Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1-38 in Rodents
Abstract
:1. Introduction
2. Results
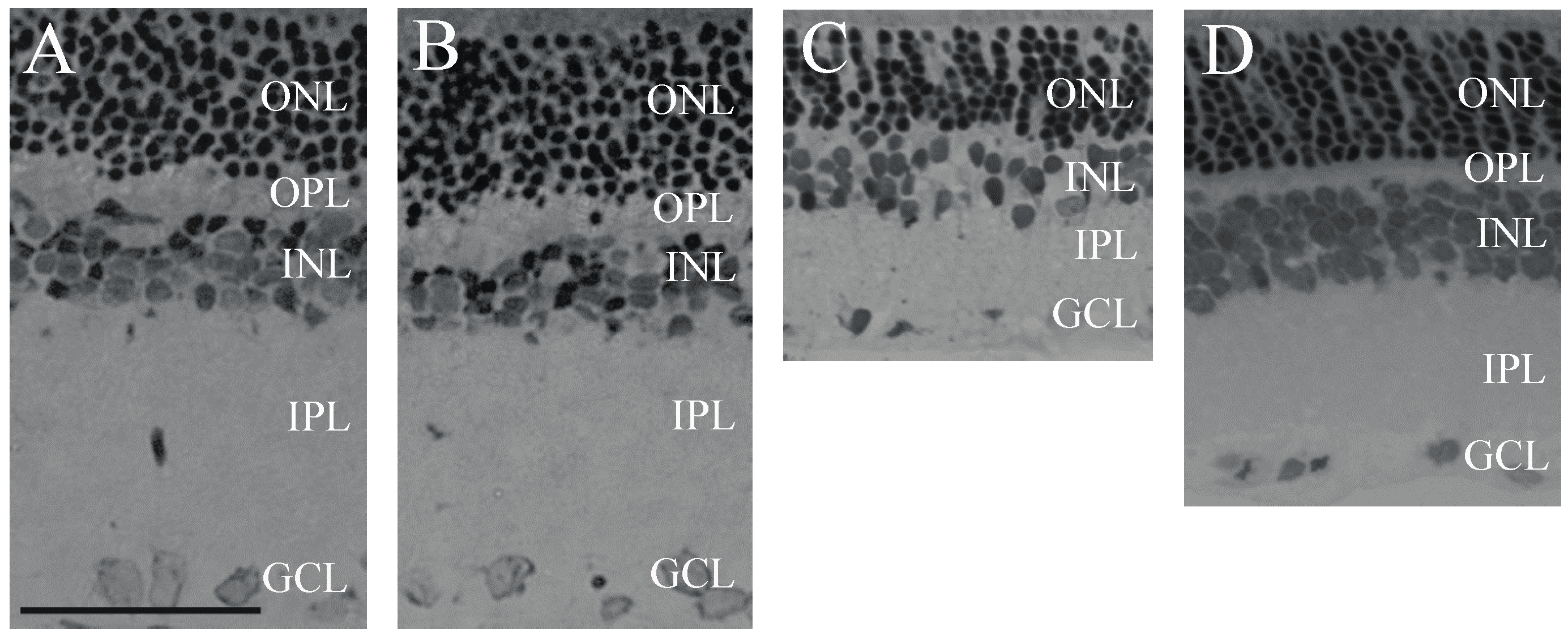
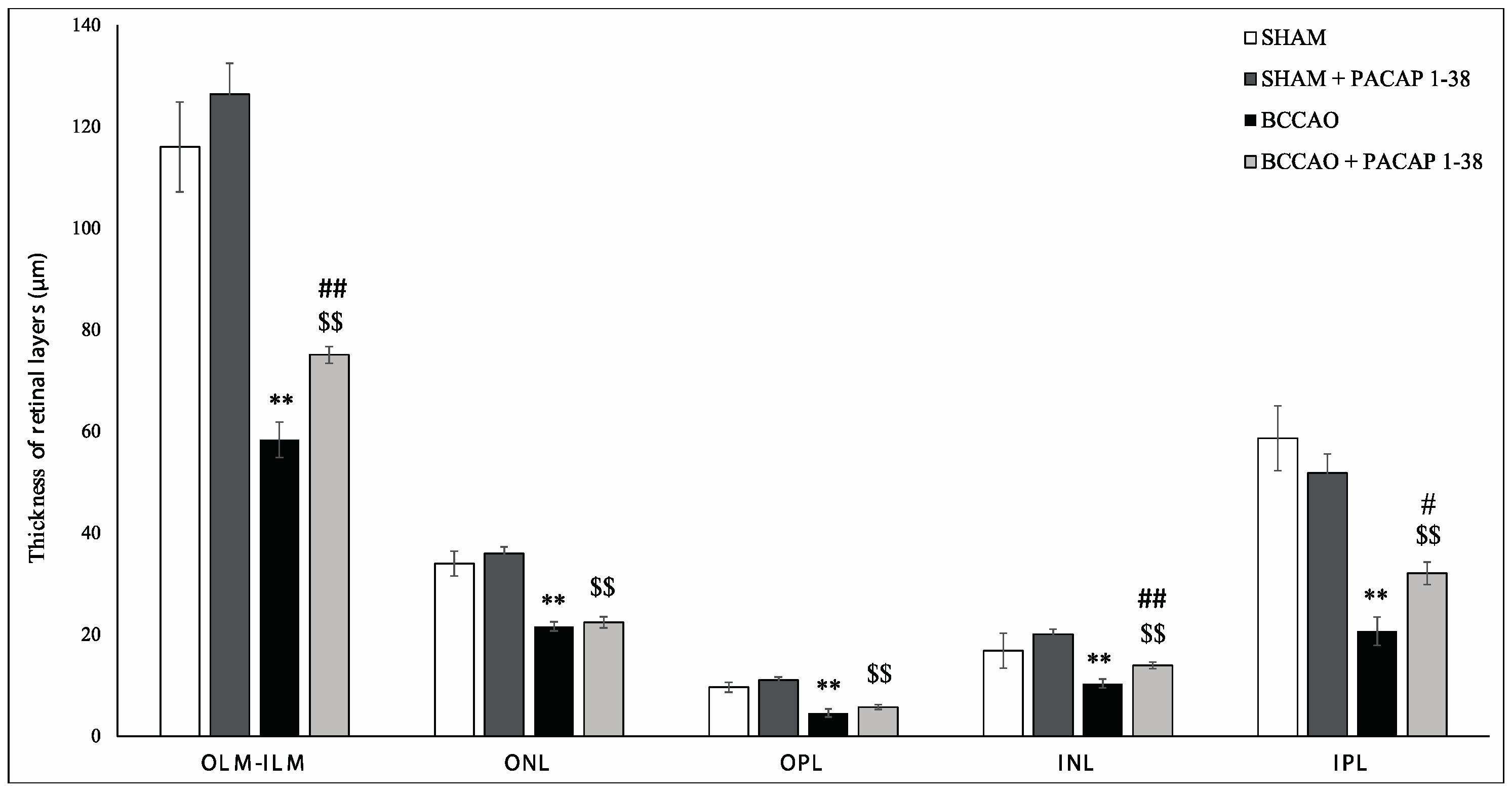
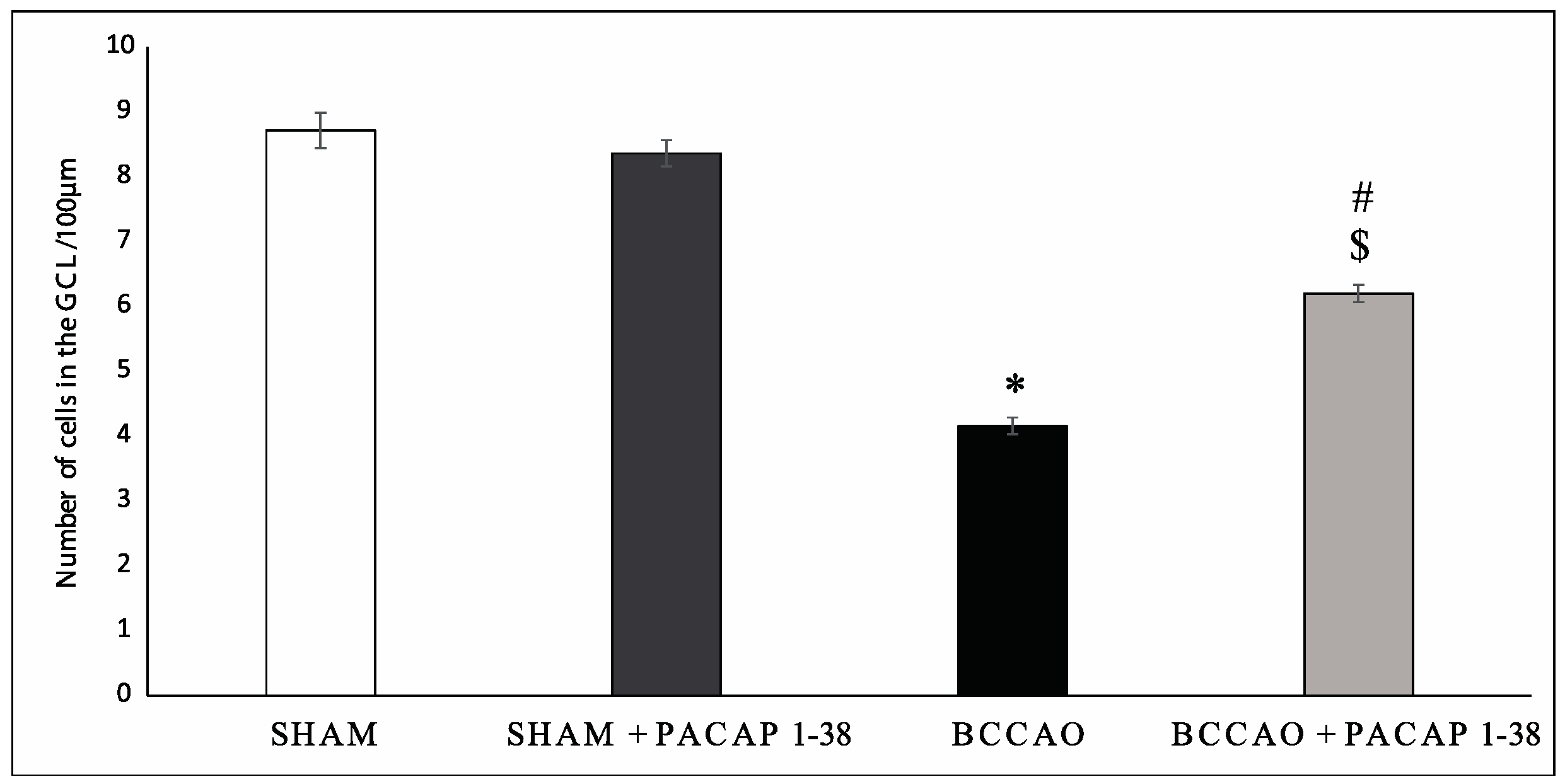
2.1. Retina Morphology and Morphometry
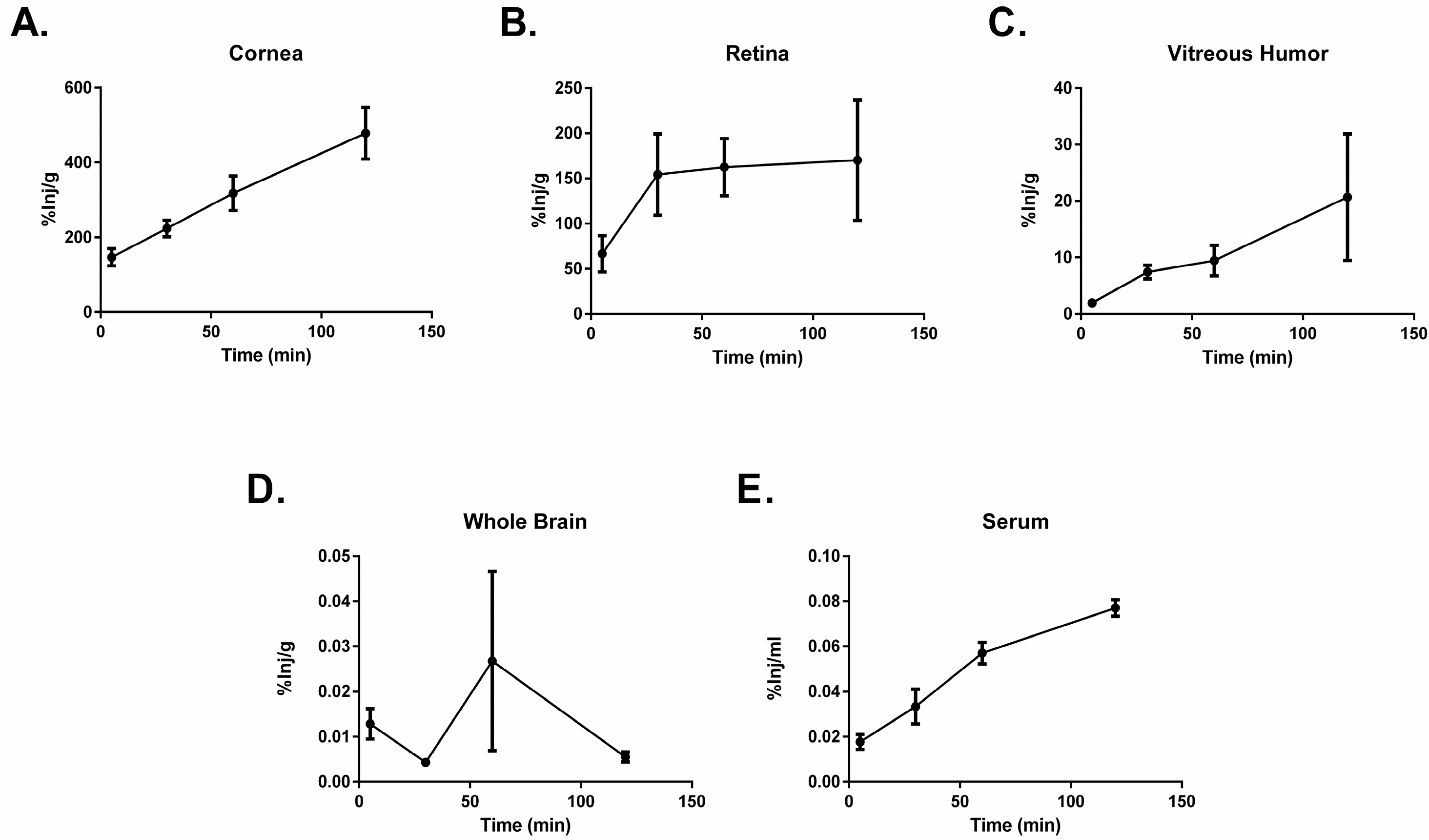
2.2. PACAP1-38 Uptake after Ocular Administration
2.3. PACAP1-38 Stability after Ocular Administration
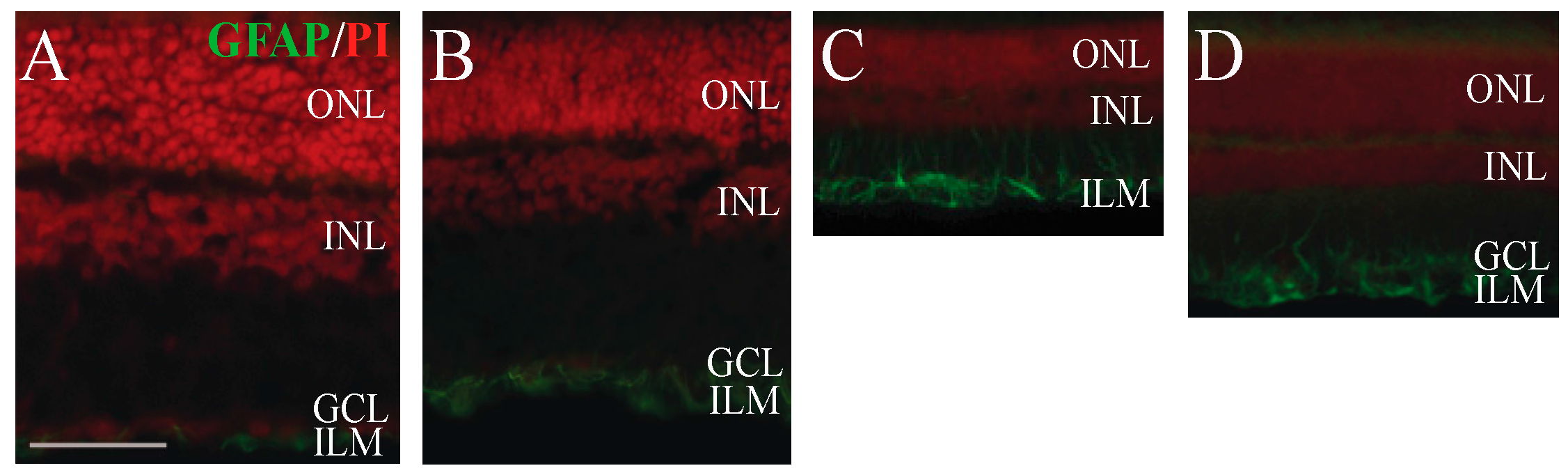
2.4. Immunohistochemical Analysis of Retinal Glial Cell Activation
3. Discussion
4. Materials and Methods
4.1. Surgery and PACAP1-38 in Benzalkonium Solution Treatment
4.2. Histological Analysis of PACAP1-38 Eye Drops
4.3. Analysis of the Passage of PACAP1-38 Eye Drops through the Ocular Barriers
4.4. In Vivo Stability of 125I-PACAP1-38
4.5. Measurement of Glial Fibrillary Acidic Protein (GFAP) Activity in the Müller Glial Cells
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garami, A.; Pakai, E.; Rumbus, Z.; Solymar, M. The role of PACAP in the regulation of body temperature. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; Volume 11, pp. 239–257. [Google Scholar]
- Clason, T.A.; Girard, B.M.; May, V.; Parsons, R.L. Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J. Mol. Neurosci. 2016, 59, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bardosi, S.; Bardosi, A.; Nagy, Z.; Reglodi, D. Expression of PACAP and PAC1 receptor in normal human thyroid gland and in thyroid papillary carcinoma. J. Mol. Neurosci. 2016, 60, 171–178. [Google Scholar] [CrossRef]
- Juhasz, T.; Szentleleky, E.; Szucs Somogyi, C.S.; Takacs, R.; Dobrosi, N.; Engler, M.; Tamas, A.; Reglodi, D.; Zakany, R. Pituitary adenylate cyclase activating polypeptide (PACAP) pathway is induced by mechanical load and reduces the activity of Hedghog signalling in chondrogenic micromass cell cultures. Int. J. Mol. Sci. 2015, 16, 17344–17367. [Google Scholar] [CrossRef] [PubMed]
- Krajcs, N.; Hernadi, L.; Pirger, Z.S.; Reglodi, D.; Toth, G.; Kiss, T. PACAP modulates acetylcholine-elicited contractions at nicotinic neuromuscular contacts of the Land snail. J. Mol. Neurosci. 2015, 57, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide: A potential neuroprotective peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review of the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effects of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, M.; Nakamachi, T.; Sugiyama, K.; Tsuchikawa, D.; Watanabe, J.; Hori, M.; Yoshikawa, A.; Imai, N.; Kagami, N.; Matkovits, A.; et al. PACAP stimulates functional recovery after spinal cord injury through axonal regeneration. J. Mol. Neurosci. 2014, 54, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Lubics, A.; Lengvari, I.; Reglodi, D. Protective effects of PACAP in excitotoxic striatal lesion. Ann. N. Y. Acad. Sci. 2006, 1070, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Lamine, A.; Létourneau, M.; Doan, N.D.; Maucotel, J.; Couvineau, A.; Vaudry, H.; Chatenet, D.; Vaudry, D.; Fournier, A. Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology 2016, 108, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socías, B.; Del-Bel, E.; Raisman-Vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Lubics, A.; Szalontay, L.; Lengvari, I. Morphological and functional effects of PACAP in a 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul. Pept. 2004, 123, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Njaine, B.; Rocha-Martins, M.; Vieira-Vieira, C.H.; de Melo, L.D.; Linden, R.; Braas, K.; May, V.; Martins, R.A.; Silveira, M.S. Pleiotropic functions of pituitary adenylyl cyclase-activating polypeptide on retinal ontogenesis: Involvement of KLF4 in the control of progenitor cell proliferation. J. Mol. Neurosci. 2014, 54, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Racz, B.; Gallyas, F.; Tamas, A.; Gaal, V.; Marton, Z.; Gabriel, R.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide in the retina: Focus on the retinoprotective effects. Ann. N. Y. Acad. Sci. 2010, 1200, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Vaczy, A.; Werling, D.; Kiss, P.; Tamas, A.; Kovacs, K.; Fabian, E.; Kvarik, T.; Mammel, B.; Danyadi, B.; et al. Neuroprotective effects of PACAP in the retina. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; Volume 11, pp. 501–527. [Google Scholar]
- Nakamachi, T.; Matkovits, A.; Seki, T.; Shioda, S. Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front. Endocrinol. Lausanne 2012, 3, 145. [Google Scholar]
- Shioda, S.; Takenoya, F.; Wada, N.; Hirabayashi, T.; Seki, T.; Nakamachi, T. Pleiotropic and retinoprotective functions of PACAP. Anat. Sci. Int. 2016, 91, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Shoge, K.; Mishima, H.K.; Saitoh, T.; Ishihara, K.; Tamura, Y.; Shiomi, H.; Sasa, M. Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res. 1999, 839, 66–73. [Google Scholar] [CrossRef]
- Fabian, E.; Reglodi, D.; Mester, L.; Szabo, A.; Szabadfi, K.; Tamas, A.; Toth, G.; Kovacs, K. Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J. Mol. Neurosci. 2012, 48, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.; D’Amico, A.G.; Castorina, A.; Imbesi, R.; Carnazza, M.L.; D’Agata, V. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides 2013, 39, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cheng, H.; Yu, R.; Tang, C.; Liu, X.; Chen, J. Effects of cyclopeptide C*HSDGIC* from the cyclization of PACAP (1–5) on the proliferation and UVB-induced apoptosis of the retinal ganglion cell line RGC-5. Peptides 2012, 36, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kiss, P.; Atlasz, T.; Szabadfi, K.; Horvath, G.; Griecs, M.; Farkas, J.; Matkovits, A.; Toth, G.; Lubics, A.; Tamas, A.; et al. Comparison between PACAP- and enriched environment-induced retinal protection in MSG-treated newborn rats. Neurosci. Lett. 2011, 487, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Nakatani, M.; Taki, C.; Shinohara, Y.; Ozawa, M.; Nishimura, S.; Ito, H.; Shioda, S. Neuroprotective effects of PACAP against kainic acid-induced neurotoxicity in rat retina. Ann. N. Y. Acad. Sci. 2006, 1070, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Nakamachi, T.; Endo, K.; Seki, T.; Ohtaki, H.; Tsuchikawa, D.; Hori, M.; Tsuchida, M.; Yoshikawa, A.; Matkovits, A.; et al. PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J. Mol. Neurosci. 2013, 51, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Itoh, H.; Nakamachi, T.; Shioda, S. Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J. Mol. Neurosci. 2008, 36, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Marton, Z.; Griecs, M.; Hamza, L.; Gaal, V.; Biro, Z.S.; Tamas, A.; Hild, G.; et al. Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J. Mol. Neurosci. 2011, 43, 51–57. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.G.; Maugeri, G.; Reitano, R.; Bucolo, C.; Saccone, S.; Drago, F.; D’Agata, V. PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 2015, 57, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Reglodi, D.; Szabo, A.; Szalontai, B.; Valasek, A.; Setalo, G.Y., Jr.; Kiss, P.; Tamas, A.; Wilhelm, M.; Gabriel, R. Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: Focus on the vertical information processing pathway. Neurotox. Res. 2016, 29, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Kvarik, T.; Mammel, B.; Reglodi, D.; Kovacs, K.; Werling, D.; Bede, B.; Vaczy, A.; Fabian, E.; Toth, G.; Kiss, P.; et al. PACAP is protective in a rat model of retinopathy of prematurity. J. Mol. Neurosci. 2016, 60, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Tamas, A.; Toth, G.; Reglodi, D.; Gabriel, R. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res. Bull. 2010, 81, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vaczy, A.; Reglodi, D.; Somoskeoy, T.; Kovacs, K.; Lokos, E.; Szabo, E.; Tamas, A.; Atlasz, T. The protective role of PAC1-receptor agonist maxadilan in BCCAO-induced retinal degeneration. J. Mol. Neurosci. 2016, 60, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Reglodi, D.; Kiss, P.; Toth, G.; Szabadfi, K.; Tamas, A.; Biro, Z.; Atlasz, T. Investigation of PACAP fragments and related peptides in chronic retinal hypoperfusion. J. Ophthalmol. 2014, 563812. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Reglodi, D.; Banks, W.A.; Salameh, T.S.; Kovacs, K.; Kvarik, T.; Vaczy, A.; Kovacs, L.; Mayer, F.; Danyadi, B.; et al. Ocular delivery of PACAP1—27 protects the retina from ischemic damage in rodents. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Erdling, A.; Sheykhzade, M.; Maddahi, A.; Bari, F.; Edvinsson, L. VIP/PACAP receptors in cerebral arteries of rat: Characterization, localization and relation to intracellular calcium. Neuropeptides 2013, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Takagi, K. Relaxant effect of pituitary adenylate cyclase-activating polypeptide on guinea-pig tracheal smooth muscle. Eur. J. Pharmacol. 1992, 216, 113–117. [Google Scholar] [CrossRef]
- Baun, M.; Pedersen, M.H.; Olesen, J.; Jansen-Olesen, I. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia 2012, 32, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kantora, O.; Molnar, J.; Arimura, A.; Koves, K. PACAP38 and PACAP27 administered intracerebroventricularly have an opposite effect on LH secretion. Peptides 2000, 21, 817–820. [Google Scholar] [CrossRef]
- Björklund, H.; Bignami, A.; Dahl, D. Immunohistochemical demonstration of glial fibrillary acidic protein in normal rat Müller glia and retinal astrocytes. Neurosci. Lett. 1985, 54, 363–368. [Google Scholar] [CrossRef]
- Wojcieszak, J.; Zawilska, J.B. PACAP38 and PACAP6-38 exert cytotoxic activity against human retinoblastoma Y79 cells. J. Mol. Neurosci. 2014, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Reglodi, D.; Kiss, P.; Tamas, A.; Toth, G.; Molnar, A.; Szabo, A.; Gabriel, R. Effects of pituitary adenylate cyclase activating polypeptide (PACAP1-38) and its fragments on retinal degeneration induced by neonatal MSG treatment. Ann. N. Y. Acad. Sci. 2009, 1163, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Danyadi, B.; Tamas, A.; Helyes, Z.S.; Hashimoto, H.; Shintani, N.; Baba, A.; Toth, G.; et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox. Res. 2012, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Danyadi, B.; Kiss, P.; Tamas, A.; Fabian, E.; Gabriel, R.; Reglodi, D. Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J. Mol. Neurosci. 2012, 48, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, S.; Wang, X.; Shen, S.; Ma, M.; Xu, W.; Hong, A. A New Recombinant PACAP-derived peptide efficiently promotes corneal wound repairing and lacrimal secretion. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4336–4349. [Google Scholar] [CrossRef] [PubMed]
- Fukiage, C.; Nakajima, T.; Takayama, Y.; Minagawa, Y.; Shearer, T.R.; Azuma, M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am. J. Ophthalmol. 2007, 143, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Gallyas, F., Jr.; Kiss, P.; Toth, G.; Hegyi, O.; Gasz, B.; Borsiczky, B.; Ferencz, A.; Roth, E.; Tamás, A.; et al. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways. Regul. Pept. 2006, 137, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Gallyas, F., Jr.; Kiss, P.; Tamas, A.; Lubics, A.; Lengvari, I.; Roth, E.; Toth, G.; Hegyi, O.; Verzar, Zs.; et al. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox. Res. 2007, 12, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Danyadi, B.; Bognar, E.; Szabadfi, K.; Fabian, E.; Kiss, P.; Mester, L.; Manavalan, S.; Atlasz, T.; Gabriel, R.; et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci. Lett. 2012, 523, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Casini, G. The neuropeptide systems and their potential role in the treatment of mammalian retinal ischemia: A developing story. Curr. Neuropharmacol. 2013, 11, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Somogyvari-Vigh, A.; Szanto, Z.; Kertes, E.; Lenard, L.; Arimura, A.; Lengvari, I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides 2002, 23, 2227–2234. [Google Scholar] [CrossRef]
- Uchida, D.; Arimura, A.; Somogyvari-Vigh, A.; Shioda, S.; Banks, W.A. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996, 736, 280–286. [Google Scholar] [CrossRef]
- Falcone, J.A.; Salameh, T.S.; Yi, X.; Cordy, B.J.; Mortell, W.G.; Kabanov, A.V.; Banks, W.A. Intranasal administration as a route for drug delivery to the brain: Evidence for a unique pathway for albumin. J. Pharmacol. Exp. Ther. 2014, 351, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Salameh, T.S.; Bullock, K.M.; Hujoel, I.A.; Niehoff, M.L.; Wolden-Hanson, T.; Kim, J.; Morley, J.E.; Farr, S.A.; Banks, W.A. Central nervous system delivery of intranasal insulin: Mechanisms of uptake and effects on cognition. J. Alzheimer Dis. 2015, 47, 715–728. [Google Scholar] [CrossRef] [PubMed]
| Region of Interest | 10 min | 60 min |
|---|---|---|
| Eye | 52.73 ± 2.35 | 48.24 ± 3.39 |
| Whole Brain | 104.71 ± 17.72 | 75.09 ± 41.13 |
| Serum | 25.57 ± 25.57 | 0 ± 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werling, D.; Banks, W.A.; Salameh, T.S.; Kvarik, T.; Kovacs, L.A.; Vaczy, A.; Szabo, E.; Mayer, F.; Varga, R.; Tamas, A.; et al. Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1-38 in Rodents. Int. J. Mol. Sci. 2017, 18, 675. https://doi.org/10.3390/ijms18030675
Werling D, Banks WA, Salameh TS, Kvarik T, Kovacs LA, Vaczy A, Szabo E, Mayer F, Varga R, Tamas A, et al. Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1-38 in Rodents. International Journal of Molecular Sciences. 2017; 18(3):675. https://doi.org/10.3390/ijms18030675
Chicago/Turabian StyleWerling, Dora, William A. Banks, Therese S. Salameh, Timea Kvarik, Laszlo Akos Kovacs, Alexandra Vaczy, Edina Szabo, Flora Mayer, Rita Varga, Andrea Tamas, and et al. 2017. "Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1-38 in Rodents" International Journal of Molecular Sciences 18, no. 3: 675. https://doi.org/10.3390/ijms18030675
APA StyleWerling, D., Banks, W. A., Salameh, T. S., Kvarik, T., Kovacs, L. A., Vaczy, A., Szabo, E., Mayer, F., Varga, R., Tamas, A., Toth, G., Biro, Z., Atlasz, T., & Reglodi, D. (2017). Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1-38 in Rodents. International Journal of Molecular Sciences, 18(3), 675. https://doi.org/10.3390/ijms18030675









