Low-Symmetry Mixed Fluorinated Subphthalocyanines as Fluorescence Imaging Probes in MDA-MB-231 Breast Tumor Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of SPcs
2.2. 19F NMR Characterization of SPcs
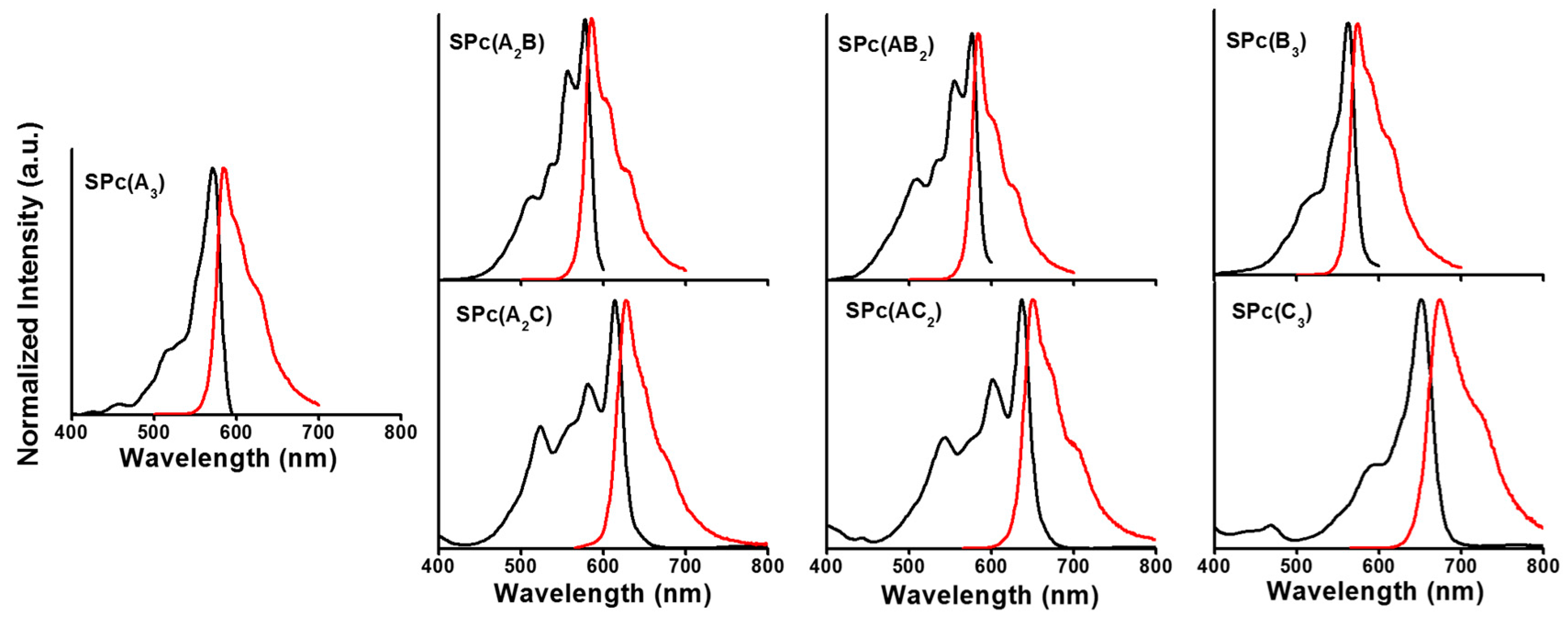
2.3. Photophysical Properties of SPcs
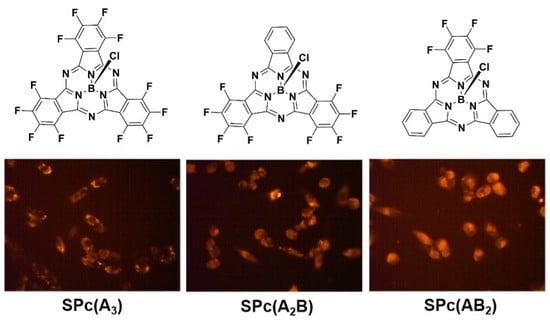
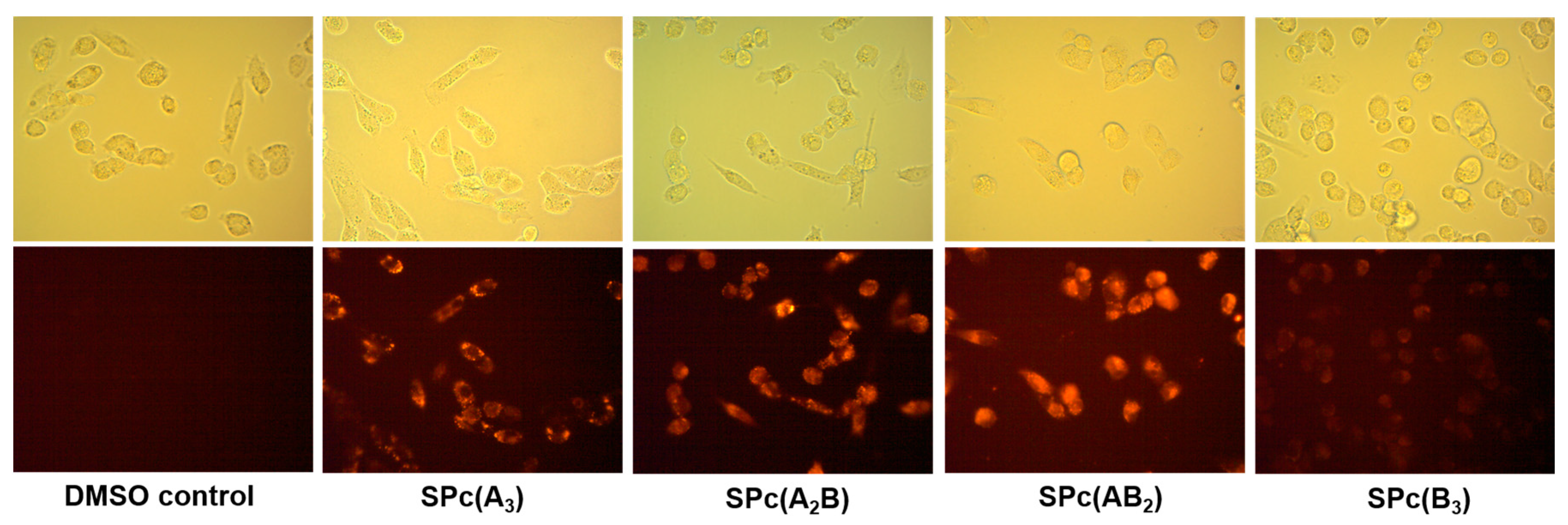
2.4. In Vitro Epifluorescence Microscopy in MDA-MB-231 Breast Tumor Cells
3. Materials and Methods
3.1. Materials
3.2. General Considerations
3.3. Synthesis
3.4. Fluorescence Quantum Yield Determination
3.5. Cell Culture and Fluorescence Imaging
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SPc | Subphthalocyanine |
| Pc | Phthalocyanine |
| NIR | Near-infrared |
| LUMO | Lowest unoccupied molecular orbital |
| THF | Tetrahydrofuran |
| HPLC | High performance liquid chromatography |
| DMSO | Dimethylsulfoxide |
| DMEM | Dulbecco’s modified eagle medium |
References
- Meller, A.; Ossko, A. Phthalocyaninartige bor-komplexe. Monatsh. Für Chem. 1972, 103, 150. [Google Scholar] [CrossRef]
- Claessens, C.G.; Gonzalez-Rodriguez, D.; Rodriguez-Morgade, M.S.; Medina, A.; Torres, T. Subphthalocyanines, subporphyrazines, and subporphyrins: Singular nonplanar aromatic systems. Chem. Rev. 2014, 114, 2192–2277. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kobayashi, N. Structurally-modified subphthalocyanines: Molecular design towards realization of expected properties from the electronic structure and structural features of subphthalocyanine. Chem. Commun. 2014, 50, 6949–6966. [Google Scholar] [CrossRef] [PubMed]
- Fulford, M.V.; Jaidka, D.; Paton, A.S.; Morse, G.E.; Brisson, E.R.L.; Lough, A.J.; Bender, T.P. Crystal structures, reaction rates, and selected physical properties of halo-boronsubphthalocyanines (halo = fluoride, chloride, and bromide). J. Chem. Eng. Data 2012, 57, 2756–2765. [Google Scholar] [CrossRef]
- Claessens, C.G.; Torres, T. Subphthalocyanine enantiomers: First resolution of a C3 aromatic compound by HPLC. Tetrahedron Lett. 2000, 41, 6361–6365. [Google Scholar] [CrossRef]
- Zyskowski, C.D.; Kennedy, V.O. Compounds in the series from boron subphthalocyanine to boron subnaphthalocyanine. J. Porphyr. Phthalocyanines 2000, 4, 707–712. [Google Scholar] [CrossRef]
- Stork, J.R.; Potucek, R.J.; Durfee, W.S.; Noll, B.C. The synthesis and structure of mixed subphthalocyanine/subnaphthalocyanine complexes. Tetrahedron Lett. 1999, 40, 8055–8058. [Google Scholar] [CrossRef]
- Shimizu, S.; Nakano, S.; Hosoya, T.; Kobayashi, N. Pyrene-fused subphthalocyanine. Chem. Commun. 2011, 47, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Nakano, S.; Kojima, A.; Kobayashi, N. A core-expanded subphthalocyanine analogue with a significantly distorted conjugated surface and unprecedented properties. Angew. Chem. Int. Ed. 2014, 53, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.E.; Ke, S.; Kwon, S.; Liang, F.; Fan, Z.; Lu, Y.; Hirschi, K.; Mawad, M.E.; Barry, M.A.; Sevick-Muraca, E.M. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J. Biomed. Opt. 2007, 12, 024017. [Google Scholar] [CrossRef] [PubMed]
- Sevick-Muraca, E.M. Translation of near-infrared fluorescence imaging technologies: Emerging clinical applications. Annu. Rev. Med. 2012, 63, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Matcher, S.J.; Ferrari, M. Identification and quantification of intrinsic optical contrast for near-infrared mammography. Photochem. Photobiol. 1998, 67, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, P.; van der Vorst, J.R.; Verbeek, F.P.R.; Oliveira, S.; Snoeks, T.J.A.; Keereweer, S.; Chan, B.; Boonstra, M.C.; Frangioni, J.V.; van Bergen en Henegouwen, P.M.P.; et al. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent anti-epidermal growth factor receptor nanobody. Int. J. Cancer 2014, 134, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Verbeek, F.P.R.; Schaafsma, B.E.; Boonstra, M.C.; van der Vorst, J.R.; Liefers, G.J.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and methylene blue. Eur. J. Surg. Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kobayashi, N. 102—The photophysical properties of phthalocyanines and related compounds. In The Porphyrin Handbook; Smith, K.M., Guilard, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 1–42. [Google Scholar]
- Lobo, A.C.S.; Silva, A.D.; Tomé, V.A.; Pinto, S.M.A.; Silva, E.F.F.; Calvete, M.J.F.; Gomes, C.M.F.; Pereira, M.M.; Arnaut, L.G. Phthalocyanine labels for near-infrared fluorescence imaging of solid tumors. J. Med. Chem. 2016, 59, 4688–4696. [Google Scholar] [CrossRef] [PubMed]
- Avşar, G.; Sari, F.A.; Yuzer, A.C.; Soylu, H.M.; Er, O.; Ince, M.; Lambrecht, F.Y. Intracellular uptake and fluorescence imaging potential in tumor cell of zinc phthalocyanine. Int. J. Pharm. 2016, 505, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; He, X.; Wu, L.; Liu, T. Lactose substituted zinc phthalocyanine: A near infrared fluorescence imaging probe for liver cancer targeting. Bioorg. Med. Chem. Lett. 2013, 23, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, E.R.; Harney, A.S.; Olive, M.B.; Podgorski, I.; Moin, K.; Sloane, B.F.; Barrett, A.G.M.; Meade, T.J.; Hoffman, B.M. Chiral porphyrazine near-IR optical imaging agent exhibiting preferential tumor accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, X.J.; Chan, E.Y.M.; Fong, W.P.; Ng, D.K.P. Synthesis, photophysical properties and in vitro photodynamic activity of axially substituted subphthalocyanines. Org. Biomol. Chem. 2007, 5, 3987–3992. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, Y.; Winckler, P.; Chassagnon, R.; Richard, P.; Gigot, E.; Perrier-Cornet, J.M.; Decreau, R.A. Subphthalocyanines: Addressing water-solubility, nano-encapsulation, and activation for optical imaging of B16 melanoma cells. Chem. Commun. 2014, 50, 13975–13978. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.; Bhupathiraju, N.V.S.D.K.; John, B.K.; Drain, C.M. Tuning the structure and photophysics of a fluorous phthalocyanine platform. J. Phys. Chem. A 2016, 120, 7451–7464. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Alarcon, E.; Munoz, M.; Scaiano, J.C.; Edwards, A.M.; Lissi, E. Photophysical behaviour and photodynamic activity of zinc phthalocyanines associated to liposomes. Photochem. Photobiol. Sci. 2011, 10, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The polar hydrophobicity of fluorinated compounds. Chembiochem 2004, 5, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; Gonzalez-Rodriguez, D.; del Rey, B.; Torres, T.; Mark, G.; Schuchmann, H.-P.; von Sonntag, C.; MacDonald, J.G.; Nohr, R.S. Highly efficient synthesis of chloro- and phenoxy-substituted subphthalocyanines. Eur. J. Org. Chem. 2003, 2547–2551. [Google Scholar] [CrossRef]
- Fifolt, M.J.; Sojka, S.A.; Wolfe, R.A.; Hojnicki, D.S.; Bieron, J.F.; Dinan, F.J. A chemical shift additivity method for the prediction of fluorine-19 chemical shifts in fluoroaromatic compounds. J. Org. Chem. 1989, 54, 3019–3023. [Google Scholar] [CrossRef]
- Gouterman, M.; Snyder, L.C.; Wagniere, G.H. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Wheeler, B.L.; Nagasubramanian, G.; Bard, A.J.; Schechtman, L.A.; Kenney, M.E. A silicon phthalocyanine and a silicon naphthalocyanine: Synthesis, electrochemistry, and electrogenerated chemiluminescence. J. Am. Chem. Soc. 1984, 106, 7404–7410. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Magde, D.; Brannon, J.H.; Cremers, T.L.; Olmsted, J. Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 1979, 83, 696–699. [Google Scholar] [CrossRef]
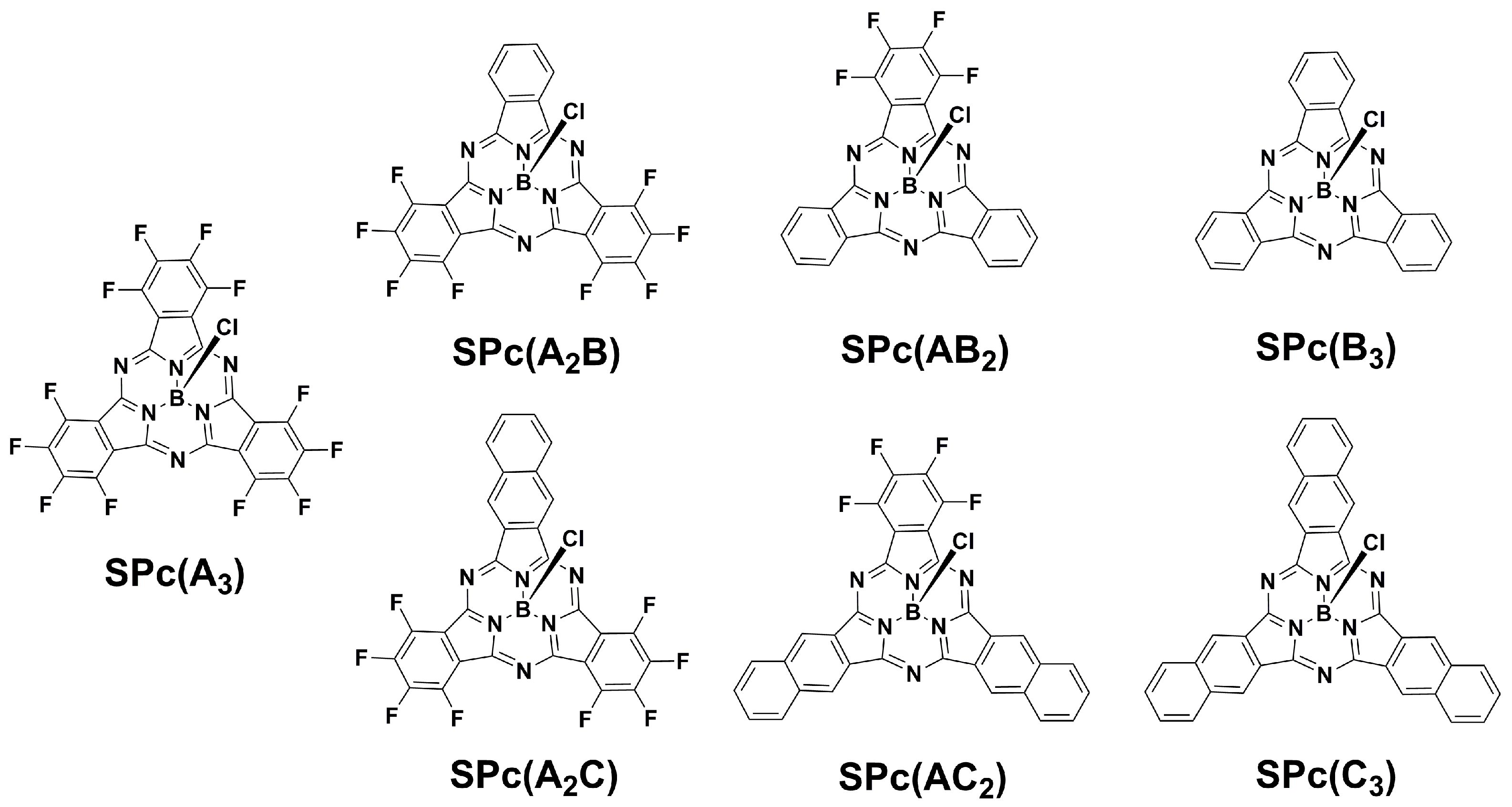
| Compound | α-F–δ (ppm) | β-F–δ (ppm) |
|---|---|---|
| SPc(A3) | −147.4 (d) 2 | −136.7 (d) |
| SPc(A2B) | −149.2 (m), −149.9 (m) | −138.5 (m), −138.8 (m) |
| SPc(AB2) | −151.7 (d) | −140.3 (d) |
| SPc(A2C) | −149.2 (m), −150.6 (m) | −138.8 (m), −138.9 (m) |
| SPc(AC2) | −153.7 (d) | −141.8 (d) |
| Compound | Absorption (λmax, nm) | Emission (λf, nm) | Stokes Shift (cm−1) | Quantum Yield (Φf) |
|---|---|---|---|---|
| SPc(A3) | 571 | 584 | 390 | 0.30 |
| SPc(A2B) | 578 | 585 | 210 | 0.19 |
| SPc(AB2) | 572 | 578 | 180 | 0.26 |
| SPc(B3) | 563 | 574 | 460 | 0.29 |
| SPc(A2C) | 613 | 628 | 390 | 0.21 |
| SPc(AC2) | 637 | 650 | 310 | 0.16 |
| SPc(C3) | 651 | 674 | 520 | 0.28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAuliffe, K.J.; Kaster, M.A.; Szlag, R.G.; Trivedi, E.R. Low-Symmetry Mixed Fluorinated Subphthalocyanines as Fluorescence Imaging Probes in MDA-MB-231 Breast Tumor Cells. Int. J. Mol. Sci. 2017, 18, 1177. https://doi.org/10.3390/ijms18061177
McAuliffe KJ, Kaster MA, Szlag RG, Trivedi ER. Low-Symmetry Mixed Fluorinated Subphthalocyanines as Fluorescence Imaging Probes in MDA-MB-231 Breast Tumor Cells. International Journal of Molecular Sciences. 2017; 18(6):1177. https://doi.org/10.3390/ijms18061177
Chicago/Turabian StyleMcAuliffe, Katherine J., Megan A. Kaster, Regina G. Szlag, and Evan R. Trivedi. 2017. "Low-Symmetry Mixed Fluorinated Subphthalocyanines as Fluorescence Imaging Probes in MDA-MB-231 Breast Tumor Cells" International Journal of Molecular Sciences 18, no. 6: 1177. https://doi.org/10.3390/ijms18061177
APA StyleMcAuliffe, K. J., Kaster, M. A., Szlag, R. G., & Trivedi, E. R. (2017). Low-Symmetry Mixed Fluorinated Subphthalocyanines as Fluorescence Imaging Probes in MDA-MB-231 Breast Tumor Cells. International Journal of Molecular Sciences, 18(6), 1177. https://doi.org/10.3390/ijms18061177