Transplantation of Embryonic Spinal Cord Derived Cells Helps to Prevent Muscle Atrophy after Peripheral Nerve Injury
Abstract
:1. Introduction
2. Results
2.1. In Vitro Characterization of Embryonic Spinal Cord Derived Cells
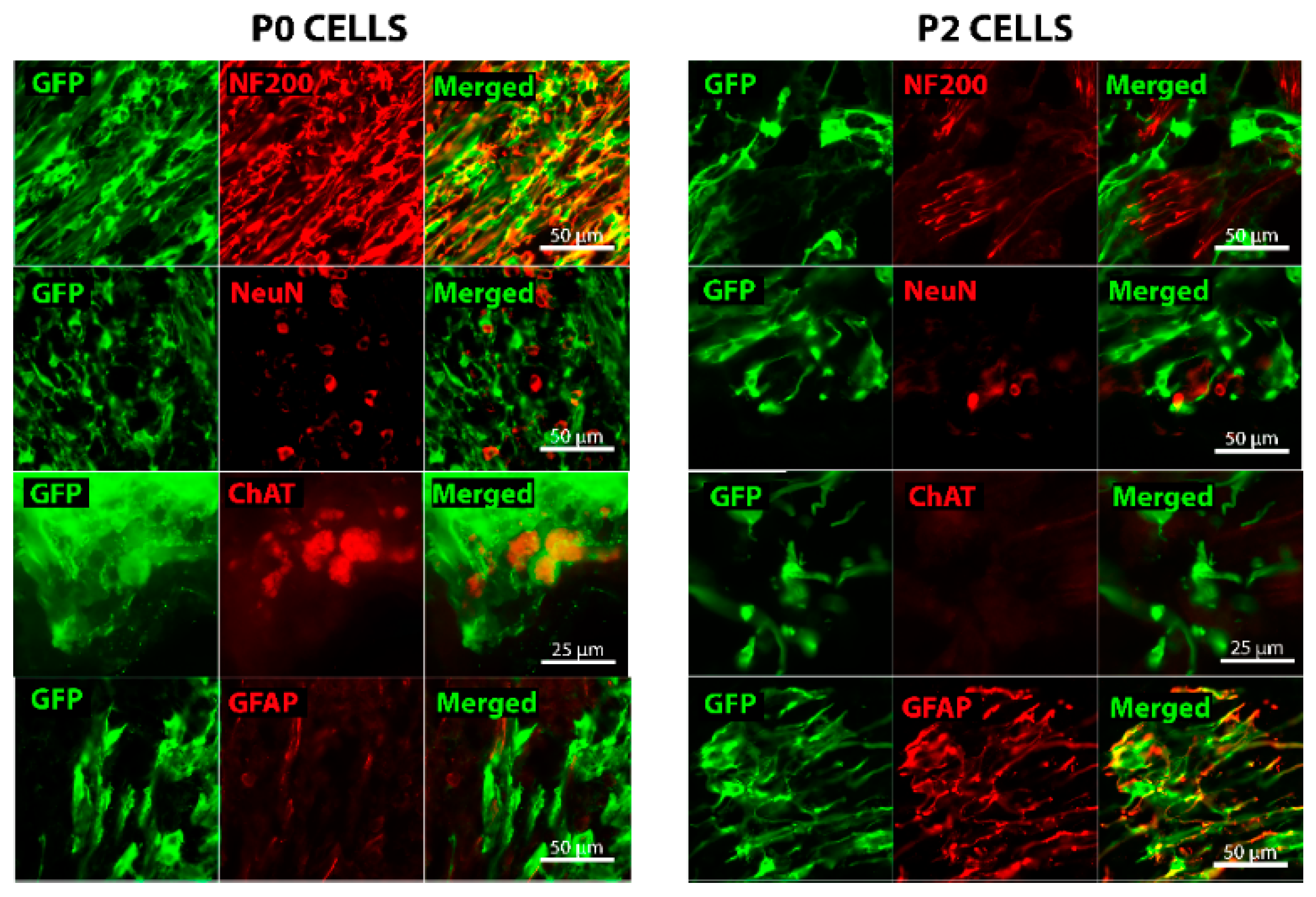
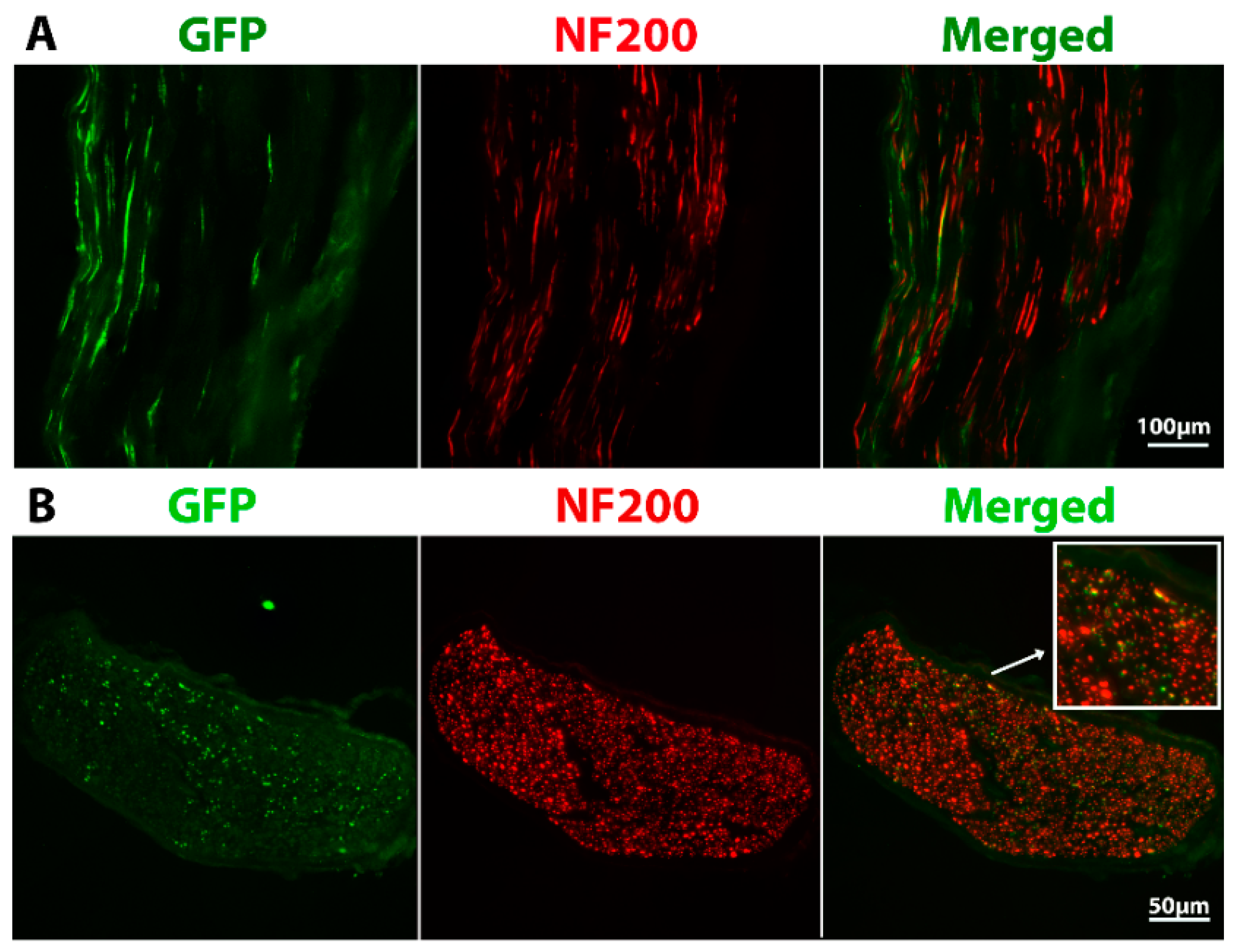
2.2. Grafted Cells Were Able to Survive and Differentiate in the Peripheral Nerve Environment
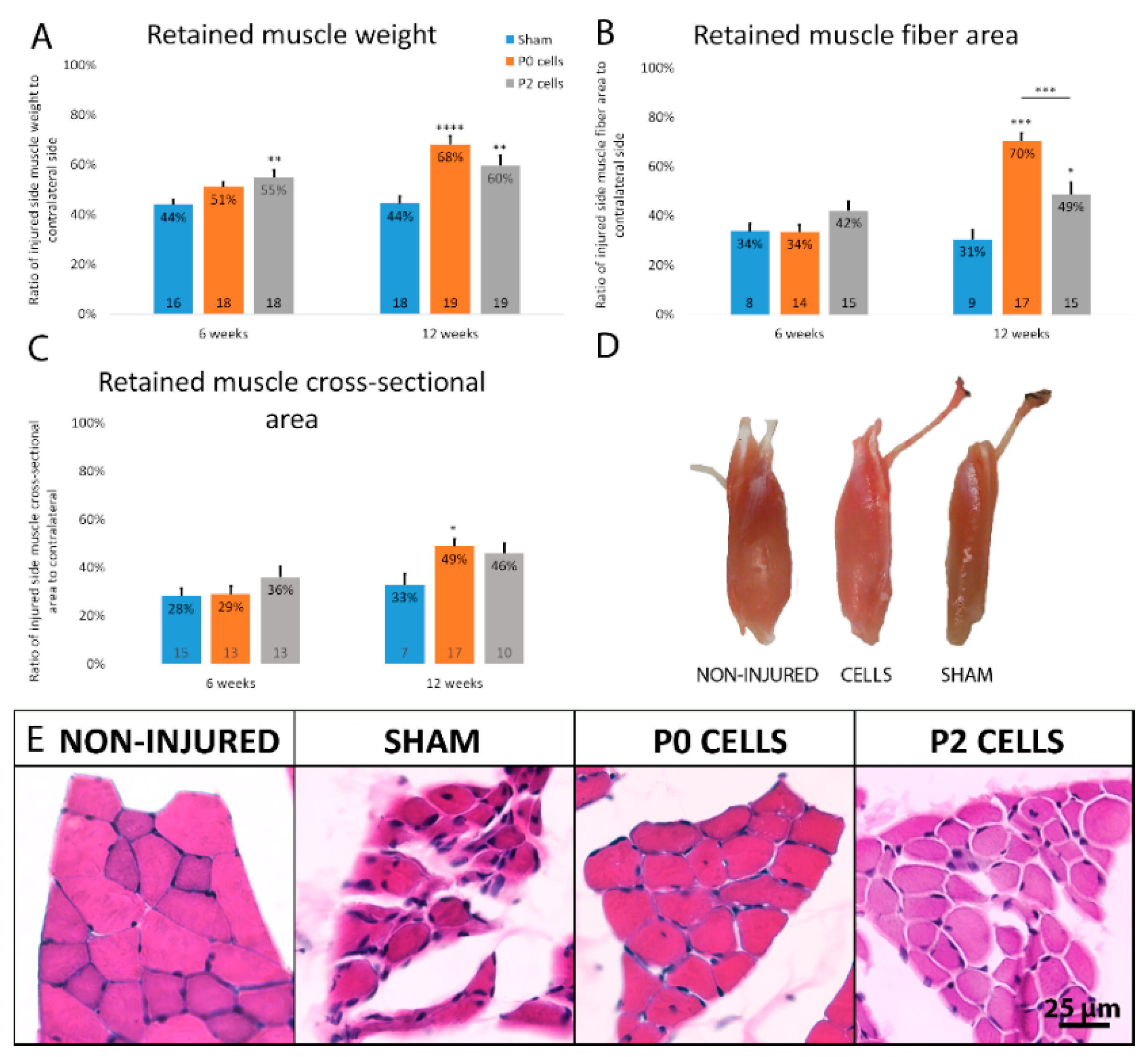
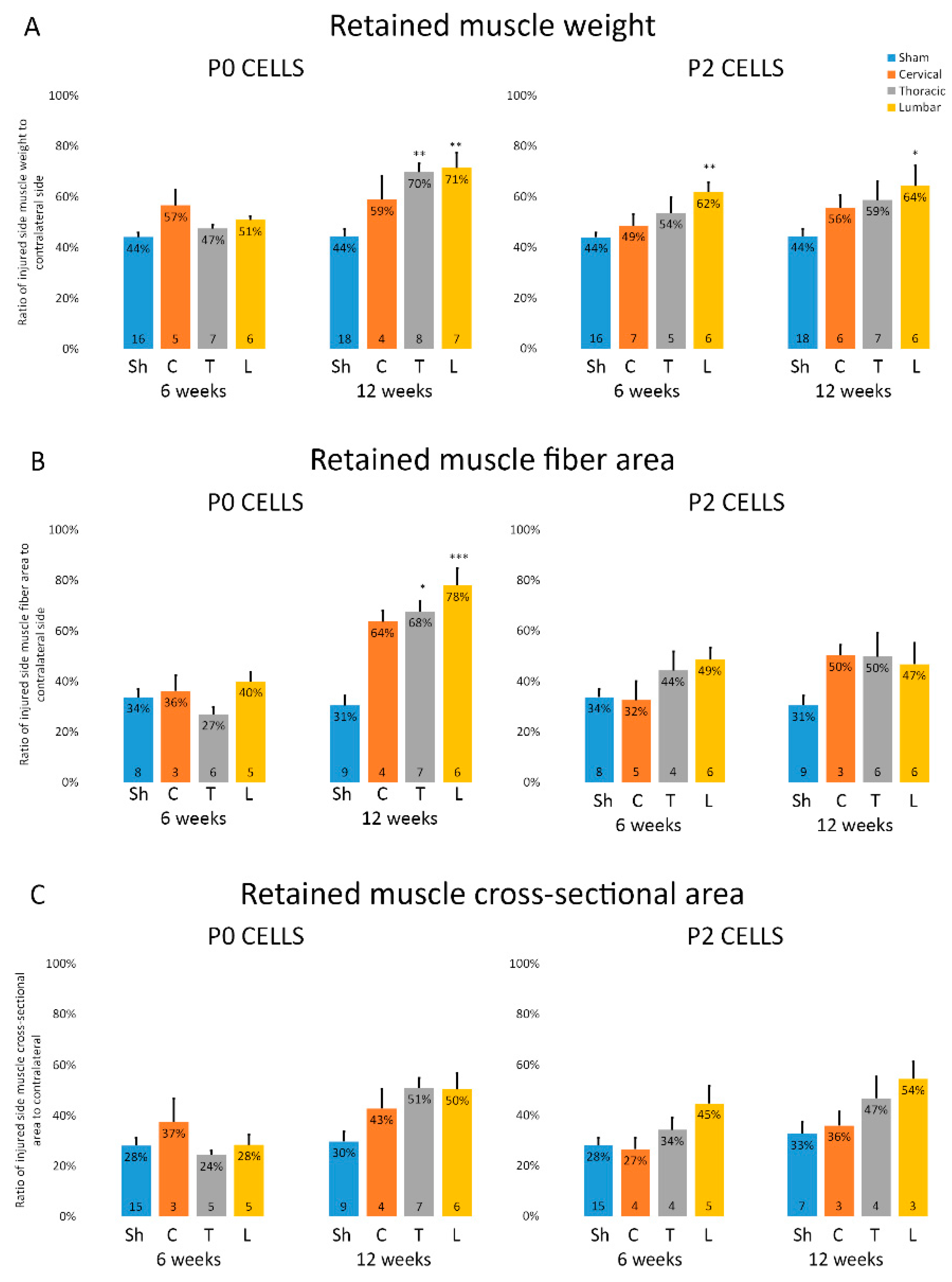
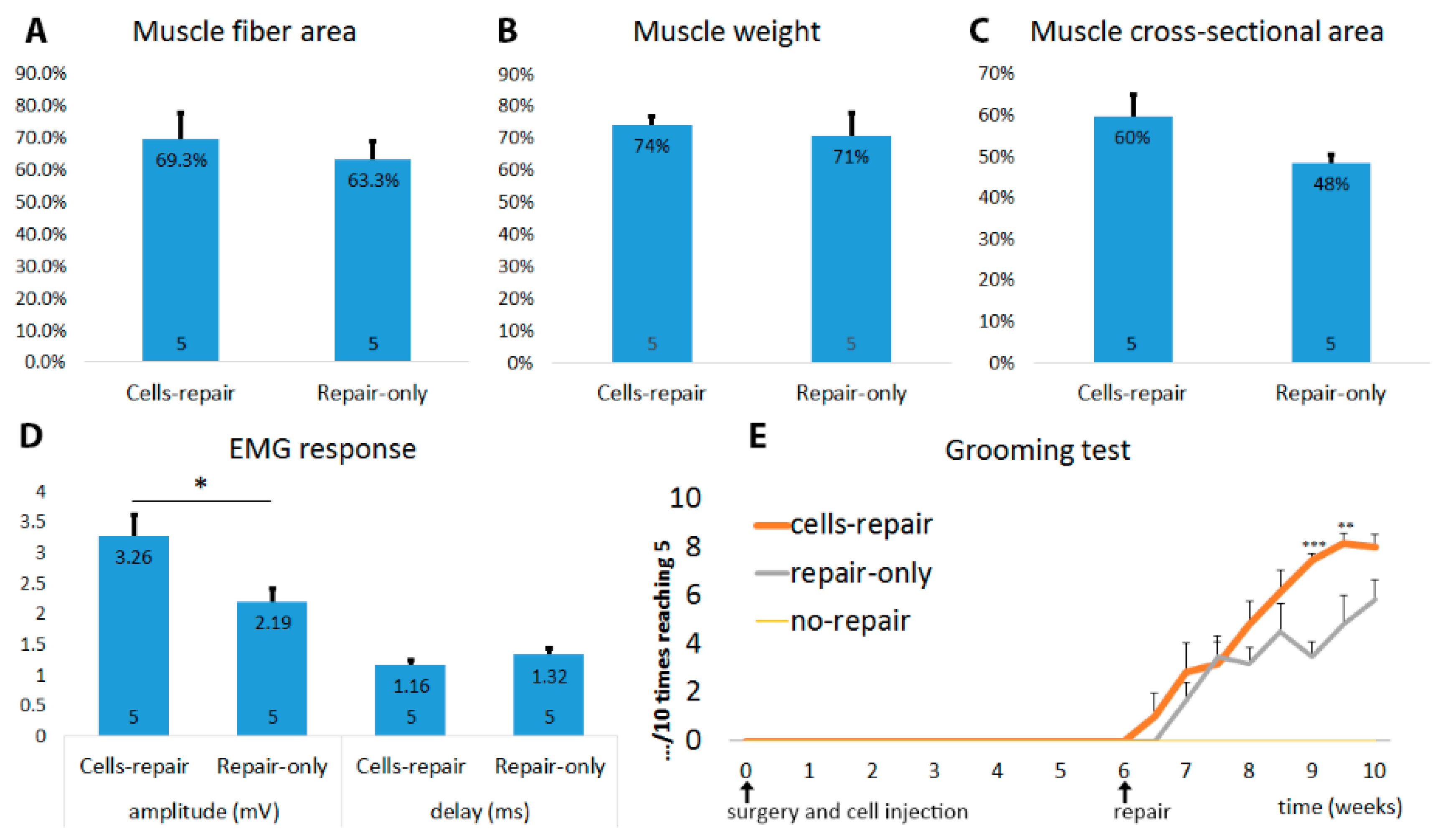
2.3. Muscles from Cell-Grafted Animals Showed Less Atrophy and Larger Muscle Fibers
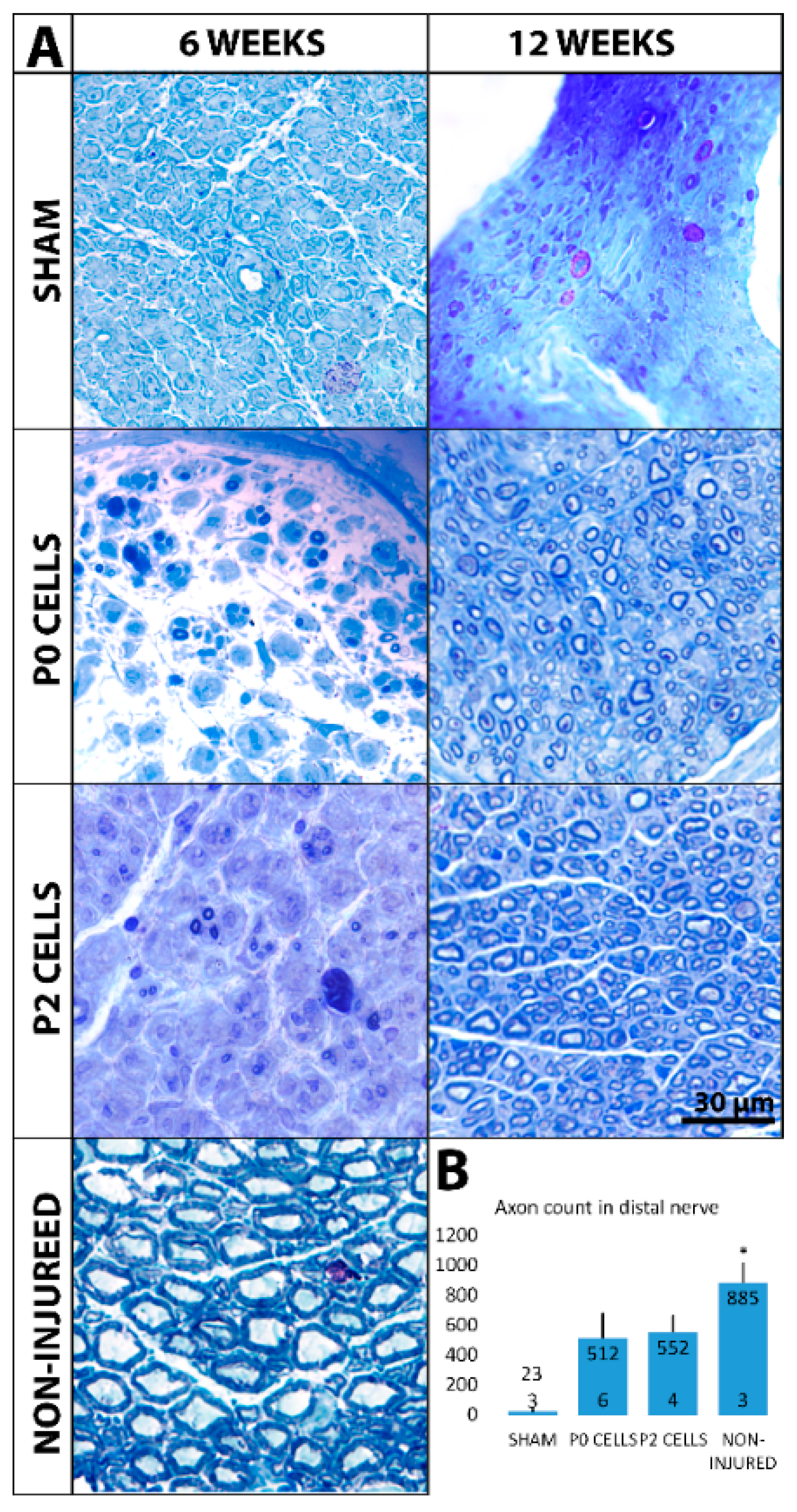
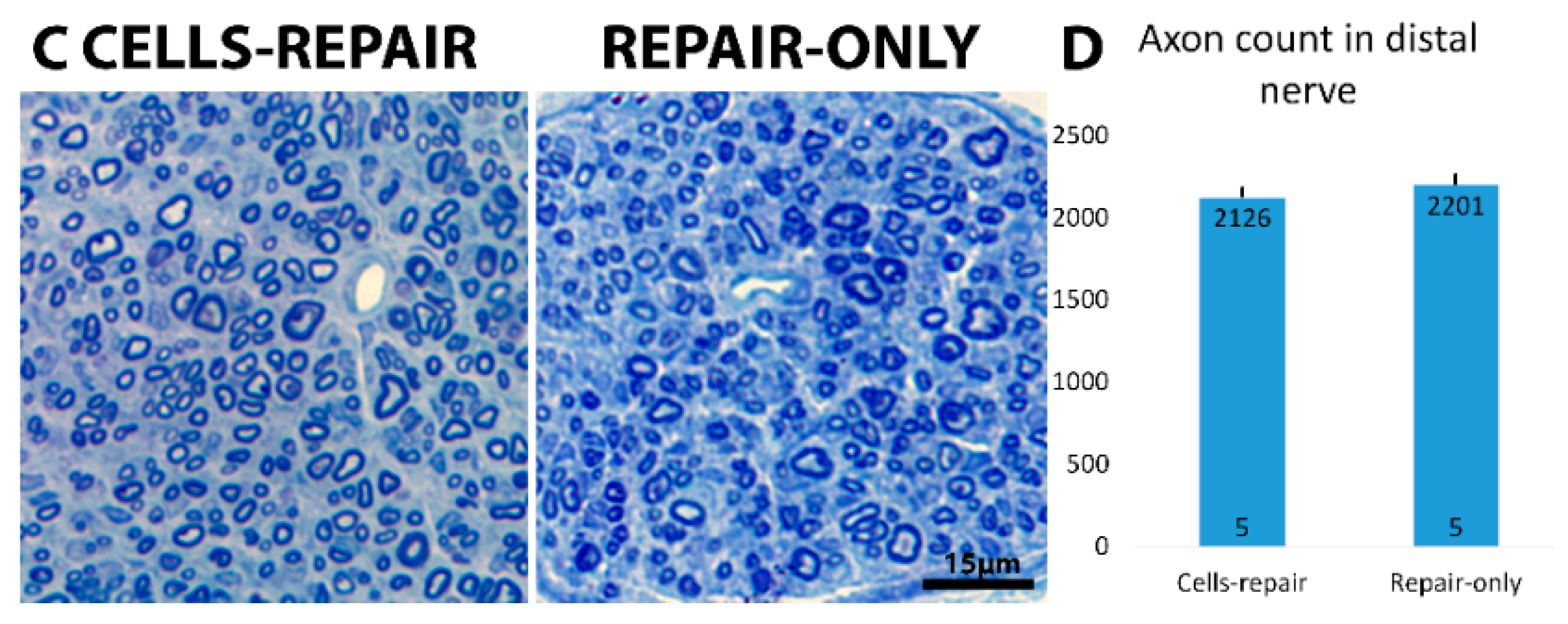
2.4. Distal Nerve of Cell-Grafted Animals Contained a Lot of Small Newly Myelinated Axons
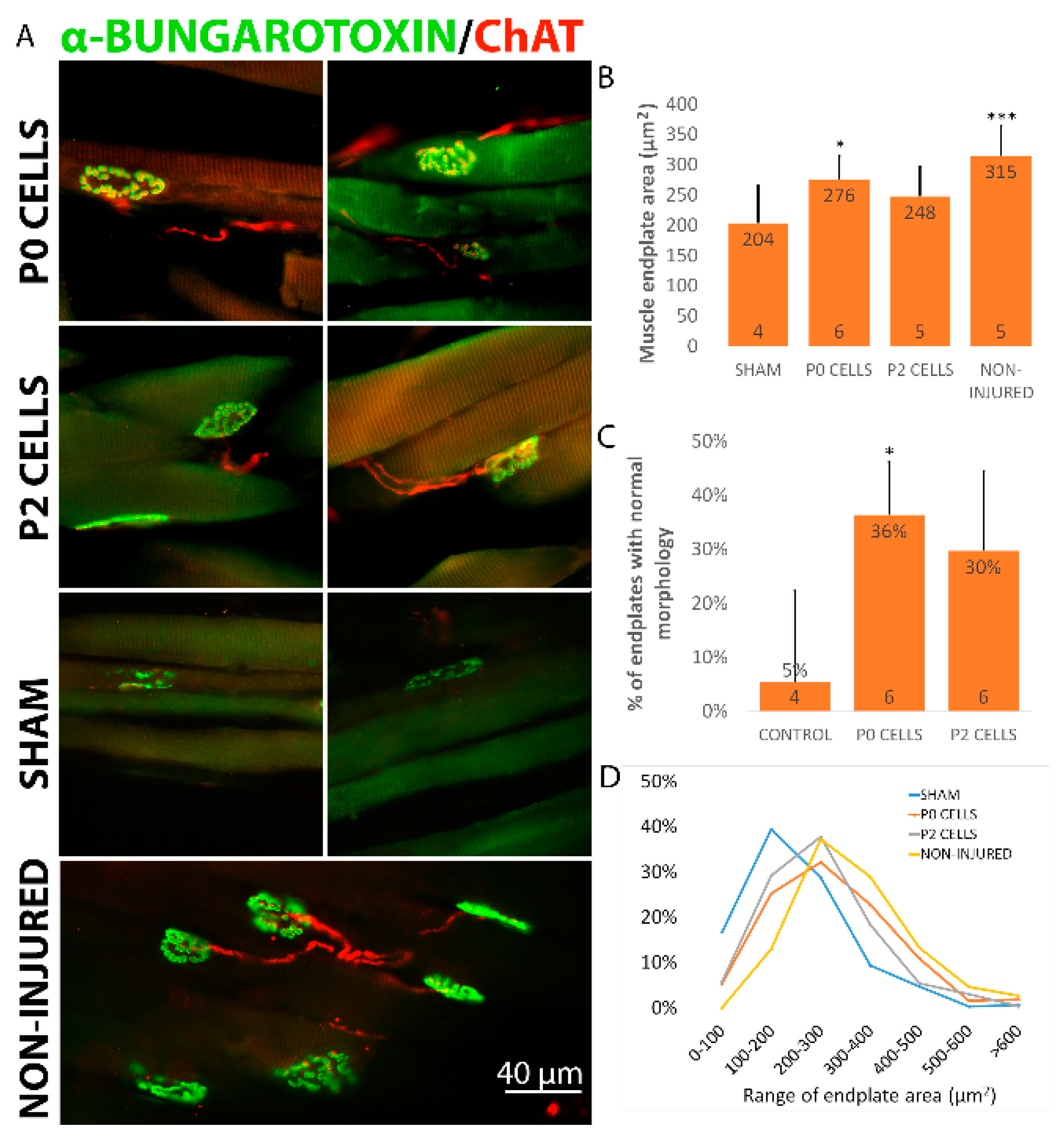
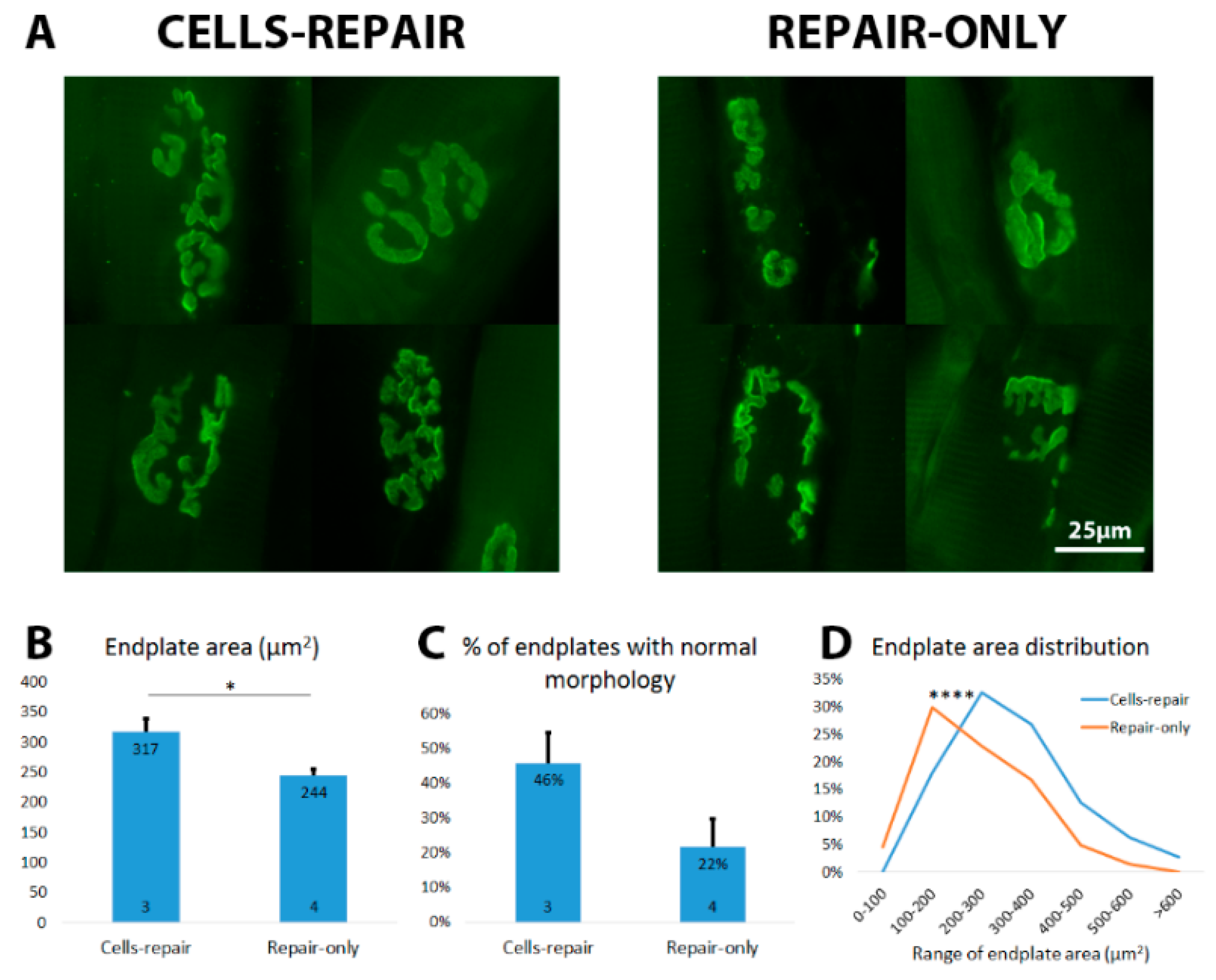
2.5. Cell Graft Helped to Preserve the Size and Morphology of Muscle Endplates
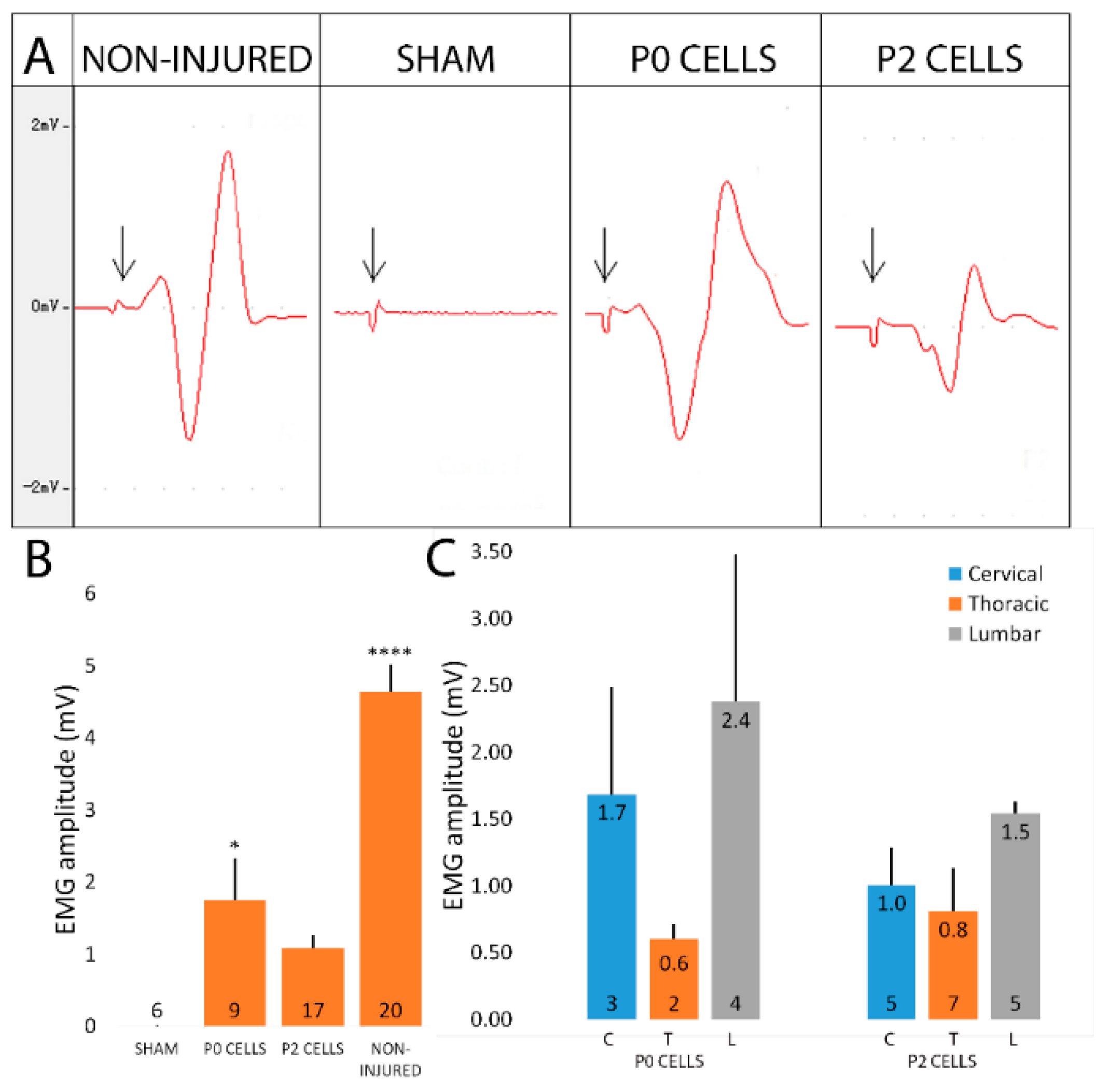
2.6. Cell-Grafted Animals Showed Functional Neuromuscular Connections
2.7. Cell Grafted Animals Show Earlier Functional Recovery after Delayed Nerve Repair
2.8. Grafted Cells Help to Preserve the Muscle Endplates until Regenerating Axons Reach the Target
3. Discussion
3.1. Cell Transplantation after Peripheral Nerve Injuries. Which Cells Should Be Used?
3.2. Do Fetal Spinal Cord Cells and NPCs Reduce Muscle Atrophy through Direct Neuronal Replacement or Can It Be the Environmental Support?
3.3. Is It Clinically Relevant?
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. In Vitro Immunocytochemistry
4.3. Animal Surgery and Cell Transplantation
4.4. Behavioral Test
4.5. Electromyographic Measurements (EMG)
4.6. Tissue Processing
4.7. Immunohistochemistry
4.8. Examination of Neuromuscular Junctions (NMJs) and Endplates
4.9. Assessment of Myelinated Axons
4.10. Histological Assessment and Muscle Fiber Measurements
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ChAT | Choline acetyltransferase |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EMG | Electromyography |
| GFAP | Glial fibrillary acidic protein |
| GFP | Green fluorescent protein |
| NMJs | Neuromuscular junctions |
| NPCs | Neural progenitor cells |
| P0 cells | Passage zero cells |
| P2 cells | Second passage cells |
| α-BTX | α-bungarotoxin |
Appendix A. Grooming Test
References
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.S.; Bastos, D.; Siqueira, M.G.; Heise, C.O.; Teixeira, M.J. Traumatic injuries of peripheral nerves: A review with emphasis on surgical indication. Arquiv. Neuro Psiquiatr. 2013, 71, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. Rate of regeneration in human peripheral nerves; analysis of the interval between injury and onset of recovery. Arch. Neurol. Psychiatry 1947, 58, 251–295. [Google Scholar] [CrossRef] [PubMed]
- Ruven, C.; Wu, W. Muscle atrophy following peripheral nerve injury and strategies to prevent it. In Horizons in Neuroscience Research; Andres Costa, E.V., Ed.; NOVA Science Publishers: Hauppauge, NY, USA, 2017; Volume 28. [Google Scholar]
- Ijkema-Paassen, J.; Meek, M.F.; Gramsbergen, A. Transection of the sciatic nerve and reinnervation in adult rats: Muscle and endplate morphology. Equine Vet. J. Suppl. 2001, 41–45. [Google Scholar] [CrossRef]
- Wang, H.; Gu, Y.; Xu, J.; Shen, L.; Li, J. Comparative study of different surgical procedures using sensory nerves or neurons for delaying atrophy of denervated skeletal muscle. J. Hand Surg. Am. 2001, 26, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.L.; Rygiel, K.; Purves-Smith, F.M.; Solbak, N.M.; Turnbull, D.M.; Hepple, R.T. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS ONE 2012, 7, e29082. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.S.; Lemay, M.A.; Tessler, A. Abnormal spontaneous potentials in distal muscles in animal models of spinal cord injury. Muscle Nerve 2005, 31, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.S.; Boyce, V.S.; Tessler, A.; Lemay, M.A. Fibrillation potentials following spinal cord injury: Improvement with neurotrophins and exercise. Muscle Nerve 2007, 35, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.L.; Zhang, L.Y.; Shen, L.Y.; Xu, J.G.; Gu, Y.D. Fibrillation potential amplitude to quantitatively assess denervation muscle atrophy. Neuromuscul. Disord. 2000, 10, 85–91. [Google Scholar] [CrossRef]
- Kraft, G.H. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve 1990, 13, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, A.; Arpa, J. Muscle fiber conduction velocity in situ (MFCV) in denervation, reinnervation and disuse atrophy. Acta Neurol. Scand. 1999, 100, 337–340. [Google Scholar] [CrossRef] [PubMed]
- al-Amood, W.S.; Lewis, D.M.; Schmalbruch, H. Effects of chronic electrical stimulation on contractile properties of long-term denervated rat skeletal muscle. J. Physiol. 1991, 441, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M.; Billington, L.; Faulkner, J. Studies on the regenerative recovery of long-term denervated muscle in rats. Restor. Neurol. Neurosci. 1996, 10, 77–84. [Google Scholar] [PubMed]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus. 2004, 16, E1. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Mackinnon, S.E.; Watanabe, O.; Ball, D.J.; Gu, X.M.; Hunter, D.A.; Kuzon, W.M., Jr. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve 1997, 20, 858–866. [Google Scholar] [CrossRef]
- Brown, D.L.; Bennett, T.M.; Dowsing, B.J.; Hayes, A.; Abate, M.; Morrison, W.A. Immediate and delayed nerve repair: Improved muscle mass and function with leukemia inhibitory factor. J. Hand Surg. Am. 2002, 27, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.A.; Mackinnon, S.E.; Gu, X.M.; Kobayashi, J.; Kuzon, W.M., Jr. Force deficits in skeletal muscle after delayed reinnervation. Plast. Reconstr. Surg. 2004, 113, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Veltri, K.L.; Chamberlain, D.; Fahnestock, M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience 2001, 103, 503–510. [Google Scholar] [CrossRef]
- Yohn, D.C.; Miles, G.B.; Rafuse, V.F.; Brownstone, R.M. Transplanted mouse embryonic stem-cell-derived motoneurons form functional motor units and reduce muscle atrophy. J. Neurosci. 2008, 28, 12409–12418. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Sonntag, V.K.; Dickman, C.; Mather, J.; Li, R.H.; Cordoba, S.C.; Bichard, B.; Berens, M. The role of cultured schwann cell grafts in the repair of gaps within the peripheral nervous system of primates. Exp. Neurol. 1997, 143, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Guenard, V.; Aebischer, P.; Bunge, R.P. The functional characteristics of schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J. Neurosci. 1994, 14, 1309–1319. [Google Scholar] [PubMed]
- Ogawa, Y.; Sawamoto, K.; Miyata, T.; Miyao, S.; Watanabe, M.; Nakamura, M.; Bregman, B.S.; Koike, M.; Uchiyama, Y.; Toyama, Y.; et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002, 69, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, W.; Yang, X.; Qin, D.; Sang, Y.; Wu, C.; Wong, W.M.; Yuan, Q.; So, K.F.; Wu, W. Neural progenitor cells generate motoneuron-like cells to form functional connections with target muscles after transplantation into the musculocutaneous nerve. Cell Transplant. 2012, 21, 2651–2663. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, L.; Cai, J.; Yuan, Q.; Yang, X.; Yao, X.; Wong, W.M.; Huang, W.; Li, Z.; Wan, J.B.; et al. Transplanted motoneurons derived from human induced pluripotent stem cells form functional connections with target muscle. Stem Cell Res. 2013, 11, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, X.; Liu, K.; Zhao, F.; Fu, Z.; Zhang, D.; Zhang, Q.; Jiang, B. Bone marrow stromal cells differentiated into functional schwann cells in injured rats sciatic nerve. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Ou, Y.C.; Liao, S.L.; Chen, W.Y.; Chen, S.Y.; Wu, C.W.; Wang, C.C.; Wang, W.Y.; Huang, Y.S.; Hsu, S.H. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp. Neurol. 2007, 204, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.L.; Zhang, Z.Y.; Xu, Y.; Zhi, Y.X.; Han, C.J.; Zhou, Y.H.; Liu, F.; Lin, H.Y.; Zhang, C.S. Bone marrow-derived, neural-like cells have the characteristics of neurons to protect the peripheral nerve in microenvironment. Stem Cells Int. 2015, 2015, 941625. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H. Adipose-derived stem cells for tissue repair and regeneration: Ten years of research and a literature review. J. Nippon. Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cheng, Y.; Guo, S.; Feng, Y.; Li, Q.; Jia, H.; Wang, Y.; Tong, L.; Tong, X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int. J. Mol. Med. 2011, 28, 565–572. [Google Scholar] [PubMed][Green Version]
- Amoh, Y.; Li, L.; Campillo, R.; Kawahara, K.; Katsuoka, K.; Penman, S.; Hoffman, R.M. Implanted hair follicle stem cells form schwann cells that support repair of severed peripheral nerves. Proc. Natl. Acad. Sci. USA 2005, 102, 17734–17738. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, C.; Hoffman, R.M. Nestin-expressing stem cells from the hair follicle can differentiate into motor neurons and reduce muscle atrophy after transplantation to injured nerves. Tissue Eng. Part A 2014, 20, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Gordon, T.; Addas, B.M.; Kemp, S.W.; Midha, R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp. Neurol. 2010, 223, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Pluderi, M.; Colleoni, F.; Belicchi, M.; Meregalli, M.; Farini, A.; Parolini, D.; Draghi, L.; Fruguglietti, M.E.; Gavina, M.; et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia 2007, 55, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Dadon-Nachum, M.; Melamed, E.; Offen, D. Stem cells treatment for sciatic nerve injury. Expert Opin. Biol. Ther. 2011, 11, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Zarbakhsh, S.; Moradi, F.; Joghataei, M.T.; Bahktiari, M.; Mansouri, K.; Abedinzadeh, M. Evaluation of the functional recovery in sciatic nerve injury following the co-transplantation of schwann and bone marrow stromal stem cells in rat. Basic Clin. Neurosci. 2013, 4, 291–298. [Google Scholar] [PubMed]
- Matsumine, H.; Takeuchi, Y.; Sasaki, R.; Kazama, T.; Kano, K.; Matsumoto, T.; Sakurai, H.; Miyata, M.; Yamato, M. Adipocyte-derived and dedifferentiated fat cells promoting facial nerve regeneration in a rat model. Plast. Reconstr. Surg. 2014, 134, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Shen, Y.; Xu, W.; Xu, L.; Li, X.; Zhou, G.; Gu, Y.; Xu, J. Application of fetal neural stem cells transplantation in delaying denervated muscle atrophy in rats with peripheral nerve injury. Microsurgery 2010, 30, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jiang, J.; Wei, L.; Zhou, X.; Fraser, J.L.; Snider, B.J.; Yu, S.P. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells 2008, 26, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chen, S.L.; Wang, D.Y.; Chiu, I.M. Stem cell-based therapy in neural repair. Biomed. J. 2013, 36, 98–105. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, H.; Zhang, G.; Ka, K.; Huang, W. Combining acellular nerve allografts with brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone. Neural Regen. Res. 2014, 9, 1814–1819. [Google Scholar] [PubMed]
- Grumbles, R.M.; Wood, P.; Rudinsky, M.; Gomez, A.M.; Thomas, C.K. Muscle reinnervation with delayed or immediate transplant of embryonic ventral spinal cord cells into adult rat peripheral nerve. Cell Transplant. 2002, 11, 241–250. [Google Scholar] [PubMed]
- MacDonald, S.C.; Fleetwood, I.G.; Hochman, S.; Dodd, J.G.; Cheng, G.K.; Jordan, L.M.; Brownstone, R.M. Functional motor neurons differentiating from mouse multipotent spinal cord precursor cells in culture and after transplantation into transected sciatic nerve. J. Neurosurg. 2003, 98, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, S.; Feng, G.Y.; Zhang, L.P.; Zhao, D.M.; Sun, Y.; Liu, Q.; Huang, F. Neural stem cells enhance nerve regeneration after sciatic nerve injury in rats. Mol. Neurobiol 2012, 46, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.N.; Duan, X.H.; Zhong, X.M.; Guo, R.M.; Zhang, F.; Zhou, C.P.; Shen, J. Transplanted neural stem cells promote nerve regeneration in acute peripheral nerve traction injury: Assessment using mri. AJR Am. J. Roentgenol. 2011, 196, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Grumbles, R.M.; Liu, Y.; Thomas, C.M.; Wood, P.M.; Thomas, C.K. Acute stimulation of transplanted neurons improves motoneuron survival, axon growth, and muscle reinnervation. J. Neurotrauma 2013, 30, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Grumbles, R.M.; Thomas, C.K. Electrical stimulation of embryonic neurons for 1 hour improves axon regeneration and the number of reinnervated muscles that function. J. Neuropathol. Exp. Neurol. 2013, 72, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.K.; Sesodia, S.; Erb, D.E.; Grumbles, R.M. Properties of medial gastrocnemius motor units and muscle fibers reinnervated by embryonic ventral spinal cord cells. Exp. Neurol. 2003, 180, 25–31. [Google Scholar] [CrossRef]
- Erb, D.E.; Mora, R.J.; Bunge, R.P. Reinnervation of adult rat gastrocnemius muscle by embryonic motoneurons transplanted into the axotomized tibial nerve. Exp. Neurol. 1993, 124, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Grumbles, R.M.; Casella, G.T.; Rudinsky, M.J.; Wood, P.M.; Sesodia, S.; Bent, M.; Thomas, C.K. Long-term delivery of FGF-6 changes the fiber type and fatigability of muscle reinnervated from embryonic neurons transplanted into adult rat peripheral nerve. J. Neurosci. Res. 2007, 85, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Grumbles, R.M.; Casella, G.T.; Rudinsky, M.J.; Godfrey, S.; Wood, P.M.; Thomas, C.K. The immunophilin ligand FK506, but not the p38 kinase inhibitor SB203580, improves function of adult rat muscle reinnervated from transplants of embryonic neurons. Neuroscience 2005, 130, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, X.; Zhang, C.; Li, W.; Wong, W.M.; Xu, Y.; Wu, W.; Lin, J. Transplantation of embryonic spinal cord neurons to the injured distal nerve promotes axonal regeneration after delayed nerve repair. Eur. J. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Toma, J.S.; Shettar, B.C.; Chipman, P.H.; Pinto, D.M.; Borowska, J.P.; Ichida, J.K.; Fawcett, J.P.; Zhang, Y.; Eggan, K.; Rafuse, V.F. Motoneurons derived from induced pluripotent stem cells develop mature phenotypes typical of endogenous spinal motoneurons. J. Neurosci. 2015, 35, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Karumbayaram, S.; Novitch, B.G.; Patterson, M.; Umbach, J.A.; Richter, L.; Lindgren, A.; Conway, A.E.; Clark, A.T.; Goldman, S.A.; Plath, K.; et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 2009, 27, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with als can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kawai, H.; Tian, F.; Ohta, Y.; Abe, K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011, 20, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.; Mitsui, Y.; Kumazaki, T.; Kawahara, Y.; Matsuo, T.; Takahashi, T. Tumorigenic risk of human induced pluripotent stem cell explants cultured on mouse snl76/7 feeder cells. Biochem. Biophys. Res. Commun. 2014, 453, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, Z.; Huang, M.; Hu, S.; Wilson, K.D.; Dey, D.; Wu, J.C. Dissecting the oncogenic and tumorigenic potential of differentiated human induced pluripotent stem cells and human embryonic stem cells. Cancer Res. 2011, 71, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.P.; Ashraf, M.; Buccini, S.; Shujia, J.; Haider, H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Chen, M.; Yang, B.; Zhang, F.; Cao, K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS ONE 2011, 6, e19012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Johe, K.; Molnar, P.; Davis, H.; Hickman, J. Characterization of a human fetal spinal cord stem cell line, nsi-566rsc, and its induction to functional motoneurons. J. Tissue Eng. Regen. Med. 2010, 4, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Salmons, S.; Ashley, Z.; Sutherland, H.; Russold, M.F.; Li, F.; Jarvis, J.C. Functional electrical stimulation of denervated muscles: Basic issues. Artif. Organs 2005, 29, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.A.; Cardozo, C.; Saez, J.C. Neuronal involvement in muscular atrophy. Front. Cell Neurosci. 2014, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin's role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Buono, R.; Catanzaro, G.; de Palma, C.; Longhi, R.; Meneveri, R.; Bresolin, N.; Bassi, M.T.; Cossu, G.; Clementi, E.; et al. The low-affinity receptor for neurotrophins p75NTR plays a key role for satellite cell function in muscle repair acting via RHOA. Mol. Biol. Cell 2009, 20, 3620–3627. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, M.; Lu, A.; Peng, H.; Cummins, J.; Huard, J. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum. Gene Ther. 2006, 17, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Parsadanian, A.S.; Snider, W.D.; Lichtman, J.W. Hyperinnervation of neuromuscular junctions caused by gdnf overexpression in muscle. Science 1998, 279, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Vekris, M.D.; Soucacos, P.N. Brachial plexus root avulsions. World J. Surg. 2001, 25, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Gorio, A.; Carmignoto, G.; Finesso, M.; Polato, P.; Nunzi, M.G. Muscle reinnervation—II. Sprouting, synapse formation and repression. Neuroscience 1983, 8, 403–416. [Google Scholar] [CrossRef]
- Kang, H.; Lichtman, J.W. Motor axon regeneration and muscle reinnervation in young adult and aged animals. J. Neurosci. 2013, 33, 19480–19491. [Google Scholar] [CrossRef] [PubMed]
- Magill, C.K.; Tong, A.; Kawamura, D.; Hayashi, A.; Hunter, D.A.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. Reinnervation of the tibialis anterior following sciatic nerve crush injury: A confocal microscopic study in transgenic mice. Exp. Neurol. 2007, 207, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Favero, M.; Busetto, G.; Cangiano, A. Spike timing plays a key role in synapse elimination at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2012, 109, E1667–E1675. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.B.; Colman, H.; Nelson, C.; Lichtman, J.W. Synaptic competition during the reformation of a neuromuscular map. J. Neurosci. 1998, 18, 7328–7335. [Google Scholar] [PubMed]
- Rich, M.M.; Lichtman, J.W. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J. Neurosci. 1989, 9, 1781–1805. [Google Scholar] [PubMed]
- Gu, H.Y.; Chai, H.; Zhang, J.Y.; Yao, Z.B.; Zhou, L.H.; Wong, W.M.; Bruce, I.C.; Wu, W.T. Survival, regeneration and functional recovery of motoneurons after delayed reimplantation of avulsed spinal root in adult rat. Exp. Neurol. 2005, 192, 89–99. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruven, C.; Li, W.; Li, H.; Wong, W.-M.; Wu, W. Transplantation of Embryonic Spinal Cord Derived Cells Helps to Prevent Muscle Atrophy after Peripheral Nerve Injury. Int. J. Mol. Sci. 2017, 18, 511. https://doi.org/10.3390/ijms18030511
Ruven C, Li W, Li H, Wong W-M, Wu W. Transplantation of Embryonic Spinal Cord Derived Cells Helps to Prevent Muscle Atrophy after Peripheral Nerve Injury. International Journal of Molecular Sciences. 2017; 18(3):511. https://doi.org/10.3390/ijms18030511
Chicago/Turabian StyleRuven, Carolin, Wen Li, Heng Li, Wai-Man Wong, and Wutian Wu. 2017. "Transplantation of Embryonic Spinal Cord Derived Cells Helps to Prevent Muscle Atrophy after Peripheral Nerve Injury" International Journal of Molecular Sciences 18, no. 3: 511. https://doi.org/10.3390/ijms18030511
APA StyleRuven, C., Li, W., Li, H., Wong, W.-M., & Wu, W. (2017). Transplantation of Embryonic Spinal Cord Derived Cells Helps to Prevent Muscle Atrophy after Peripheral Nerve Injury. International Journal of Molecular Sciences, 18(3), 511. https://doi.org/10.3390/ijms18030511





