2111. [Google Scholar] [PubMed]
- Flora, S.J.; Mehta, A.; Gupta, R. Prevention of arsenic-induced hepatic apoptosis by concomitant administration of garlic extracts in mice. Chem. Biol. Interact. 2009, 177, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Tsukamoto, I. Arsenite induces apoptosis in hepatocytes through an enhancement of the activation of Jun N-terminal kinase and p38 mitogen-activated protein kinase caused by partial hepatectomy. Toxicol. Lett. 2006, 165, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.I.; Jin, B.; Youn, P.; Park, C.; Park, J.D.; Ryu, D.Y. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol. Appl. Pharmacol. 2007, 218, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, X.; Zhao, W.; Zhang, Z. Dual role of resveratrol in modulation of genotoxicity induced by sodium arsenite via oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 59, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, G.H.; Yu, Z.H.; Chen, G.; Zhang, X. Effect of the lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009, 113, 872–877. [Google Scholar]
- Jung, C.H.; Kim, Y.; Kim, M.S.; Lee, S.; Yoo, S.H. The establishment of efficient bioconversion, extraction, and isolation processes for the production of phyllodulcin, a potential high intensity sweetener, from sweet hydrangea leaves (hydrangea macrophylla thunbergii). Phytochem. Anal. 2016, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, C.; Dulac, A.; Pencreac'h, G.; Ergan, F.; Richomme, P.; Soultani-Vigneron, S. Lipase-catalyzed synthesis of two new antioxidants: 4-O- and 3-O-palmitoyl chlorogenic acids. Biotechnol. Lett. 2010, 32, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Matsuda, H.; Yamashita, C.; Nakamura, S.; Yoshikawa, M. Hydrangeic acid from the processed leaves of hydrangea macrophylla var. Thunbergii as a new type of anti-diabetic compound. Eur. J. Pharmacol. 2009, 606, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ishih, A.; Miyase, T.; Terada, M. Comparison of antimalarial activity of the alkaloidal fraction of hydrangea macrophylla var. Otaksa leaves with the hot-water extract in ICR mice infected with plasmodium yoelii 17 XL. Phytother. Res. 2003, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Dewanjee, S.; Gangopadhyay, M.; Khanra, R.; Zia-Ul-Haq, M.; De Feo, V. Ameliorative effect of water spinach, ipomea aquatica (convolvulaceae), against experimentally induced arsenic toxicity. J. Transl. Med. 2015, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; de Silva, P.M. Arsenic and human health effects: A review. Environ Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Sinha, K.; Sil, P.C. An update on oxidative stress-mediated organ pathophysiology. Food Chem. Toxicol. 2013, 62, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Byass, P. The global burden of liver disease: A challenge for methods and for public health. BMC Med. 2014, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Eloff, J.N. Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J. Ethnopharmacol. 2015, 160, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, X.; Bao, J.; Chen, S.; Wang, K.; Zhang, Y.; Li, P.; Wan, J.B.; Su, H.; Wang, Y.; et al. Polyphyllin vii induces apoptosis in HepG2 cells through Ros-mediated mitochondrial dysfunction and MAPK pathways. BMC Complement. Altern. Med. 2016, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, T.; Zhang, H.; Wu, Y.; Wang, X.; Li, G. The antiapoptosis effect of glycyrrhizate on HepG2 cells induced by hydrogen peroxide. Oxid. Med. Cell Longev. 2016, 2016, 6849758. [Google Scholar] [CrossRef] [PubMed]
- Arciello, M.; Gori, M.; Balsano, C. Mitochondrial dysfunctions and altered metals homeostasis: New weapons to counteract HCV-related oxidative stress. Oxid. Med. Cell Longev. 2013, 2013, 971024. [Google Scholar] [CrossRef] [PubMed]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Wang, J.; Zhou, W.; Sun, R.; Xia, M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2015, 102, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, L.; Cheng, Y.; Jiang, J.; Chen, Y.; Jiang, H.; Yu, H.; Shan, A.; Cheng, B. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. Biomed. Res. Int. 2014, 2014, 617202. [Google Scholar] [CrossRef] [PubMed]
- Laouar, A.; Klibet, F.; Bourogaa, E.; Benamara, A.; Boumendjel, A.; Chefrour, A.; Messarah, M. Potential antioxidant properties and hepatoprotective effects of juniperus phoenicea berries against ccl4 induced hepatic damage in rats. Asian Pac. J. Trop. Med. 2017, 10, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and necrosis in the liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar] [PubMed]
- Pearson, G.; Robinson, F.; Gibson, T.B.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Huttemann, M.; Pecina, P.; Rainbolt, M.; Sanderson, T.H.; Kagan, V.E.; Samavati, L.; Doan, J.W.; Lee, I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011, 11, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Santra, A.; Chowdhury, A.; Ghatak, S.; Biswas, A.; Dhali, G.K. Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicol. Appl. Pharmacol. 2007, 220, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Choi, G.; Yoon, T.; Cheon, M.S.; Choo, B.K.; Kim, H.K. Anti-inflammatory activity of chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2009, 123, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Akanda, M.R.; Kim, M.J.; Kim, I.S.; Ahn, D.; Tae, H.J.; Rahman, M.M.; Park, Y.G.; Seol, J.W.; Nam, H.H.; Choo, B.K.; et al. Neuroprotective effects of Sigesbeckia pubescens extract on glutamate-induced oxidative stress in HT22 cells via downregulation of MAPK/Caspase-3 pathways. Cell. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
| Plant Extract | Total Phenolics (mg Gallic Acid Equivalent/g Extract) | Total Flavonoids (mg Rutin/g Extract) | Total Yield (%) |
|---|---|---|---|
| HM extract | 92.358 ± 0.342 | 220.725 ± 3.263 | 26.9 |
| Gene | Primers Sequence (5′–3′) | Size (bp) | Genebank Accession No. |
|---|---|---|---|
| ERK | TCAGAGGCAGGTGGATCTCT ACGGGGAGGACTCTGTTTTT | 109 | NM_011949.3 |
| JNK | CGGAACACCTTGTCCTGAAT CACATCGGGGAACAGTTTCT | 93 | NM_016700.4 |
| p38 | AGCCAATTCCAGTGTTGGAC TTCTGGGCTCCAAATGATTC | 120 | NM_011951.3 |
| β-actin | AGAAGATCTGGCACCACACC TACGACCAGAGGCATACAGG | 195 | NM_007393.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akanda, M.R.; Tae, H.-J.; Kim, I.-S.; Ahn, D.; Tian, W.; Islam, A.; Nam, H.-H.; Choo, B.-K.; Park, B.-Y. Hepatoprotective Role of Hydrangea macrophylla against Sodium Arsenite-Induced Mitochondrial-Dependent Oxidative Stress via the Inhibition of MAPK/Caspase-3 Pathways. Int. J. Mol. Sci. 2017, 18, 1482. https://doi.org/10.3390/ijms18071482
Akanda MR, Tae H-J, Kim I-S, Ahn D, Tian W, Islam A, Nam H-H, Choo B-K, Park B-Y. Hepatoprotective Role of Hydrangea macrophylla against Sodium Arsenite-Induced Mitochondrial-Dependent Oxidative Stress via the Inhibition of MAPK/Caspase-3 Pathways. International Journal of Molecular Sciences. 2017; 18(7):1482. https://doi.org/10.3390/ijms18071482
Chicago/Turabian StyleAkanda, Md Rashedunnabi, Hyun-Jin Tae, In-Shik Kim, Dongchoon Ahn, Weishun Tian, Anowarul Islam, Hyeon-Hwa Nam, Byung-Kil Choo, and Byung-Yong Park. 2017. "Hepatoprotective Role of Hydrangea macrophylla against Sodium Arsenite-Induced Mitochondrial-Dependent Oxidative Stress via the Inhibition of MAPK/Caspase-3 Pathways" International Journal of Molecular Sciences 18, no. 7: 1482. https://doi.org/10.3390/ijms18071482
APA StyleAkanda, M. R., Tae, H.-J., Kim, I.-S., Ahn, D., Tian, W., Islam, A., Nam, H.-H., Choo, B.-K., & Park, B.-Y. (2017). Hepatoprotective Role of Hydrangea macrophylla against Sodium Arsenite-Induced Mitochondrial-Dependent Oxidative Stress via the Inhibition of MAPK/Caspase-3 Pathways. International Journal of Molecular Sciences, 18(7), 1482. https://doi.org/10.3390/ijms18071482