YKL-40-Induced Inhibition of miR-590-3p Promotes Interleukin-18 Expression and Angiogenesis of Endothelial Progenitor Cells
Abstract
:1. Introduction
2. Results
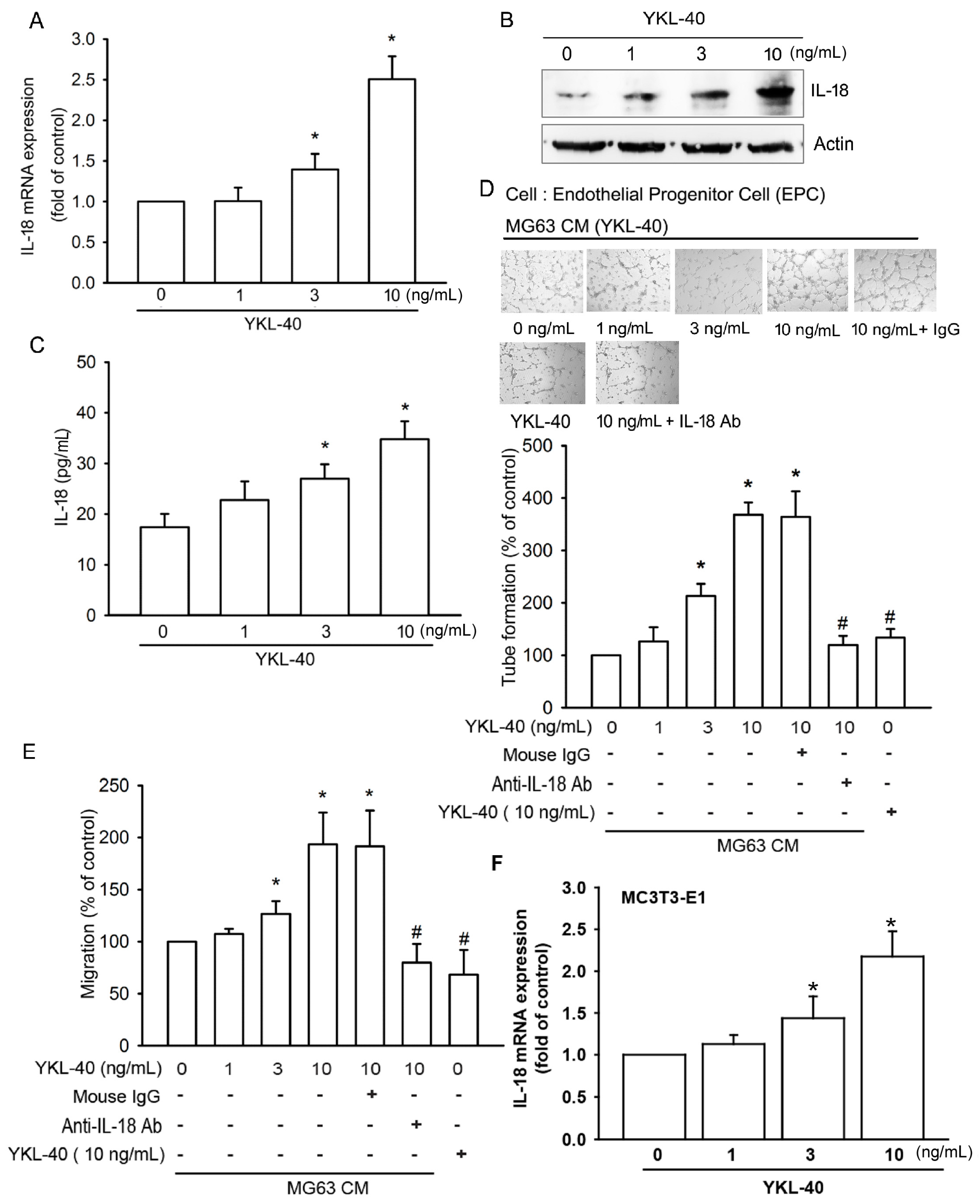
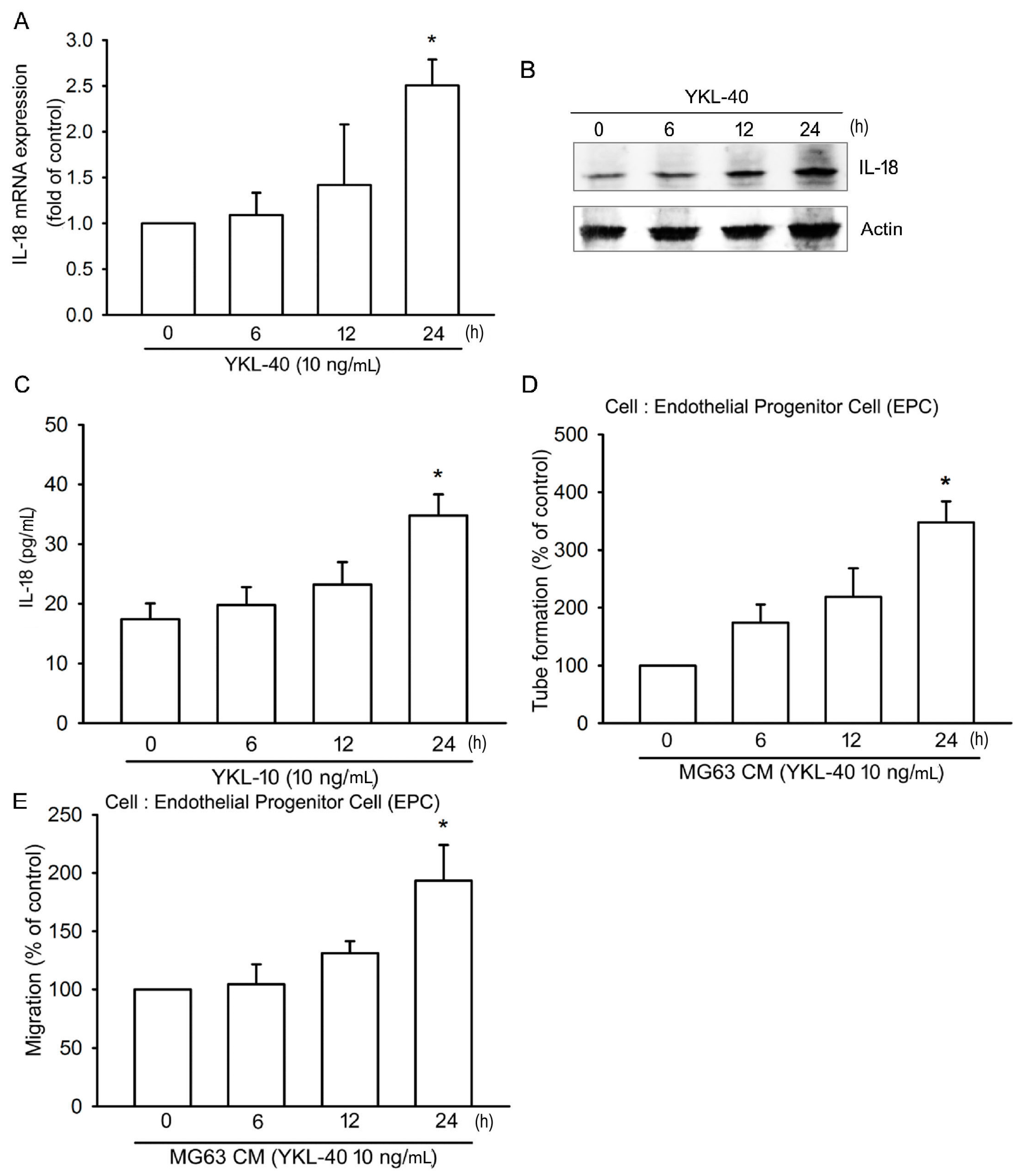
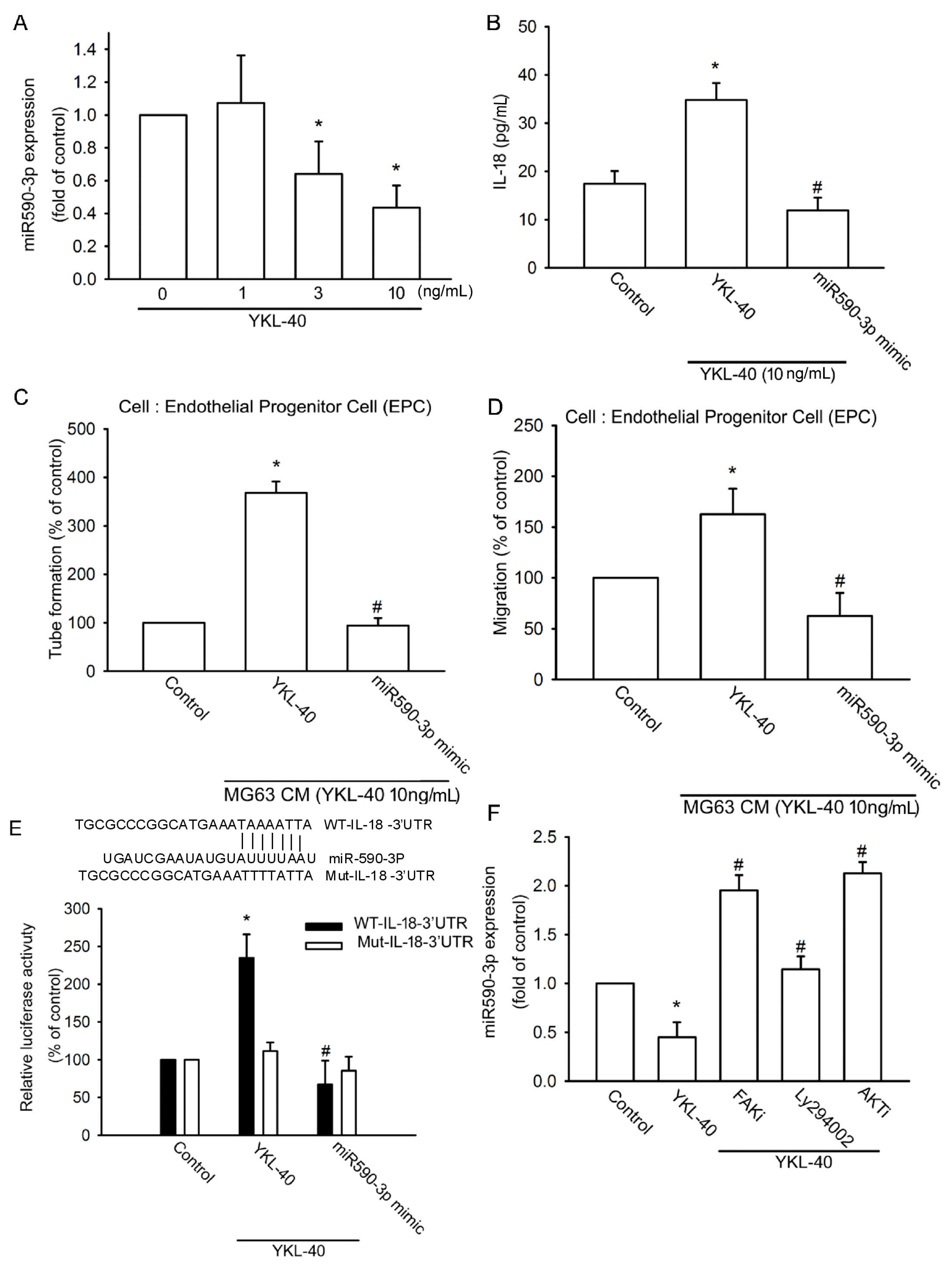
2.1. YKL-40 Promotes IL-18 Production in Osteoblasts and Facilitates EPC Angiogenesis
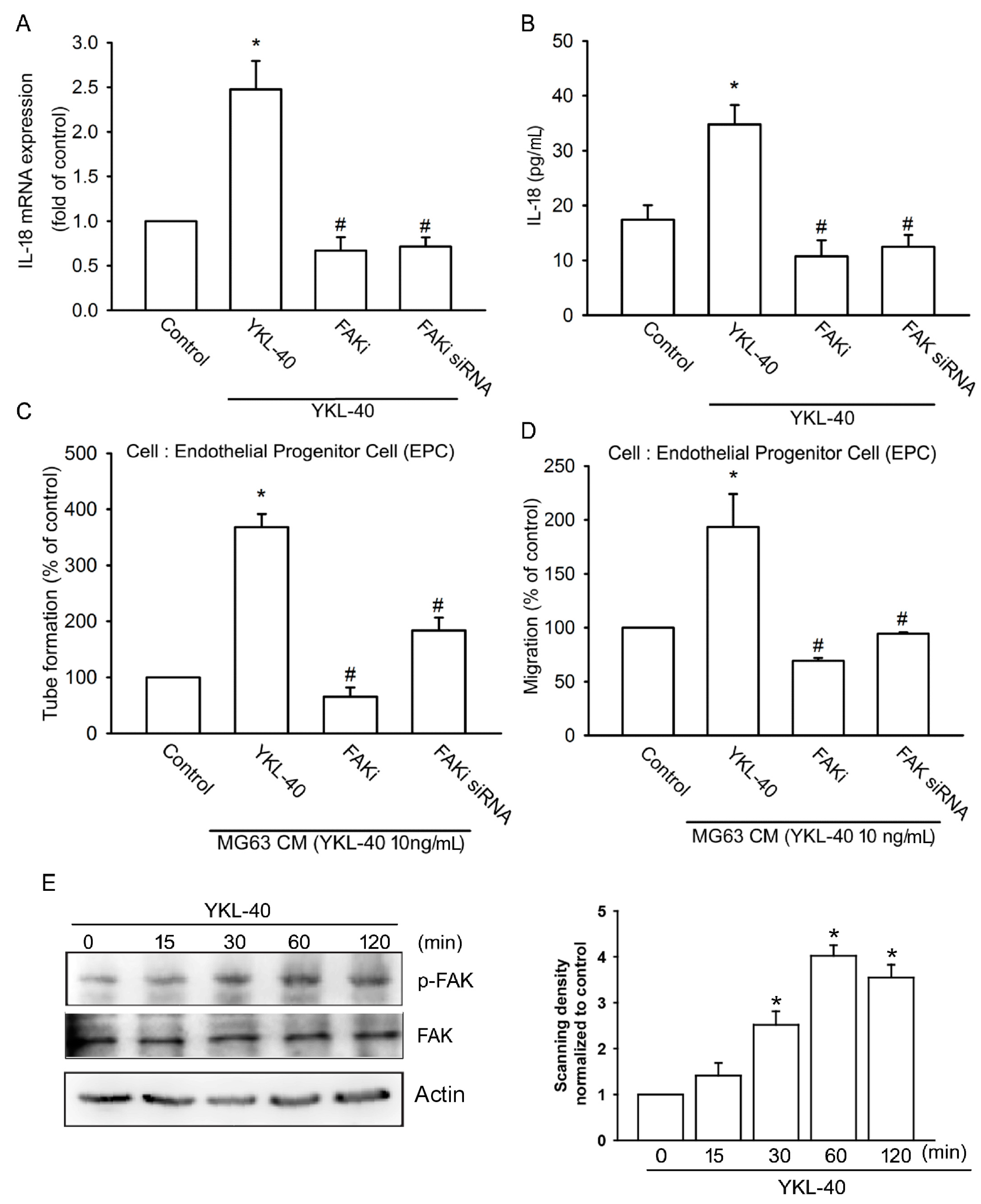
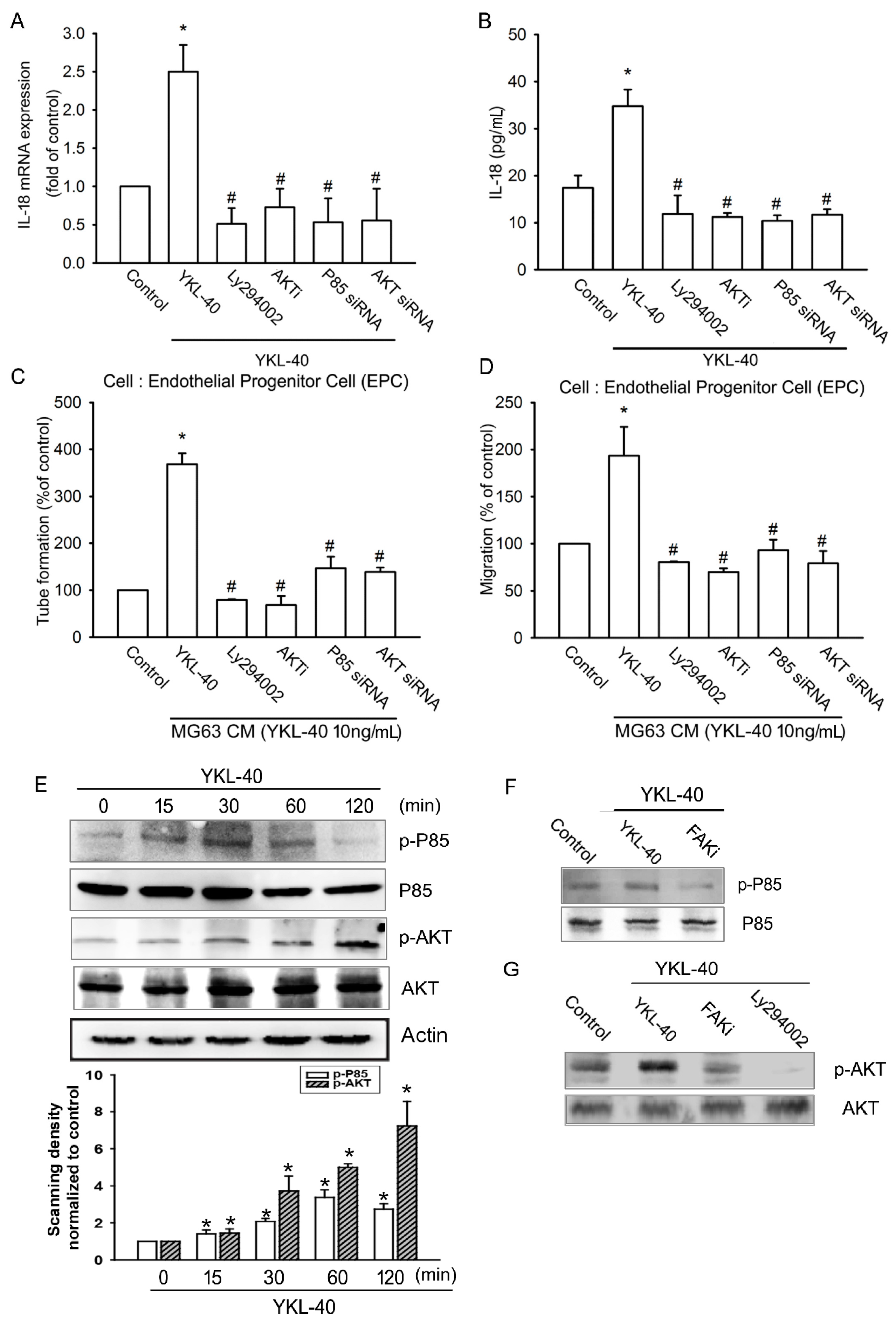
2.2. YKL-40 Promotes IL-18 Expression and Stimulates EPC Angiogenesis through the FAK/PI3K/Akt Signaling Pathway
2.3. YKL-40 Promotes IL-18 Expression via Inhibition of miR-590-3p Expression
2.4. Inhibition of YKL-40 Reduces Angiogenesis In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. ELISA Assay
4.4. Migration and Tube Formation of EPCs
4.5. Quantification of mRNA and miRNA
4.6. Western Blotting
4.7. Plasmid Construction and Luciferase Assay
4.8. Chick Chorioallantoic Membrane Assay
4.9. In Vivo Matrigel Plug Assay
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [PubMed]
- Anderson, K.O.; Bradley, L.A.; Young, L.D.; McDaniel, L.K.; Wise, C.M. Rheumatoid arthritis: Review of psychological factors related to etiology, effects, and treatment. Psychol. Bull. 1985, 98, 358–387. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Salaffi, F.; Di Franco, M.; Bazzichi, L.; Cassisi, G.; Casale, R.; Cazzola, M.; Stisi, S.; Battellino, M.; Atzeni, F. Pain in rheumatoid arthritis: A critical review. Reumatismo 2014, 66, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lisignoli, G.; Piacentini, A.; Cristino, S.; Grassi, F.; Cavallo, C.; Cattini, L.; Tonnarelli, B.; Manferdini, C.; Facchini, A. Ccl20 chemokine induces both osteoblast proliferation and osteoclast differentiation: Increased levels of Ccl20 are expressed in subchondral bone tissue of rheumatoid arthritis patients. J. Cell. Physiol. 2007, 210, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Zini, N.; Lisignoli, G.; Solimando, L.; Bavelloni, A.; Grassi, F.; Guidotti, L.; Trimarchi, C.; Facchini, A.; Maraldi, N.M. Il1-β and TNF-α induce changes in the nuclear polyphosphoinositide signalling system in osteoblasts similar to that occurring in patients with rheumatoid arthritis: An immunochemical and immunocytochemical study. Histochem. Cell Biol. 2003, 120, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Fuh, L.J.; Huang, C.C.; Hsu, C.J.; Su, C.M.; Liu, S.C.; Lin, Y.M.; Tang, C.H. Enhancement of ccl2 expression and monocyte migration by ccn1 in osteoblasts through inhibiting mir-518a-5p: Implication of rheumatoid arthritis therapy. Sci. Rep. 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Chiang, Y.C.; Huang, C.Y.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Osteopontin promotes oncostatin m production in human osteoblasts: Implication of rheumatoid arthritis therapy. J. Immunol. 2015, 195, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Su, C.M.; Hsu, C.J.; Huang, C.C.; Wang, S.W.; Liu, S.C.; Chen, W.C.; Fuh, L.J.; Tang, C.H. Ccn1 promotes vegf production in osteoblasts and induces endothelial progenitor cell angiogenesis by inhibiting mir-126 expression in rheumatoid arthritis. J. Bone Miner. Res. 2017, 32, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Morel, J.C.; Amin, M.A.; Connors, M.A.; Harlow, L.A.; Koch, A.E. Evidence of il-18 as a novel angiogenic mediator. J. Immunol. 2001, 167, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Volin, M.V.; Koch, A.E. Interleukin-18: A mediator of inflammation and angiogenesis in rheumatoid arthritis. J. Interferon Cytokine Res. 2011, 31, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Ruth, J.H.; Park, C.C.; Amin, M.A.; Lesch, C.; Marotte, H.; Shahrara, S.; Koch, A.E. Interleukin-18 as an in vivo mediator of monocyte recruitment in rodent models of rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R118. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.M.; Matsuno, H.; Nakamura, H.; Nishioka, K.; Yudoh, K. Interleukin-18 enhances monocyte tumor necrosis factor α and interleukin-1β production induced by direct contact with T lymphocytes: Implications in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Rabquer, B.J.; Mansfield, P.J.; Ruth, J.H.; Marotte, H.; Haas, C.S.; Reamer, E.N.; Koch, A.E. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann. Rheum. Dis. 2010, 69, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Huang, C.Y.; Tang, C.H. Characteristics of resistin in rheumatoid arthritis angiogenesis. Biomark. Med. 2016, 10, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Noel, D.; Pers, Y.M.; Apparailly, F.; Jorgensen, C. Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat. Rev. Rheumatol. 2016, 12, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, G.; Suffert, G.; Semaan, N.; Juncker, T.; Frenzel, L.; Gottenberg, J.E.; Sibilia, J.; Pfeffer, S.; Wachsmann, D. Bruton’s tyrosine kinase is involved in miR-346-related regulation of IL-18 release by lipopolysaccharide-activated rheumatoid fibroblast-like synoviocytes. J. Immunol. 2009, 182, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Seike, M.; Takeuchi, S.; Soeno, C.; Miyanaga, A.; Noro, R.; Minegishi, Y.; Kubota, K.; Gemma, A. Mir-379/411 cluster regulates IL-18 and contributes to drug resistance in malignant pleural mesothelioma. Oncol. Rep. 2014, 32, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Chen, P.C.; Tsao, C.W.; Chang, A.C.; Lein, M.Y.; Lin, C.C.; Wang, S.W.; Lin, C.W.; Tang, C.H. WISP-1, a novel angiogenic regulator of the CCN family, promotes oral squamous cell carcinoma angiogenesis through VEGF-A expression. Oncotarget 2015, 6, 4239–4252. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Cheng, H.C.; Wang, J.; Wang, S.W.; Tai, H.C.; Lin, C.W.; Tang, C.H. Prostate cancer-derived CCN3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment. Oncotarget 2014, 5, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Chang, A.C.; Wang, S.W.; Wang, S.J.; Chang, Y.S.; Chang, T.M.; Hsu, S.K.; Fong, Y.C.; Tang, C.H. Leptin promotes VEGF-C production and induces lymphangiogenesis by suppressing miR-27b in human chondrosarcoma cells. Sci. Rep. 2016, 6, 28647. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck, F.; Gaufillier, S.; Bonnaud, A.; Sabatini, M.; Lesur, C.; Pastoureau, P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Biophys. Res. Commun. 2001, 285, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.G.; Vaughan, A.; Dent, A.G.; O’Hare, P.E.; Goh, F.; Bowman, R.V.; Fong, K.M.; Yang, I.A. Biomarkers of progression of chronic obstructive pulmonary disease (COPD). J. Thorac. Dis. 2014, 6, 1532–1547. [Google Scholar] [PubMed]
- Specjalski, K.; Chelminska, M.; Jassem, E. YKL-40 protein correlates with the phenotype of asthma. Lung 2015, 193, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hakala, B.E.; White, C.; Recklies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [PubMed]
- Volck, B.; Johansen, J.S.; Stoltenberg, M.; Garbarsch, C.; Price, P.A.; Ostergaard, M.; Ostergaard, K.; Lovgreen-Nielsen, P.; Sonne-Holm, S.; Lorenzen, I. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthr. Cartil. 2001, 9, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Dodds, R.A.; Emery, J.G.; Kirkpatrick, R.B.; Rosenberg, M.; Gowen, M. Human cartilage glycoprotein 39 (HC gp-39) mRNA expression in adult and fetal chondrocytes, osteoblasts and osteocytes by in-situ hybridization. Osteoarthr. Cartil. 2000, 8, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chim, S.M.; Tickner, J.; Chow, S.T.; Kuek, V.; Guo, B.; Zhang, G.; Rosen, V.; Erber, W.; Xu, J. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013, 24, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Qin, L.; Tamasi, J.; Jefcoat, S.C., Jr.; Shimizu, E.; Selvamurugan, N.; Liew, F.Y.; Bevelock, L.; Feyen, J.H.; Partridge, N.C. Interleukin-18 is regulated by parathyroid hormone and is required for its bone anabolic actions. J. Biol. Chem. 2008, 283, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tsurumoto, T. Serum YKL-40 levels in rheumatoid arthritis: Correlations between clinical and laborarory parameters. Clin. Exp. Rheumatol. 2001, 19, 655–660. [Google Scholar] [PubMed]
- Huang, C.Y.; Chen, S.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. Thrombin induces epidermal growth factor receptor transactivation and CCL2 expression in human osteoblasts. Arthritis Rheum. 2012, 64, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Lee, W.L.; Hsu, C.J.; Lu, T.T.; Wang, L.H.; Xu, G.H.; Tang, C.H. Adiponectin induces oncostatin m expression in osteoblasts through the PI3K/Akt signaling pathway. Int. J. Mol. Sci. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Hammaker, D.; Firestein, G.S. Phosphoinositide 3-kinase δ regulates migration and invasion of synoviocytes in rheumatoid arthritis. J. Immunol. 2014, 192, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Lau, S.H.; Lv, Z.L.; Xie, D.; Li, W.; Lai, Y.R.; Zhong, J.M.; Wu, H.Q.; Su, Q.; He, Y.L.; et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum. Pathol. 2009, 40, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, A.S.; Sayed, A.; Broskova, Z.; Teoh, J.P.; Wilson, J.; Su, H.; Tang, Y.L.; Kim, I.M. Crosstalk between long noncoding RNAs and microRNAs in health and disease. Int. J. Mol. Sci. 2016, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Hsu, C.J.; Tsai, C.H.; Huang, C.Y.; Wang, S.W.; Tang, C.H. Resistin promotes angiogenesis in endothelial progenitor cells through inhibition of microRNA206: Potential implications for rheumatoid arthritis. Stem Cells 2015, 33, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, C.Y.; Lin, J.A.; Wang, S.W.; Peng, C.Y.; Cheng, H.C.; Tang, C.H. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene 2014, 33, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Huang, C.Y.; Tsai, C.H.; Wang, S.W.; Lin, Y.M.; Tang, C.H. Interleukin-1β induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clin. Sci. 2016, 130, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Liu, L.C.; Hsiao, C.L.; Su, C.H.; Wang, H.C.; Ji, H.X.; Tsai, C.W.; Maa, M.C.; Bau, D.T. The contributions of the tissue inhibitor of metalloproteinase-1 genotypes to triple negative breast cancer risk. Biomedicine 2016, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Lien, C.H.; Lee, M.F.; Huang, C.Y. Quercetin suppresses cellular migration and invasion in human head and neck squamous cell carcinoma (HNSCC). Biomedicine 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Tsai, H.C.; Chou, P.Y.; Wang, S.W.; Chen, H.T.; Lin, Y.M.; Chiang, I.P.; Chang, T.M.; Hsu, S.K.; Chou, M.C.; et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/VEGF-A axis in human osteosarcoma cells. Oncotarget 2016, 7, 4310–4325. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-M.; Liu, S.-C.; Huang, Y.-H.; Huang, C.-C.; Hsu, C.-J.; Tsai, C.-H.; Wang, S.-W.; Tang, C.-H. YKL-40-Induced Inhibition of miR-590-3p Promotes Interleukin-18 Expression and Angiogenesis of Endothelial Progenitor Cells. Int. J. Mol. Sci. 2017, 18, 920. https://doi.org/10.3390/ijms18050920
Li T-M, Liu S-C, Huang Y-H, Huang C-C, Hsu C-J, Tsai C-H, Wang S-W, Tang C-H. YKL-40-Induced Inhibition of miR-590-3p Promotes Interleukin-18 Expression and Angiogenesis of Endothelial Progenitor Cells. International Journal of Molecular Sciences. 2017; 18(5):920. https://doi.org/10.3390/ijms18050920
Chicago/Turabian StyleLi, Te-Mao, Shan-Chi Liu, Ya-Hsin Huang, Chien-Chung Huang, Chin-Jung Hsu, Chun-Hao Tsai, Shih-Wei Wang, and Chih-Hsin Tang. 2017. "YKL-40-Induced Inhibition of miR-590-3p Promotes Interleukin-18 Expression and Angiogenesis of Endothelial Progenitor Cells" International Journal of Molecular Sciences 18, no. 5: 920. https://doi.org/10.3390/ijms18050920
APA StyleLi, T.-M., Liu, S.-C., Huang, Y.-H., Huang, C.-C., Hsu, C.-J., Tsai, C.-H., Wang, S.-W., & Tang, C.-H. (2017). YKL-40-Induced Inhibition of miR-590-3p Promotes Interleukin-18 Expression and Angiogenesis of Endothelial Progenitor Cells. International Journal of Molecular Sciences, 18(5), 920. https://doi.org/10.3390/ijms18050920





