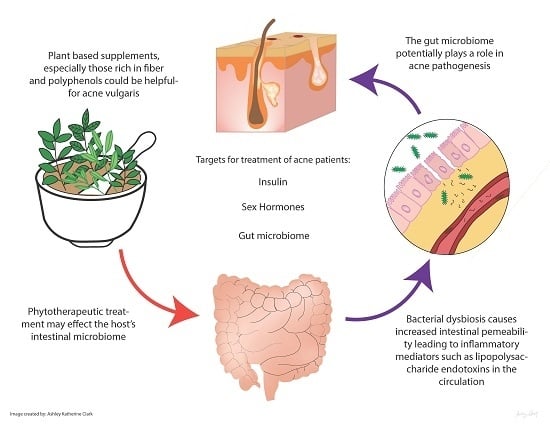
Edible Plants and Their Influence on the Gut Microbiome and Acne
Abstract
1. Introduction
2. Methods
3. Gut Microbiome and the Skin
3.1. Altered Gut Function Impacts the Skin
3.2. Gut Microbiome Dysbiosis and Acne
4. Probiotics Improve Acne
5. Edible Plants and Acne
5.1. Insulin
5.2. Sex Hormones
5.3. Antimicrobial
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- White, G.M. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J. Am. Acad. Dermatol. 1998, 39, S34–S37. [Google Scholar] [CrossRef]
- Morohashi, M.; Toyoda, M. Pathogenesis of acne: Medical electron microscopy. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Paugam, C.; Corvec, S.; Saint-Jean, M.; Le Moigne, M.; Khammari, A.; Boisrobert, A.; Nguyen, J.; Gaultier, A.; Dréno, B. Propionibacterium acnes phylotypes and acne severity: An observational prospective study. J. Eur. Acad. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lheure, C.; Grange, P.A.; Ollagnier, G.; Morand, P.; Désiré, N.; Sayon, S.; Corvec, S.; Raingeaud, J.; Marcelin, A.-G.; Calvez, V. Tlr-2 recognizes propionibacterium acnes camp factor 1 from highly inflammatory strains. PLoS ONE 2016, 11, e0167237. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Suh, D.H. Recent progress in the research about propionibacterium acnes strain diversity and acne: Pathogen or bystander? Int. J. Dermatol. 2016, 55, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Toossi, P.; Farshchian, M.; Malekzad, F.; Mohtasham, N.; Kimyai-Asadi, A. Subantimicrobial-dose doxycycline in the treatment of moderate facial acne. J. Drugs Dermatol. 2008, 7, 1149–1152. [Google Scholar] [PubMed]
- Culic, O.; Erakovic, V.; Parnham, M.J. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 2001, 429, 209–229. [Google Scholar] [CrossRef]
- Eady, E.A.; Ingham, E.; Walters, C.E.; Cove, J.H.; Cunliffe, W.J. Modulation of comedonal levels of interleukin-1 in acne patients treated with tetracyclines. J. Investig. Dermatol. 1993, 101, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: A review. J. Drugs Dermatol. 2010, 9, 655–664. [Google Scholar] [PubMed]
- Milstone, E.B.; McDonald, A.J.; Scholhamer, C.F., Jr. Pseudomembranous colitis after topical application of clindamycin. Arch. Dermatol. 1981, 117, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Tulkens, P.M. Hepatic safety of antibiotics used in primary care. J. Antimicrob. Chemother. 2011, 66, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Lalla, J.K.; Nandedkar, S.Y.; Paranjape, M.H.; Talreja, N.B. Clinical trials of ayurvedic formulations in the treatment of acne vulgaris. J. Ethnopharmacol. 2001, 78, 99–102. [Google Scholar] [CrossRef]
- He, J.M.; Mu, Q. The medicinal uses of the genus mahonia in traditional chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; KoSt'álová, D.; Labudová, D.; Kotulová, D.; Kettmann, V. Antimicrobial activity of mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004, 18, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, L.; Qiang, Q.; Ji, L.; Wang, X.; Luo, H.; Wu, H.; Jiang, Y.; Wang, G.; Shen, T. The dichloromethane fraction from Mahonia bealei (Fort.) Carr. Leaves exerts an anti-inflammatory effect both in vitro and in vivo. J. Ethnopharmacol. 2016, 188, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Ziereis, K.; Gawlik, I. The antipsoriatic Mahonia Aquifolium and its active constituents; II. Antiproliferative activity against cell growth of human keratinocytes. Planta Med. 1995, 61, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Morohashi, M. Effect of some alkaloids, flavonoids and triterpenoids, contents of Japanese-Chinese traditional herbal medicines, on the lipogenesis of sebaceous glands. Skin Pharmacol. Physiol. 1993, 6, 56–60. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.H.; Hsu, C.H. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement. Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K.; Hirakawa, H.; Oikawa, D.; Nakanishi, T.; Takagi, T.; Tachibana, T.; Furuse, M. Effect of garcinia cambogia extract on serum leptin and insulin in mice. Fitoterapia 2003, 74, 267–273. [Google Scholar] [CrossRef]
- Pan-In, P.; Wongsomboon, A.; Kokpol, C.; Chaichanawongsaroj, N.; Wanichwecharungruang, S. Depositing α-mangostin nanoparticles to sebaceous gland area for acne treatment. J. Pharmacol. Sci. 2015, 129, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Thappa, D.M.; Dogra, J. Nodulocystic acne: Oral gugulipid versus tetracycline. J. Dermatol. 1994, 21, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Goyal, C.; Ahuja, M.; Sharma, S.K. Preparation and evaluation of anti-inflammatory activity of gugulipid-loaded proniosomal gel. Acta. Pol. Pharm. 2011, 68, 147–150. [Google Scholar] [PubMed]
- Paranjpe, P.; Kulkarni, P.H. Comparative efficacy of four ayurvedic formulations in the treatment of acne vulgaris: A double-blind randomised placebo-controlled clinical evaluation. J. Ethnopharmacol. 1995, 49, 127–132. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, H.-Y. In vitro anti-propionibacterium activity by curcumin containing vesicle system. Chem. Pharm. Bull. 2013, 61, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Hegge, A.B.; Bruzell, E.; Kristensen, S.; Tønnesen, H. Photoinactivation of Staphylococcus Epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin–effect of selected nanocarrier: Studies on curcumin and curcuminoides XLVII. Eur. J. Pharm. Sci. 2012, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.H.; Pillsbury, D.M. The effect on the skin of emotional and nervous states: I II. Theoretical and practical consideration of a gastro-intestinal mechanism. Arch. Derm. Syphilol. 1930, 22, 962–993. [Google Scholar] [CrossRef]
- Reddymasu, S.C.; Sostarich, S.; McCallum, R.W. Small intestinal bacterial overgrowth in irritable bowel syndrome: Are there any predictors? BMC Gastroenterol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Foti, M.; Ruggia, O.; Chiecchio, A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin. Gastroenterol. Hepatol. 2010, 8, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.; Patel, N.; Logan, A. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef. Microbes 2014, 5, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Strickler, A.; Kolmer, J.A.; Schamberg, J.F. Complement fixation in acne vulgaris. J. Cutan. Dis. 1916, 34, 166–178. [Google Scholar]
- Juhlin, L.; Michaëlsson, G. Fibrin microclot formation in patients with acne. Acta Derm. Venereol. 1983, 63, 538–540. [Google Scholar] [PubMed]
- Possemiers, S.; Grootaert, C.; Vermeiren, J.; Gross, G.; Marzorati, M.; Verstraete, W.; van de Wiele, T. The intestinal environment in health and disease-Recent insights on the potential of intestinal bacteria to influence human health. Curr. Pharm. Des. 2009, 15, 2051–2065. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Loveman, D.E.; Noojin, R.O.; Winkler, C.H., Jr. Comparative studies of enteric bacterial flora in acne vulgaris. J. Investig. Dermatol. 1955, 25, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Volkova, L.A.; Khalif, I.L.; Kabanova, I.N. Impact of the impaired intestinal microflora on the course of acne vulgaris. Klin. Med. 2001, 79, 39–41. [Google Scholar]
- Watanabe, S.; Narisawa, Y.; Arase, S.; Okamatsu, H.; Ikenaga, T.; Tajiri, Y.; Kumemura, M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 2003, 111, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Karlsson, C.; Olsson, C.; Adlerberth, I.; Wold, A.E.; Strachan, D.P.; Martricardi, P.M.; Aberg, N.; Perkin, M.R.; Tripodi, S.; et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Schroten, H.; Adam, R. The role of prebiotics and probiotics in prevention and treatment of childhood infectious diseases. Pediatr. Infect. Dis. J. 2012, 31, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Veereman-Wauters, G.; de Greef, E.; Peeters, S.; Casteels, A.; Mahler, T.; Devreker, T.; Hauser, B. Probiotics and prebiotics in prevention and treatment of diseases in infants and children. J. Pediatr. 2011, 87, 292–300. [Google Scholar] [CrossRef]
- Wold, A.E. The hygiene hypothesis revised: Is the rising frequency of allergy due to changes in the intestinal flora? Allergy 1998, 53, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.J. Probiotics, gut microbiota and health. Méd. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hacini-Rachinel, F.; Gheit, H.; Le Luduec, J.-B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory t cells. PLoS ONE 2009, 4, e4903. [Google Scholar] [CrossRef] [PubMed]
- Ereaux, L. Facts, fads and fancies in the treatment of acne vulgaris. CMAJ 1938, 39, 257–261. [Google Scholar]
- Siver, R. Lactobacillus for the control of acne. J. Med. Soc. N. J. 1961, 59, 52–53. [Google Scholar]
- Marchetti, F.; Capizzi, R.; Tulli, A. Efficacy of regulators of the intestinal bacterial flora in the therapy of acne vulgaris. Clin. Ther. 1987, 122, 339–343. [Google Scholar]
- Bowe, W.P.; Logan, A.C. Clinical implications of lipid peroxidation in acne vulgaris: Old wine in new bottles. Lipids Health Dis. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Thomas, D.R.; Kumar, V.B.; Brown, C.; Hager, C.; Van't Hof, M.A.; Morley, J.E.; Guigoz, Y. Systemic inflammatory markers in older persons: The effect of oral nutritional supplementation with prebiotics. J. Nutr. Health Aging 2007, 11, 475–479. [Google Scholar] [PubMed]
- Cazzola, M.; Tompkins, T.A.; Matera, M.G. Immunomodulatory impact of a synbiotic in th1 and th2 models of infection. Ther. Adv. Respir. Dis. 2010, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Glück, U.; Gebbers, J.-O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (staphylococcus aureus, streptococcus pneumoniae, and β-hemolytic streptococci). Am. J. Clin. Nutr. 2003, 77, 517–520. [Google Scholar] [PubMed]
- Smith, R.N.; Mann, N.J.; Braue, A.; Mäkeläinen, H.; Varigos, G.A. The effect of a high-protein, low glycemic–load diet versus a conventional, high glycemic–load diet on biochemical parameters associated with acne vulgaris: A randomized, investigator-masked, controlled trial. J. Am. Acad. Dermatol. 2007, 57, 247–256. [Google Scholar] [CrossRef] [PubMed]
- McGill, C.R.; Devareddy, L. Ten-year trends in fiber and whole grain intakes and food sources for the united states population: National health and nutrition examination survey 2001–2010. Nutrients 2015, 7, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [PubMed][Green Version]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Ellekilde, M.; Krych, L.; Hansen, C.H.F.; Hufeldt, M.R.; Dahl, K.; Hansen, L.H.; Sørensen, S.J.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Characterization of the gut microbiota in leptin deficient obese mice–correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.; Inglis, G.D. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Emiroğlu, N.; Cengiz, F.P.; Kemeriz, F. Insulin resistance in severe acne vulgaris. Adv. Dermatol. Allergol. 2015, 32, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, M.; Mauriello, M.C.; Faggiano, A.; Di Somma, C.; Monfrecola, G.; Fabbrocini, G.; Colao, A. Insulin resistance and acne: A new risk factor for men? Endocrine 2012, 42, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Matye, D.J.; Li, T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J. Biol. Chem. 2015, 290, 11526–11536. [Google Scholar] [CrossRef] [PubMed]
- Sripradha, R.; Magadi, S.G. Efficacy of garcinia cambogia on body weight, inflammation and glucose tolerance in high fat fed male wistar rats. J. Clin. Diagn. Res. 2015, 9, BF01–BF04. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chitchumroonchokchai, C.; Chuang, C.-C.; West, T.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. 2009, 139, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, K.; Li, H.; Cen, J.; Yang, Y.; Wu, H.; Wei, Q. Isogarcinol extracted from garcinia mangostana l. Ameliorates imiquimod-induced psoriasis-like skin lesions in mice. J. Agric. Food Chem. 2017, 65, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Tg Zakaria, T.M.F.S.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of garcinia mangostana linn. In normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Vetere Amedeo, C.A.; Burns Sean, M.; Wagner Bridget, K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Burton, J.; Heiman, M.; Greenway, F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015, 29, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Savcheniuk, O.; Kobyliak, N.; Kondro, M.; Virchenko, O.; Falalyeyeva, T.; Beregova, T. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates ampk and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, R.; Venables, Z.; Millington, G. The menstrual cycle and the skin. Clin. Exp. Dermatol. 2015, 40, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Zouboulis, C.C.; Orfanos, C.E. Control of human sebocyte proliferation in vitro by testosterone and 5-α-dihydrotestosterone is dependent on the localization of the sebaceous glands. J. Investig. Dermatol. 1992, 99, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Sansone, G.; Reisner, R.M. Differential rates of conversion of testerone to dihydrotestosterone in acne and in normal human skin-A possible pathogenic factor in acne. J. Investig. Dermatol. 1971, 56, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Turrion-Merino, L.; Urech-García-de-la-Vega, M.; Miguel-Gomez, L.; Harto-Castaño, A.; Jaen-Olasolo, P. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015, 151, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Wierckx, K.; Van de Peer, F.; Verhaeghe, E.; Dedecker, D.; van Caenegem, E.; Toye, K.; Kaufman, J.M.; T'sjoen, G. Short-and long-term clinical skin effects of testosterone treatment in trans men. J. Sex. Med. 2014, 11, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.; Gooren, L. Effects of sex steroid deprivation/administration on hair growth and skin sebum production in transsexual males and females. J. Clin. Endocrinol. Metab. 2000, 85, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Risch, J.; McElwee, K.; Marchessault, P.; Bolduc, C.; Nigen, S.; Maari, C. Changes in serum free testosterone, sleep patterns, and 5-α-reductase type i activity influence changes in sebum excretion in female subjects. Skin Res. Technol. 2015, 21, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Al-Tikriti, A.; Al-Khateeb, E.; Abbas, M. Teucrium polium hexane extract downregulated androgen receptor in testis and decreased fertility index in rats. Hum. Exp. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D. Acne: Hormonal concepts and therapy. Clin. Dermatol. 2004, 22, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Vogel, K.; Fimmel, S.; Oeff, M.; Seltmann, H.; Zouboulis, C.C. Interplay of IGF-I and 17β-estradiol at age-specific levels in human sebocytes and fibroblasts in vitro. Exp. Gerontol. 2008, 43, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Pochi, P.E.; Strauss, J.S. Sebaceous gland suppression with ethynyl estradiol and diethylstilbestrol. Arch. Dermatol. 1973, 108, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Reinli, K.; Block, G. Phytoestrogen content of foods—A compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. In Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.K.; Shenefelt, P.D. Herbal therapy in dermatology. Arch. Dermatol. 2002, 138, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Najimi, S.A.; Farbood, Y. Effects of vitex agnus-castus fruit on sex hormones and antioxidant indices in a d-galactose-induced aging female mouse model. J. Chin. Med. Assoc. 2016, 79, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; Van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Allahtavakoli, M.; Honari, N.; Pourabolli, I.; Arababadi, M.K.; Ghafarian, H.; Roohbakhsh, A.; Nadimi, A.E.; Shamsizadeh, A. Vitex agnus castus extract improves learning and memory and increases the transcription of estrogen receptor α in hippocampus of ovariectomized rats. Basic Clin. Neurosci. 2015, 6, 185–192. [Google Scholar] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
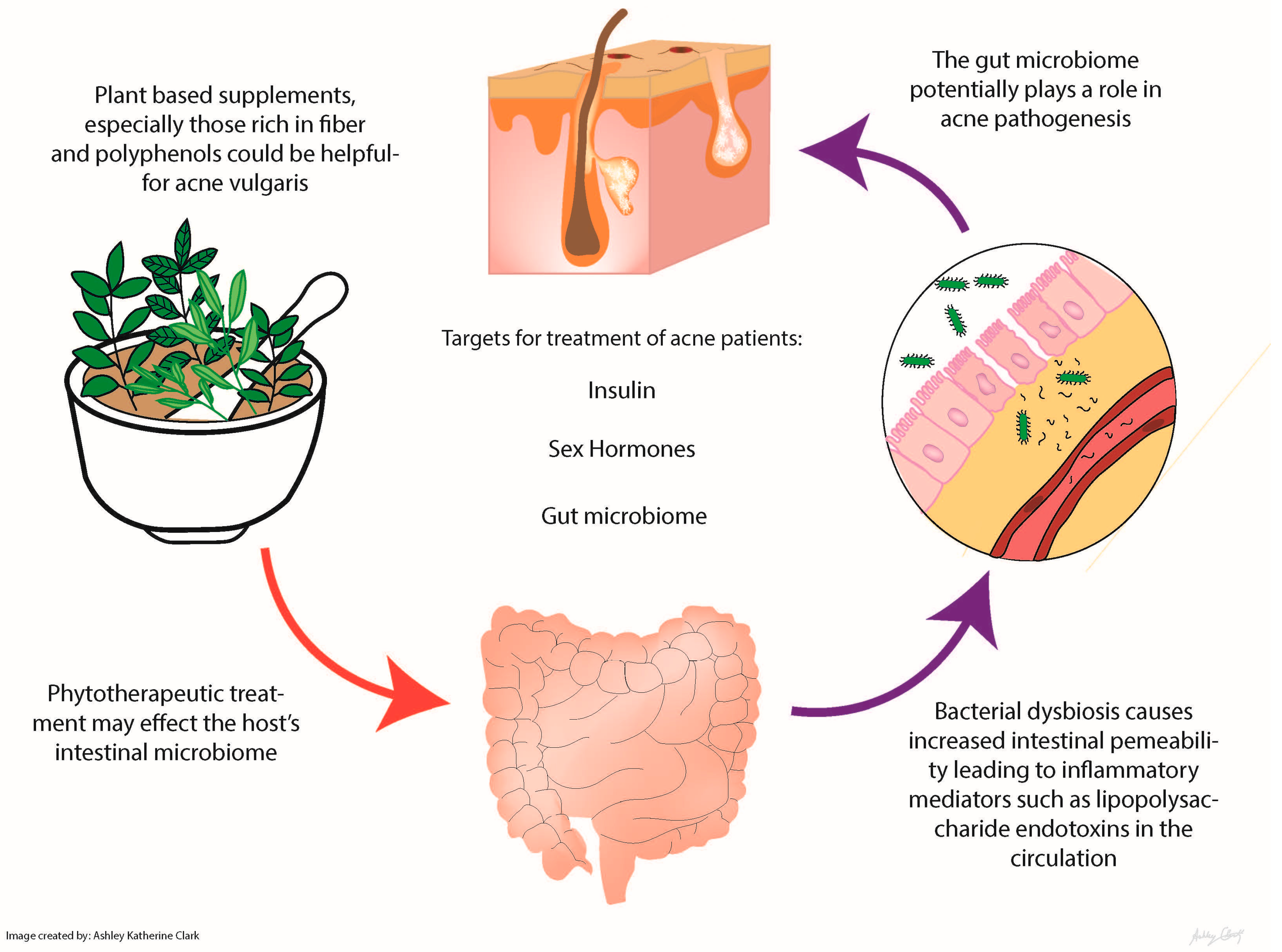
| Phytotherapeutic | Mechanisms | Study Type | Comparison | Number of Subjects | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Ayurvedic plant extracts: Aloe barbadensis, Azadirachta indica, Curcuma longa, Hemidesmus indicus, Terminalia chebula, Terminalia arjuna and Withania somnifera | Antibacterial and anti-inflammatory activity Azadirachta indica = antipyretic and anthelmintic Curcuma longa (turmeric) and Azadirachta indica = anti-inflammatory effects by suppressing Propionibacterium acnes-induced reactive oxygen species and pro-inflammatory cytokines | In vitro Double-blind, placebo-controlled clinical trial in India | Placebo | 53 mild to moderately severe acne patients 14–28 years old tested | The study found that the combined treatment of tablets and topical formulation of the plant extracts showed better results than the tablets alone, but the oral preparation was more efficacious than the topical alone | Lalla et al. (2001) [14] |
| Berberine | Relieve insulin resistance Antimicrobial against common skin microbes, like Propionibacterium acnes, Staphylococcus spp. and Malassezia spp. Antiproliferative effect on keratinocytes Decrease lipogenesis of sebaceous glands | Randomized controlled trial Hepatic cells in vitro In hamsters | Herbal supplement vs. control group taking minocycline | 92 patients with acne vulgaris | No difference between the berberine and minocycline group; this suggests that herbal supplementation may be just as effective as the standard antibiotics without the drawbacks [15] | He et al. (2015) [15]; Slobodníková et al. (2004) [16]; Hu et al. (2016) [17]; Muller et al. (1995) [18]; Seki et al. (1993) [19] |
| Berry extract (most data on strawberries) | Polyphenol with unknown molecular mechanism | In vitro | None | None | Berry extract reduced glucose uptake by human intestinal epithelial cells | Kim et al. (2016) [20] Lu et al. (2016) [21] |
| Garcinia (α mangostin) | Antibacterial, attenuation of de novo lipid synthesis | In vitro Human | None | None | Improved insulin sensitivity and attenuated LPS-induced inflammation Topical application improved acne severity | Hayamizu et al. (2003) [22]; Pan-In et al. (2015) [23] |
| Green tea extract | Epigallocatechin-3-gallate (EGCG), the major polyphenol in green tea, has potent anticarcinogenic, anti-inflammatory and antimicrobial activities; EGCG can modulate several key pathological factors of acne, including hyperseborrhea, lipogenesis, inflammation and P. acnes overgrowth | Randomized, double-blind, placebo controlled clinical trial | 1500 mg of decaffeinated green tea extract vs. placebo (cellulose) | 80 25–45 year-old women with post-adolescent acne | Decreased acne lesions in postpubescent females with a trending decrease in fasting blood sugar | Lu et al. (2016) [21] |
| Gugulipid | Potent hypolipidemic agent; Antimicrobial, anthelmintic, anti-inflammatory, anti-arthritic and antioxidant properties | Randomized controlled trial | Tetracycline 500 mg vs. gugulipid 25 mg | Twenty patients with nodulocystic acne | Both produced a progressive reduction in the lesions; with tetracycline, the percentage reduction in the inflammatory lesions was 65.2% as compared to 68% with gugulipid (p > 0.05) | Thappa et al. (1994) [24]; Goyal et al. (2011) [25] |
| Sunder Vati (Holarrhena antidysenterica, Emblica officinalis and Zingiber officinale) | Unknown | An Indian double-blind placebo-controlled trial | Placebo | 20 | Treatment was associated with significant reduction (p < 0.01) in lesion count of approximately 60%; there was a significant reduction in the total number of inflammatory lesions within 2 weeks with further reduction at each observation period during the 6-week treatment | Paranjpe et al. (1995) [26] |
| Turmeric | Antimicrobial, anti-inflammatory and antidiabetic | In vitro | None | None | Several studies have shown growth inhibition of the common skin bacteria Propionibacterium acnes, Staphylococcus epidermidis and Staphylococcus aureus when curcumin is used topically | Liu et al (2013) [27]; Mun et al. (2013) [28]; Hegge et al. (2012) [29] |
| Vitex | Binds to estrogen receptors Acts on follicle-stimulating hormone and luteinizing hormone levels in the pituitary to increase progesterone levels Increase in estrogen level | In vitro | None | None | Commonly used in traditional Chinese medicine for menopausal symptoms, but their role as estrogen analogues also makes them promising for attenuation of acne | Allahtavakoli et al. (2015); Bedi et al. (2002); Ahangarpour et al. (2016) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, A.K.; Haas, K.N.; Sivamani, R.K. Edible Plants and Their Influence on the Gut Microbiome and Acne. Int. J. Mol. Sci. 2017, 18, 1070. https://doi.org/10.3390/ijms18051070
Clark AK, Haas KN, Sivamani RK. Edible Plants and Their Influence on the Gut Microbiome and Acne. International Journal of Molecular Sciences. 2017; 18(5):1070. https://doi.org/10.3390/ijms18051070
Chicago/Turabian StyleClark, Ashley K., Kelly N. Haas, and Raja K. Sivamani. 2017. "Edible Plants and Their Influence on the Gut Microbiome and Acne" International Journal of Molecular Sciences 18, no. 5: 1070. https://doi.org/10.3390/ijms18051070
APA StyleClark, A. K., Haas, K. N., & Sivamani, R. K. (2017). Edible Plants and Their Influence on the Gut Microbiome and Acne. International Journal of Molecular Sciences, 18(5), 1070. https://doi.org/10.3390/ijms18051070