Expression Profiling of Strawberry Allergen Fra a during Fruit Ripening Controlled by Exogenous Auxin
Abstract
:1. Introduction
2. Results
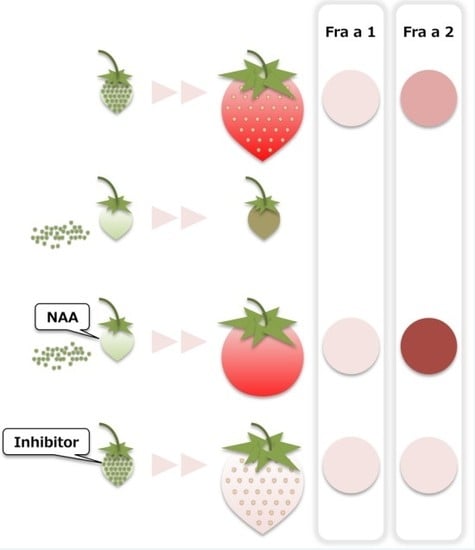
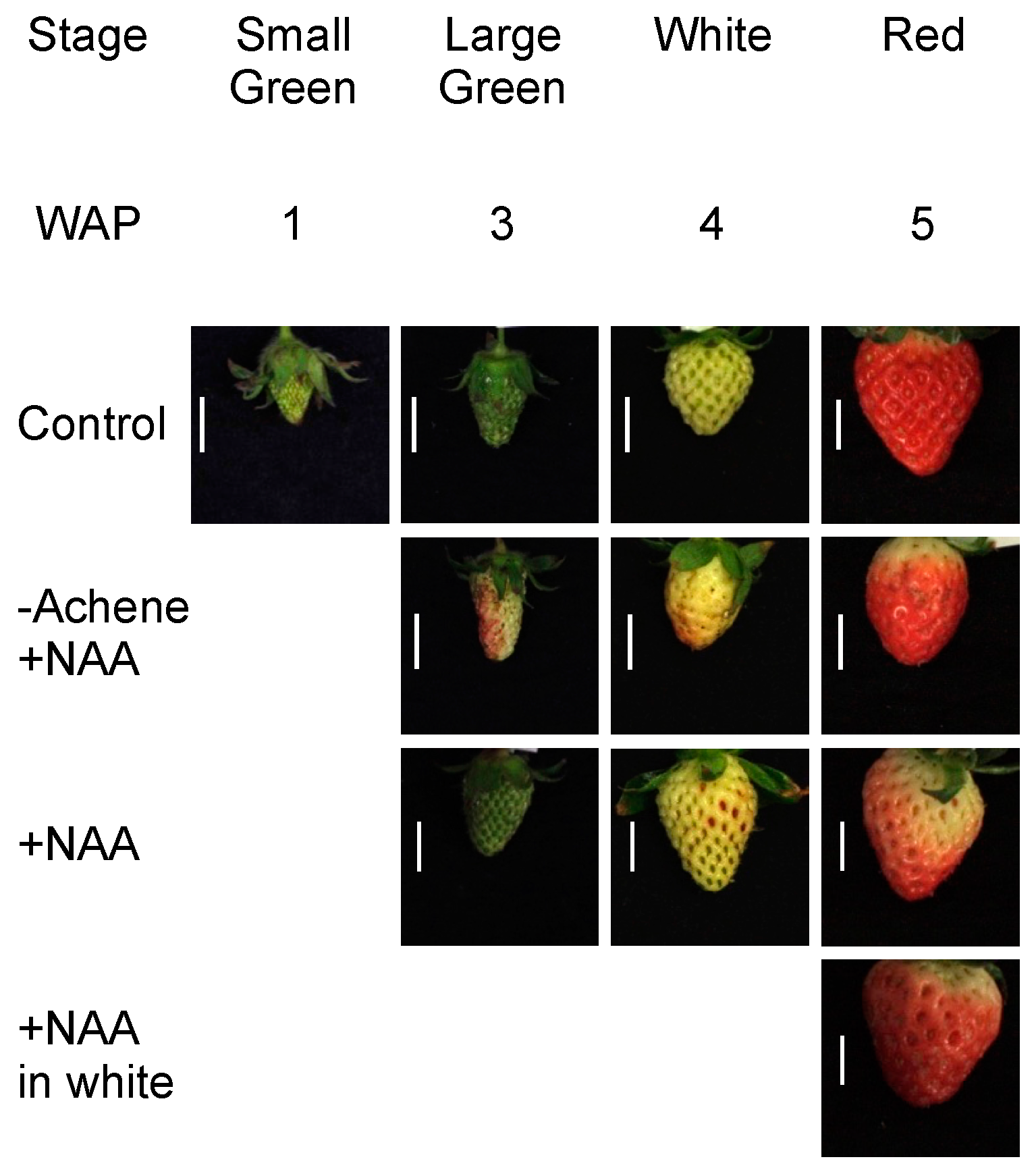
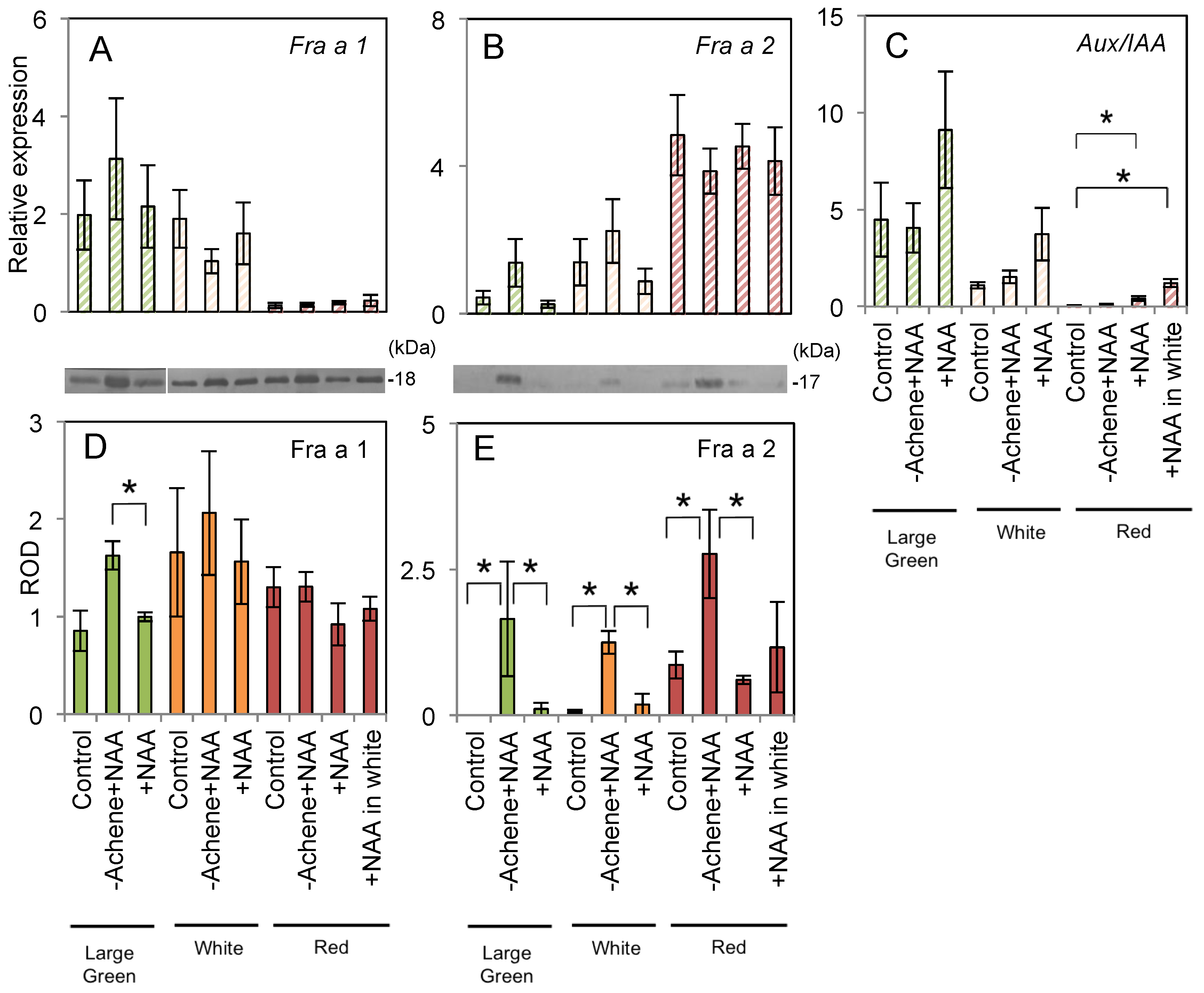
2.1. Fruit Morphology and Response of Fra a to Pre-Harvest Auxin Treatments
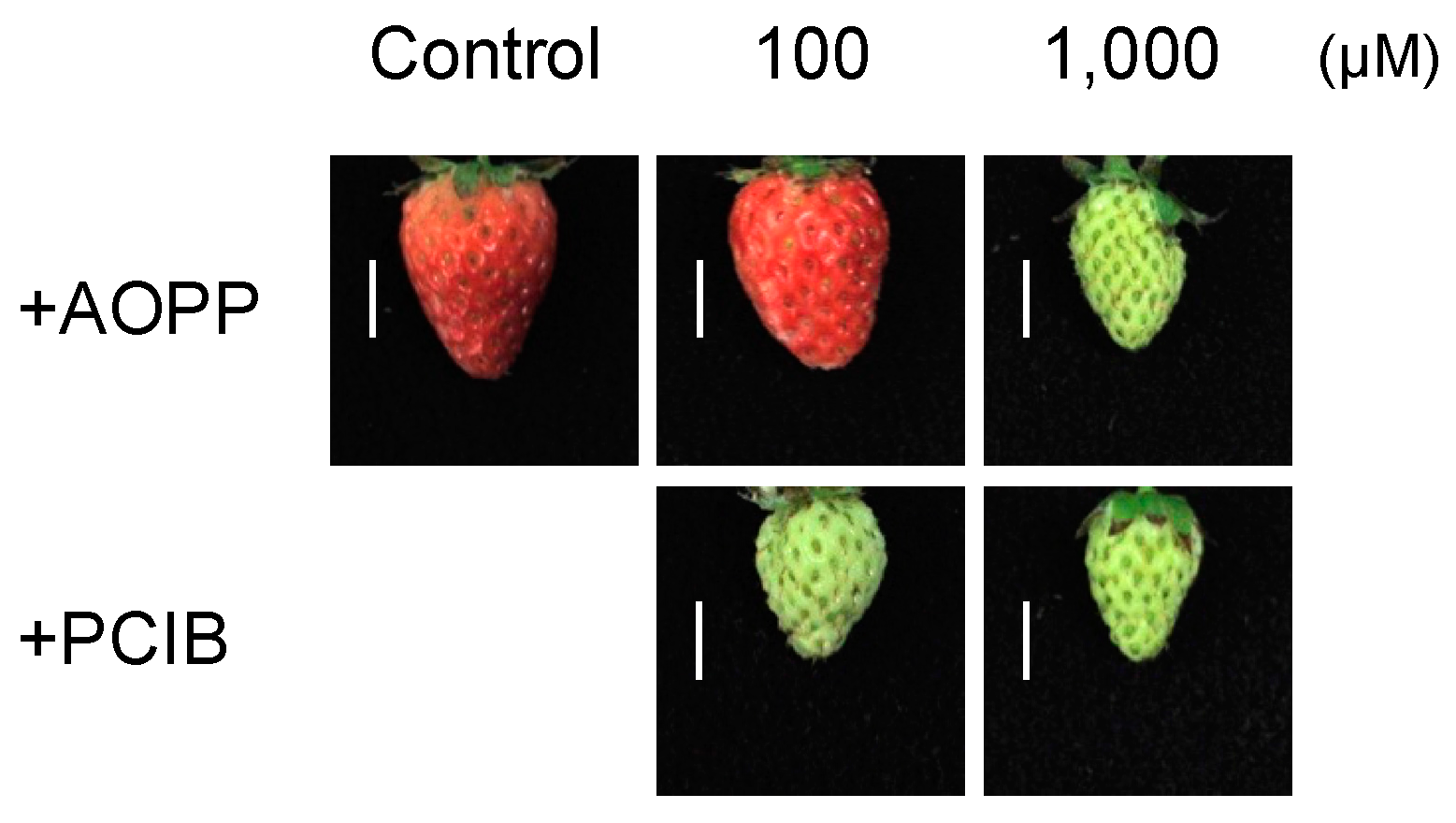
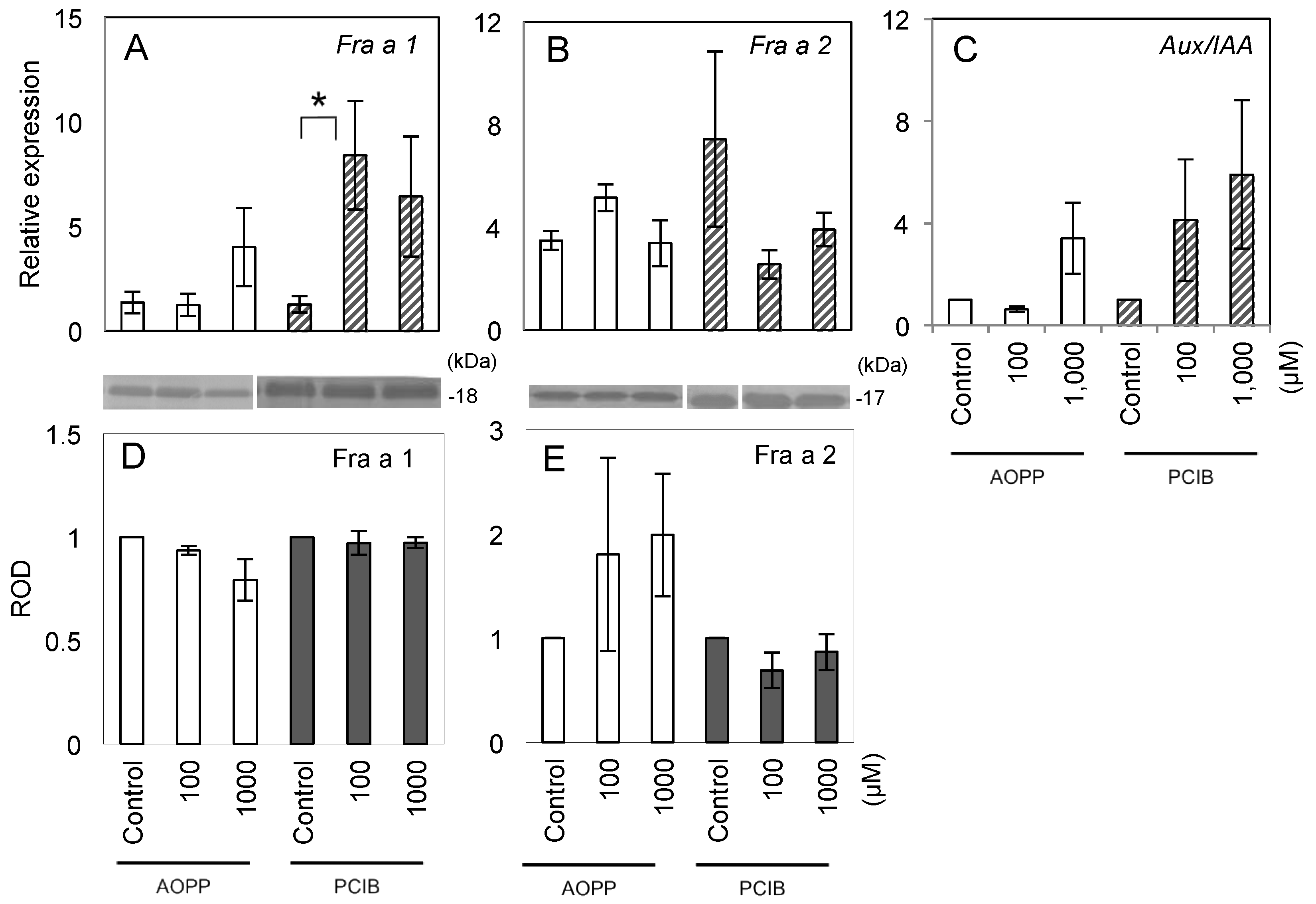
2.2. Fruit Morphology and Response of Fra a to Pre-Harvest Auxin-Inhibitor Treatments
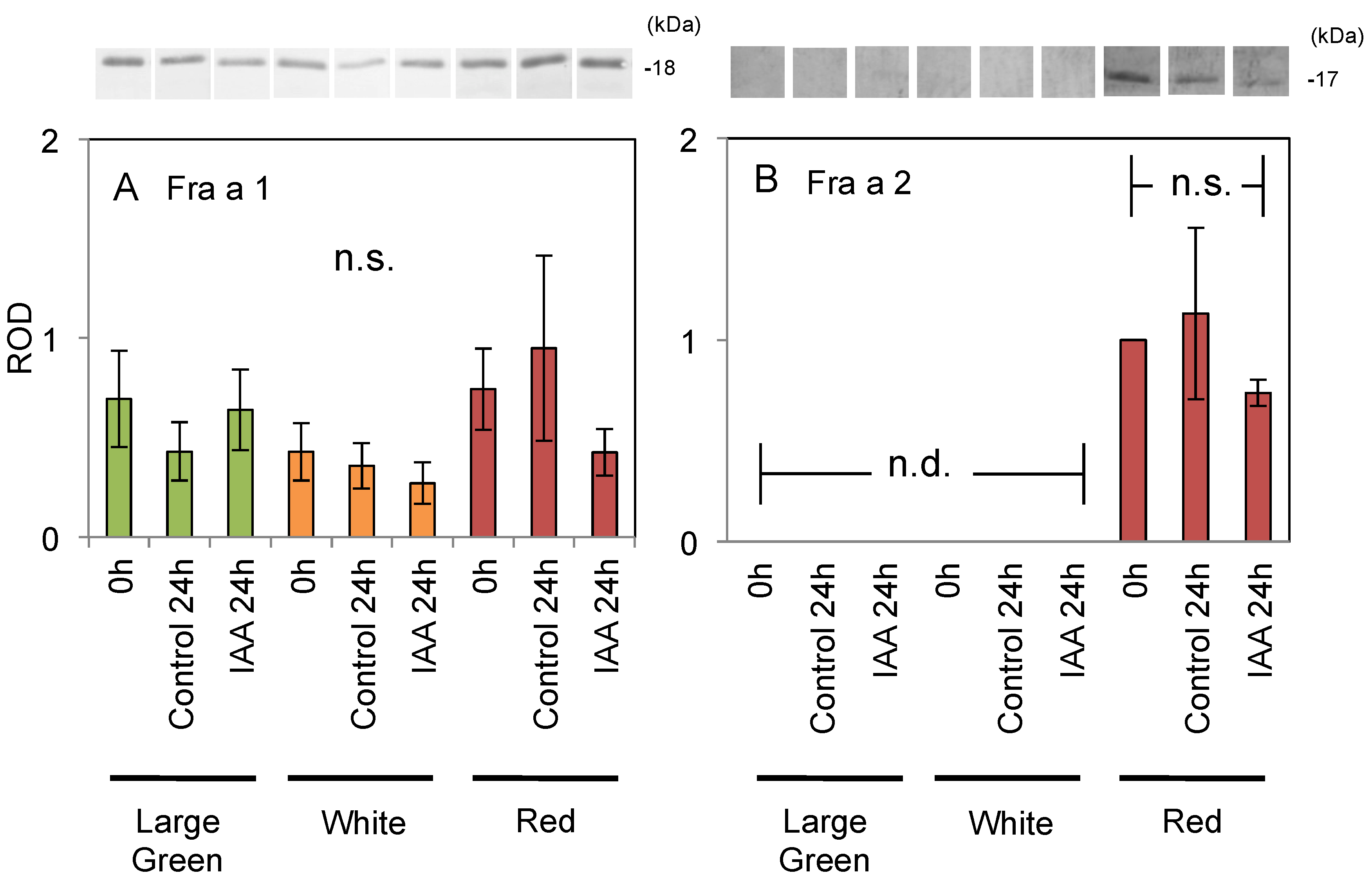
2.3. Expression of Fra a Protein in Response to Post-Harvest Auxin Treatments
3. Discussion
3.1. Response of Fra a to Auxin
3.2. Fra a Expression Profile during the Ripening Process
4. Materials and Methods
4.1. Plant Materials
4.2. Pre-Harvest Treatment
4.3. Post-Harvest Treatment
4.4. Characterization of Fruit
4.5. RNA Extraction and Quantitative Real-Time PCR
4.6. Protein Extraction and SDS-PAGE
4.7. Immunoblotting
4.8. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AOPP | l-2-Aminooxy-3-phenylpropionic acid |
| EF1α | Elongation factor 1α |
| IAA | Indole-3-acetic acid |
| NAA | 1-Naphthaleneacetic acid |
| OAS | Oral allergic syndrome |
| PCIB | p-Chlorophenoxyisobutyric acid |
| PR-10 | Pathogenesis-related 10 |
| ROD | Relative optical density |
| WAP | Weeks after pollination |
References
- Giampieri, F.; Alvarez-suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Hjernø, K.; Alm, R.; Canbäck, B.; Matthiesen, R.; Trajkovski, K.; Björk, L.; Roepstorff, P.; Emanuelsson, C. Down-regulation of the strawberry Bet v 1-homologous allergen in concert with the flavonoid biosynthesis pathway in colorless strawberry mutant. Proteomics 2006, 6, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Musidlowska-Persson, A.; Alm, R.; Emanuelsson, C. Cloning and sequencing of the Bet v 1-homologous allergen Fra a 1 in strawberry (Fragaria ananassa) shows the presence of an intron and little variability in amino acid sequence. Mol. Immunol. 2007, 44, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Hoffmann, T.; Escobar, N.M.; Ludemann, F.; Botella, M.A.; Valpuesta, V.; Schwab, W. The strawberry fruit Fra a allergen functions in flavonoid biosynthesis. Mol. Plant 2010, 3, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Franz-Oberdorf, K.; Eberlein, B.; Edelmann, K.; Hücherig, S.; Besbes, F.; Darsow, U.; Ring, J.; Schwab, W. Fra a 1.02 is the most potent isoform of the Bet v 1-like allergen in strawberry fruit. J. Agric. Food Chem. 2016, 64, 3688–3696. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kim, J.S. Genomic identification of putative allergen genes in woodland strawberry (Fragaria vesca) and mandarin orange (Citrus clementina). Plant Omics 2011, 4, 428–434. [Google Scholar]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.; Petzoldt, R.; Harman, G.E. Use of honey bees and bumble bees to disseminate trichoderma harzianum 1295–22 to strawberries for botrytis control. Biol. Control 2000, 18, 235–242. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, B.; Landi, L.; Pandolfini, T.; Spena, A. The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, J.P. Growth and morphogenesis of the strawberry as related to auxin. Am. J. Bot. 1950, 37, 211–215. [Google Scholar] [CrossRef]
- Manning, K. Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta 1994, 194, 62–68. [Google Scholar] [CrossRef]
- Karlsson, A.L.; Aim, R.; Ekstrand, B.; Fjelkner-Modig, S.; Schiött, A.; Bengtsson, U.; Björk, L.; Hjerno, K.; Roepstorff, P.; Emanuelsson, C.S. Bet v 1 homologues in strawberry identified as IgE-binding proteins and presumptive allergens. Allergy Eur. J. Allergy Clin. Immunol. 2004, 59, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Amil-Ruiz, F.; Blanco-Portales, R.; Muñoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Midoro-Horiuti, T.; Brooks, E.G.; Goldblum, R.M. Pathogenesis-related proteins of plants as allergens. Ann. Allergy Asthma Immunol. 2001, 87, 261–271. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K.M. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006, 68, 3–13. [Google Scholar] [CrossRef]
- Poupard, P.; Brunel, N.; Leduc, N.; Viémont, J.-D.; Strullu, D.-G.; Simoneau, P. Expression of a Bet v 1 homologue gene encoding a PR 10 protein in birch roots: Induction by auxin and localization of the transcripts by in situ hybridization. Aust. J. Plant Physiol. 2001, 28, 57–63. [Google Scholar] [CrossRef]
- Xie, Y.R.; Chen, Z.Y.; Brown, R.L.; Bhatnagar, D. Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. J. Plant Physiol. 2010, 167, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.; de Oliveira, I.R.; Tiecher, A.; Chaves, F.C.; Rombaldi, C.V. Postharvest UV-C treatment increases bioactive, ester volatile compounds and a putative allergenic protein in strawberry. LWT Food Sci. Technol. 2015, 64, 685–692. [Google Scholar] [CrossRef]
- Toljamo, A.; Blande, D.; Kärenlampi, S.; Kokko, H. Reprogramming of strawberry (Fragaria vesca) root transcriptome in response to Phytophthora cactorum. PLoS ONE 2016, 11, e0161078. [Google Scholar] [CrossRef] [PubMed]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Scossa, F.; Fernie, A.R. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Stasinopoulos, T.C.; Hangarter, R.P. Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol. 1990, 93, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A. The effect of applied growth substances on development of the strawberry fruit I. Introduction of parthenocarpy. J. Exp. Bot. 1964, 15, 347–358. [Google Scholar] [CrossRef]
- Abel, S.; Oeller, P.W.; Theologis, A. Early auxin-induced genes encode short-lived nuclear proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latche, A.; Pech, J.-C.; Bouzayen, M. The Tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.J.; Chen, J.Y.; Lu, W.J. Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol. Biol. Rep. 2011, 38, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Soeno, K.; Goda, H.; Ishii, T.; Ogura, T.; Tachikawa, T.; Sasaki, E.; Yoshida, S.; Fujioka, S.; Asami, T.; Shimada, Y. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol. 2010, 51, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Ooura, C.; Rahman, A.; Aspuria, E.T.; Hayashi, K.; Tanaka, A.; Uchimiya, H. p-Chlorophenoxyisobutyric acid impairs auxin response in arabidopsis root. Plant Physiol. 2003, 133, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, N.; Gödeke, K.-H. α-aminooxy-β-phenylpropionic acid—A potent inhibitor of L-phenylalanine ammonia-lyase in vitro and in vivo. Plant Sci. Lett. 1977, 8, 313–317. [Google Scholar] [CrossRef]
- Given, N.K.; Venis, M.A.; Grierson, D. Phenylalanine ammonia-lyase activity and anthocyanin synthesis in ripening strawberry fruit. J. Plant Physiol. 1988, 133, 25–30. [Google Scholar] [CrossRef]
- Liu, J.J.; Ekramoddoullah, A.K.M.; Yu, X. Differential expression of multiple PR10 proteins in western white pine following wounding, fungal infection and cold-hardening. Physiol. Plant. 2003, 119, 544–553. [Google Scholar] [CrossRef]
- Archbold, D.D.; Dennis, F.G.J. Quantification of free ABA and free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. J. Am. Soc. Hortic. Sci. 1984, 109, 330–335. [Google Scholar]
- Chai, Y.M.; Jia, H.F.; Li, C.L.; Dong, Q.H.; Shen, Y.Y. FaPYR1 is involved in strawberry fruit ripening. J. Exp. Bot. 2011, 62, 5079–5089. [Google Scholar] [CrossRef] [PubMed]
- Csukasi, F.; Osorio, S.; Gutierrez, J.R.; Kitamura, J.; Giavalisco, P.; Nakajima, M.; Fernie, A.R.; Rathjen, J.P.; Botella, M.A.; Valpuesta, V.; et al. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol. 2011, 191, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.M.; Zhang, Q.; Tian, L.; Li, C.L.; Xing, Y.; Qin, L.; Shen, Y.Y. Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul. 2013, 69, 63–69. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nelson, C.J.; Trösch, J.; Castleden, I.; Huang, S.; Millar, A.H. Protein degradation rate in Arabidopsis thaliana leaf growth and development. Plant Cell 2017, 29, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Serrani, J.C.; Carrera, E.; Ruiz-Rivero, O.; Gallego-Giraldo, L.; Peres, L.E.P.; García-Martínez, J.L. Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol. 2010, 153, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Faurobert, M.; Pelpoir, E.; Chaïb, J. Phenol extraction of proteins for proteomic studies of recalcitrant plant tissues. Plant Proteom. 2007, 335, 9–14. [Google Scholar]
| Treatment z | L* y | a* y | b* y | LD x (mm) | TD x (mm) | FW x (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Large green | ||||||||||||||
| Control | 57.7 ± 2.8 | a w | −11.8 ± 4.0 | b | 26.6 ± 1.6 | a | 22.2 ± 0.7 | a | 14.8 ± 0.9 | a | 2.1 ± 0.4 | a | ||
| −Achene+NAA | 59.6 ± 2.3 | a | 4.8 ± 2.5 | a | 28.3 ± 2.0 | a | 22.4 ± 0.6 | a | 13.1 ± 1.3 | a | 1.9 ± 0.3 | a | ||
| +NAA | 56.9 ± 1.8 | a | −7.5 ± 3.1 | a | b | 28.3 ± 1.1 | a | 24.3 ± 2.0 | a | 15.5 ± 2.0 | a | 2.7 ± 0.8 | a | |
| White | ||||||||||||||
| Control | 62.0 ± 1.5 | a | −0.6 ± 3.3 | a | 24.6 ± 2.0 | a | 27.9 ± 2.4 | a | 17.8 ± 1.1 | a | b | 3.9 ± 0.9 | a | |
| −Achene+NAA | 62.2 ± 1.9 | a | 4.0 ± 9.1 | a | 24.3 ± 0.7 | a | 22.8 ± 1.7 | a | 15.2 ± 1.0 | b | 2.8 ± 0.3 | a | ||
| +NAA | 59.4 ± 1.6 | a | −2.1 ± 3.0 | a | 23.4 ± 0.2 | a | 29.2 ± 1.8 | a | 19.7 ± 1.0 | a | 4.5 ± 0.7 | a | ||
| Red | ||||||||||||||
| Control | 40.6 ± 3.5 | a | 35.1 ± 2.8 | a | b | 26.1 ± 1.6 | a | 35.1 ± 1.8 | a | 22.8 ± 1.6 | a | 8.3 ± 1.0 | a | |
| −Achene+NAA | 40.6 ± 3.2 | a | 38.4 ± 2.4 | a | 24.4 ± 3.4 | a | 29.8 ± 1.9 | a | 21.7 ± 0.9 | a | 6.9 ± 1.1 | a | ||
| +NAA | 45.2 ± 3.2 | a | 33.1 ± 3.7 | a | b | 25.7 ± 0.2 | a | 38.6 ± 3.2 | a | 25.9 ± 2.0 | a | 10.8 ± 2.7 | a | |
| +NAA in white | 48.4 ± 5.6 | a | 23.7 ± 5.8 | b | 20.4 ± 1.2 | a | 37.4 ± 3.9 | a | 26.5 ± 1.0 | a | 10.3 ± 1.4 | a | ||
| Treatment z | L* y | a* y | b* y | LD y (mm) | TD y (mm) | FW y (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOPP | ||||||||||||||
| Control | 42.8 ± 1.7 | b x | 30.4 ± 1.7 | a | 21.6 ± 2.1 | a | 34.0 ± 2.7 | a | 22.6 ± 0.8 | a | 6.8 ± 0.6 | a | ||
| 100 μM | 45.0 ± 1.9 | b | 24.1 ± 2.3 | a | 20.7 ± 0.7 | a | 33.3 ± 3.5 | a | 23.3 ± 1.5 | a | 7.4 ± 1.4 | a | ||
| 1000 μM | 59.1 ± 3.0 | a | 11.3 ± 2.5 | b | 25.5 ± 2.0 | a | 29.8 ± 3.8 | a | 19.8 ± 2.5 | a | 5.8 ± 1.8 | a | ||
| PCIB | ||||||||||||||
| Control | 40.2 ± 1.1 | b | 31.2 ± 0.8 | a | 24.5 ± 1.7 | a | 32.5 ± 1.9 | a | 24.2 ± 1.4 | a | 7.3 ± 1.2 | a | ||
| 100 μM | 57.4 ± 3.8 | a | b | 0.6 ± 3.8 | b | 20.0 ± 2.0 | a | 26.5 ± 2.1 | a | 21.1 ± 2.0 | a | 5.2 ± 0.6 | a | |
| 1000 μM | 53.4 ± 4.5 | a | b | 11.8 ± 9.7 | b | 21.9 ± 1.8 | a | 25.7 ± 2.4 | a | 19.4 ± 2.2 | a | 4.8 ± 0.9 | a | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, M.; Yoshikawa, H.; Uno, Y. Expression Profiling of Strawberry Allergen Fra a during Fruit Ripening Controlled by Exogenous Auxin. Int. J. Mol. Sci. 2017, 18, 1186. https://doi.org/10.3390/ijms18061186
Ishibashi M, Yoshikawa H, Uno Y. Expression Profiling of Strawberry Allergen Fra a during Fruit Ripening Controlled by Exogenous Auxin. International Journal of Molecular Sciences. 2017; 18(6):1186. https://doi.org/10.3390/ijms18061186
Chicago/Turabian StyleIshibashi, Misaki, Hiroki Yoshikawa, and Yuichi Uno. 2017. "Expression Profiling of Strawberry Allergen Fra a during Fruit Ripening Controlled by Exogenous Auxin" International Journal of Molecular Sciences 18, no. 6: 1186. https://doi.org/10.3390/ijms18061186
APA StyleIshibashi, M., Yoshikawa, H., & Uno, Y. (2017). Expression Profiling of Strawberry Allergen Fra a during Fruit Ripening Controlled by Exogenous Auxin. International Journal of Molecular Sciences, 18(6), 1186. https://doi.org/10.3390/ijms18061186