Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer
Abstract
:1. Introduction
2. Results
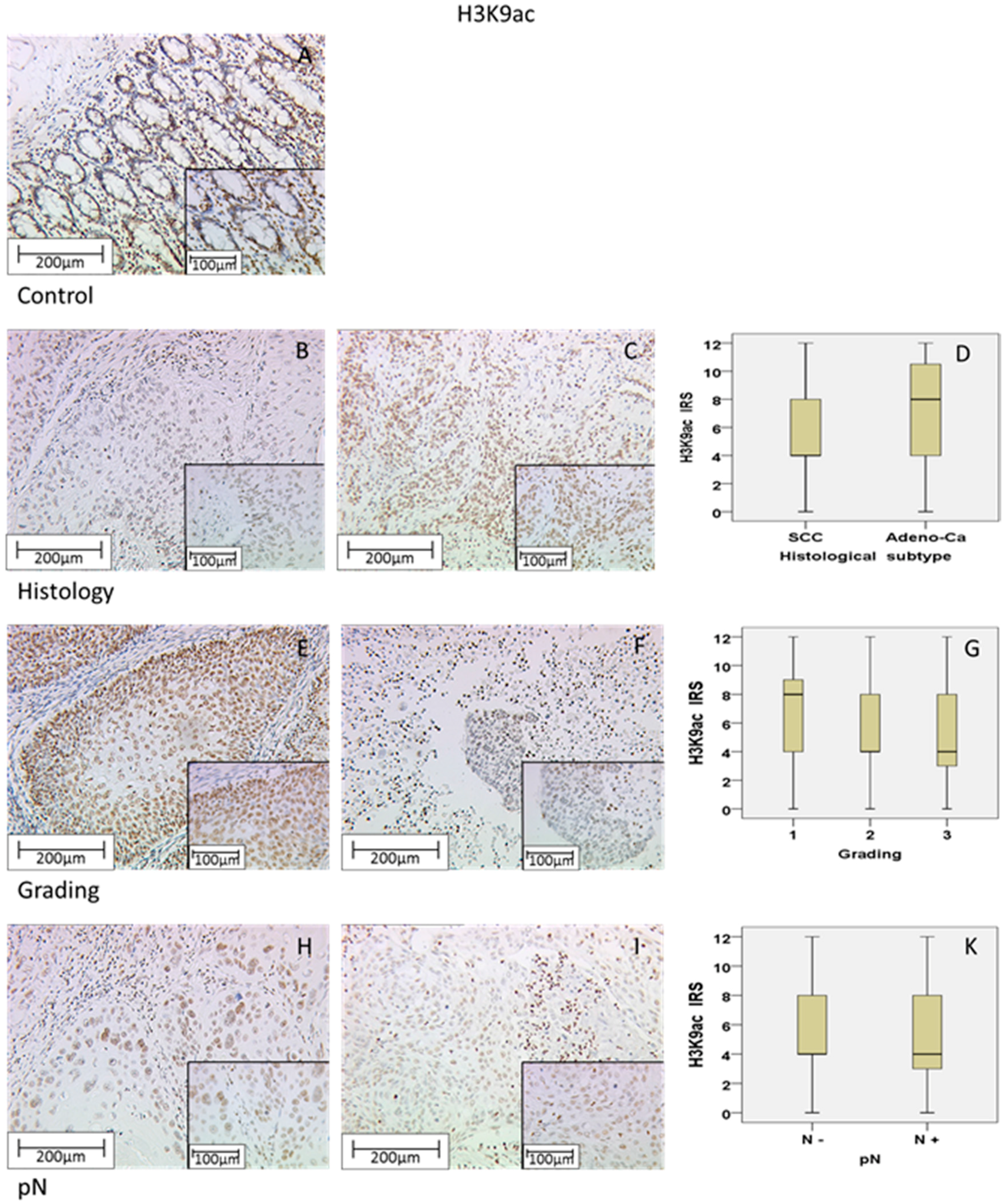
2.1. H3K9ac Staining in Cervical Carcinoma
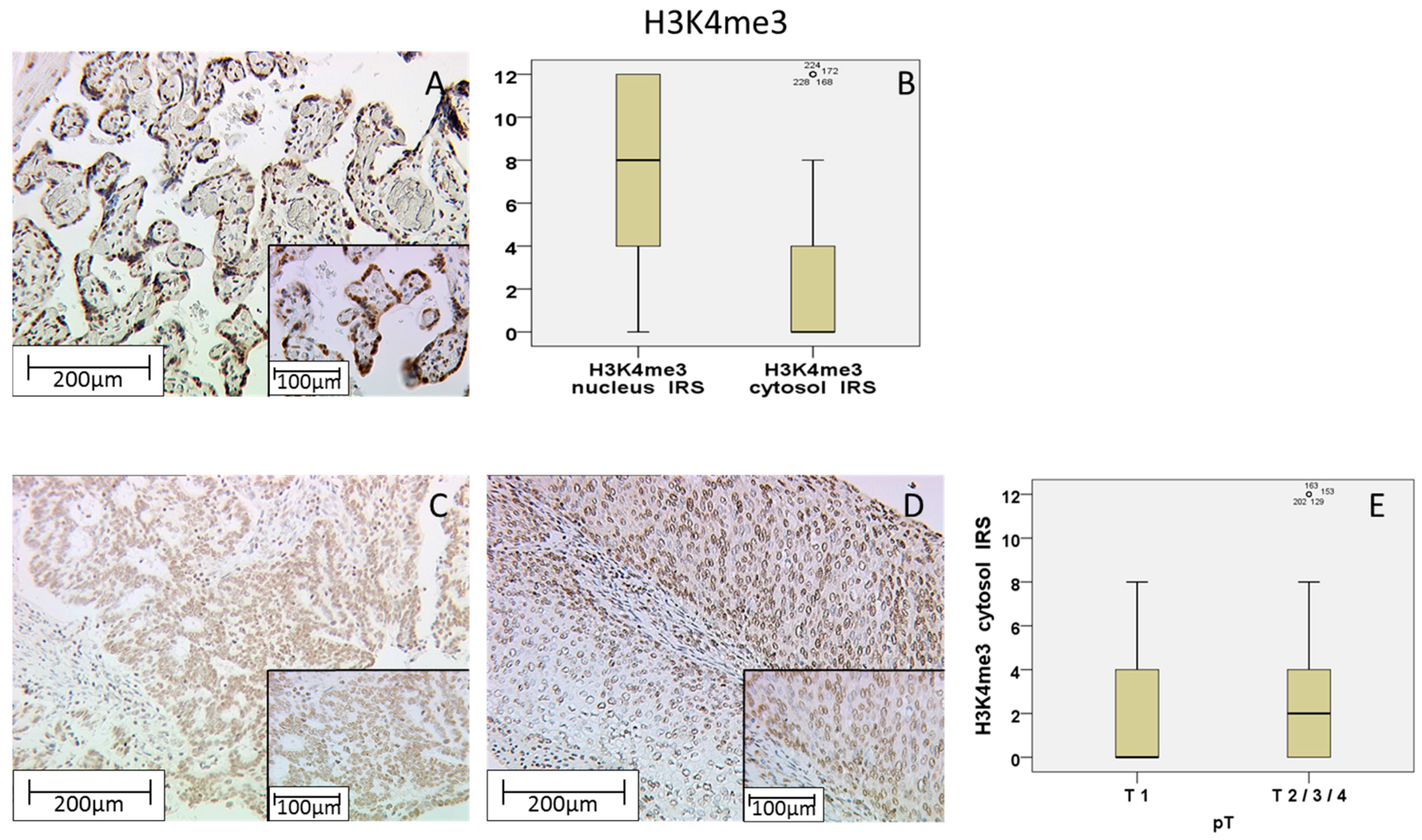
2.2. H3K4me3 Staining in Cervical Cancer
2.3. Correlation Analysis between H3K4me3 and p16 Oncoprotein
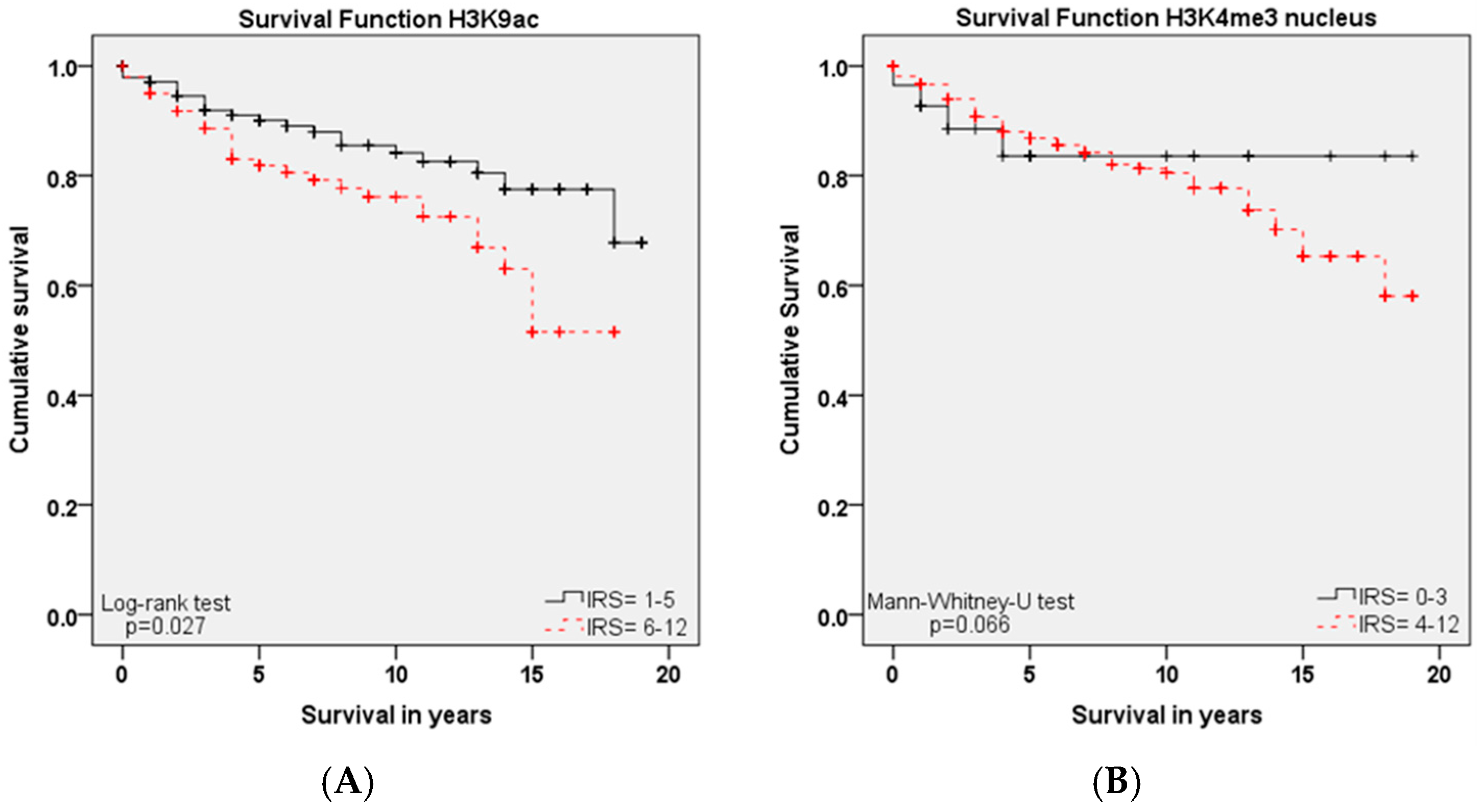
2.4. Role of H3K9ac and H3K4me3 for Overall Survival
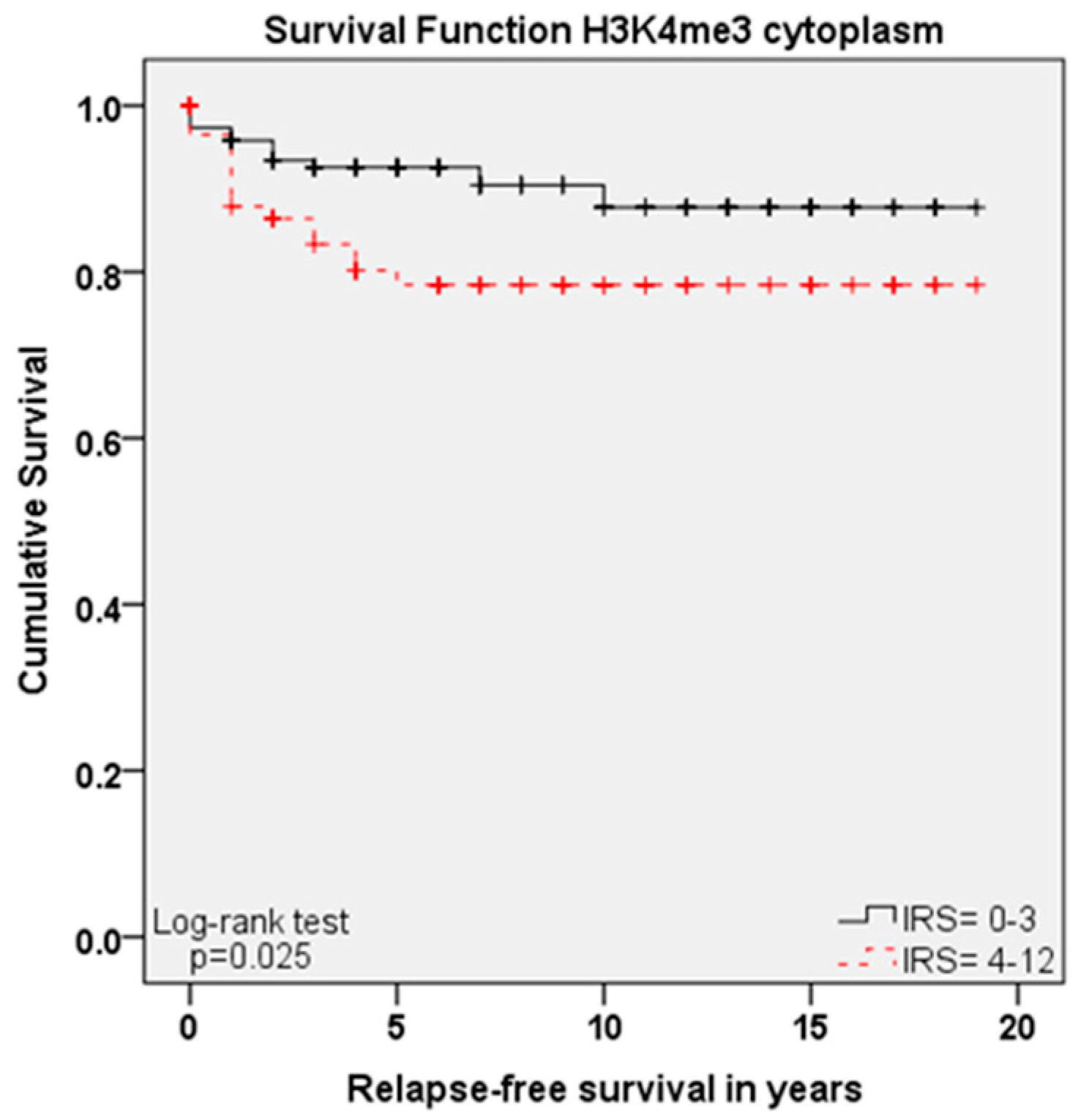
2.5. Role of H3K9ac and H3K4me3 for Progress-Free Survival
2.6. Cox Regression of H3K9ac and H3K4me3 and Clinic Pathological Variables
3. Discussion
4. Materials and Methods
4.1. Patients and Specimens
4.2. Ethics Approval
4.3. Immunohistochemistry
4.4. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| H3K9ac | histone H3 acetyl K9 |
| H3K4me3 | histone H3 tri methyl K4 |
| SCC | squamous cell carcinoma |
References
- Chatterjee, S.; Chattopadhyay, A.; Samanta, L.; Panigrahi, P. HPV and cervical cancer epidemiology—Current status of HPV vaccination in India. Asian Pac. J. Cancer Prev. 2016, 17, 3663–3673. [Google Scholar] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Wittekindt, C.; Wagner, S.; Mayer, C.S.; Klussmann, J.P. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. Laryngorhinootologie 2012, 91 (Suppl. 1), S1–S26. [Google Scholar] [PubMed]
- Liu, X.M.; Pan, C.W.; Wang, G.D.; Cai, X.H.; Chen, L.; Meng, C.F.; Huang, J.C. Finite element analysis of the stability of combined plate internal fixation in posterior wall fractures of acetabulum. Int. J. Clin. Exp. Med. 2015, 8, 13393–13397. [Google Scholar] [PubMed]
- Liu, J.; Cheng, Y.; He, M.; Yao, S. Vascular endothelial growth factor C enhances cervical cancer cell invasiveness via upregulation of galectin-3 protein. Gynecol. Endocrinol. 2014, 30, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takhar, P.P.; Degenkolbe, R.; Koh, C.H.; Zimmermann, H.; Yang, C.M.; Guan Sim, K.; Hsu, S.I.; Bernard, H.U. The human papillomavirus type 11 and 16 E6 proteins modulate the cell-cycle regulator and transcription cofactor TRIP-Br1. Virology 2003, 317, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.F.; Su, B.; Li, W. DNA demethylation is superior to histone acetylation for reactivating cancer-associated genes in ovarian cancer cells. Mol. Med. Rep. 2011, 4, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Basilio, J.; Serafin-Higuera, N.; Reyes-Hernandez, O.D.; Serafin-Higuera, I.; Leija-Montoya, G.; Blanco-Morales, M.; Sierra-Martinez, M.; Ramos-Mondragon, R.; Garcia, S.; Lopez-Hernandez, L.B.; et al. Low proteolytic clipping of histone H3 in cervical cancer. J. Cancer 2016, 7, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.H.; Laban, M.; Leung, C.H.; Lee, L.; Lee, C.K.; Salto-Tellez, M.; Raju, G.C.; Hooi, S.C. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 2005, 12, 395–404. [Google Scholar] [CrossRef] [PubMed]
- You, J. Papillomavirus interaction with cellular chromatin. Biochim. Biophys. Acta 2010, 1799, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, T.R.; Laimins, L.A. Regulation of human papillomavirus type 31 gene expression during the differentiation-dependent life cycle through histone modifications and transcription factor binding. Virology 2008, 374, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.F.; Zhu, X.J.; Peng, G.; Dai, D.Q. Promoter histone H3 lysine 9 di-methylation is associated with DNA methylation and aberrant expression of p16 in gastric cancer cells. Oncol. Rep. 2009, 22, 1221–1227. [Google Scholar] [PubMed]
- Freier, C.P.; Stiasny, A.; Kuhn, C.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Jeschke, U.; Kost, B. Immunohistochemical evaluation of the role of p53 mutation in cervical cancer: Ser-20 p53-mutant correlates with better prognosis. Anticancer Res. 2016, 36, 3131–3137. [Google Scholar] [PubMed]
- Stiasny, A.; Kuhn, C.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Jeschke, U.; Kost, B. Immunohistochemical evaluation of E6/E7 HPV oncoproteins staining in cervical cancer. Anticancer Res. 2016, 36, 3195–3198. [Google Scholar] [PubMed]
- Iwasaki, W.; Miya, Y.; Horikoshi, A.; Taguchi, H.; Tachiwana, H.; Shibata, T.; Kagawa, W.; Kurumizaka, H. Contribution of histone N-terminals to the structure and stability of nucleosomes. FEBS Open Bio 2013, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.D.; Li, H.; Ruthenburg, A.J.; Allis, C.D.; Patel, D.J. How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 2007, 14, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Yavartanoo, M.; Choi, J.K. Encode: A sourcebook of epigenomes and chromatin language. Genom. Inform. 2013, 11, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, P.; Xiong, J.; Liu, Z.; Berger, S.L.; Merlino, G. Epigenetic drugs can stimulate metastasis through enhanced expression of the pro-metastatic ezrin gene. PLoS ONE 2010, 5, e12710. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Balasubramanian, A.; Yu, M.; Kiviat, N.; Ridder, R.; Reichert, A.; Herkert, M.; von Knebel Doeberitz, M.; Koutsky, L.A. Evaluation of a new p16(INK4a) ELISA test and a high-risk HPV DNA test for cervical cancer screening: Results from proof-of-concept study. Int. J. Cancer 2007, 120, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Melkane, A.E.; Mirghani, H.; Auperin, A.; Saulnier, P.; Lacroix, L.; Vielh, P.; Casiraghi, O.; Griscelli, F.; Temam, S. HPV-related oropharyngeal squamous cell carcinomas: A comparison between three diagnostic approaches. Am. J. Otolaryngol. 2014, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin-Drubin, M.E.; Crum, C.P.; Munger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. USA 2011, 108, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin-Drubin, M.E.; Park, D.; Munger, K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA 2013, 110, 16175–16180. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, H.; Jin, S. Epigenetics and cervical cancer: From pathogenesis to therapy. Tumour Biol. 2014, 35, 5083–5093. [Google Scholar] [CrossRef] [PubMed]
- Duenas-Gonzalez, A.; Lizano, M.; Candelaria, M.; Cetina, L.; Arce, C.; Cervera, E. Epigenetics of cervical cancer. An overview and therapeutic perspectives. Mol. Cancer 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Hernandez, E.; Perez-Cardenas, E.; Contreras-Paredes, A.; Cantu, D.; Mohar, A.; Lizano, M.; Duenas-Gonzalez, A. The effects of DNA methylation and histone deacetylase inhibitors on human papillomavirus early gene expression in cervical cancer, an in vitro and clinical study. Virol. J. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ghazawi, F.M.; Bakkar, W.; Li, Q. Valproic acid and butyrate induce apoptosis in human cancer cells through inhibition of gene expression of Akt/protein kinase B. Mol. Cancer 2006, 5. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L.; Gu, W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell 2011, 2, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Su, Z.; Yang, L.; Guo, W.; Liu, W.; Zuo, J. Synergistic induction of apoptosis in HeLa cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitor SAHA. Mol. Med. Rep. 2010, 3, 613–619. [Google Scholar] [PubMed]
| Item | No./Total No. | % |
|---|---|---|
| Age, years | ||
| <49 | 139/250 | 55.6 |
| >49 | 111/250 | 44.4 |
| Number of Positive Nodes | ||
| 0 | 151/250 | 60.4 |
| ≥1 | 97/250 | 38.8 |
| Not available (NA’s) | 2/250 | 0.8 |
| Tumor Size, pT | ||
| pT1 | 110250 | 44 |
| pT2/3/4 | 137/250 | 54.8 |
| Not available (NA’s) | 3/250 | 1.2 |
| FIGO | ||
| I | 64/250 | 25.6 |
| II/III/IV | 92/250 | 36.8 |
| Not available (NA’s) | 94/250 | 37.6 |
| Tumor Grade | ||
| I | 21/250 | 8.4 |
| II | 143/250 | 57.2 |
| III | 78/250 | 31.2 |
| Not available (NA’s) | 8/250 | 3.2 |
| Tumor Subtype | ||
| Squamous | 202/250 | 80.8 |
| Adenocarcinoma | 48/250 | 19.2 |
| Progression (over 235 months) | ||
| None | 210/250 | 84 |
| At least one | 21/250 | 11.6 |
| Not available (NA’s) | 11/250 | 4.4 |
| Survival (over 235 months) | ||
| Right censured | 190/250 | 76 |
| Died | 49/250 | 19.6 |
| Not available (NA’s) | 11/250 | 4.4 |
| H3K9ac | H3K4me3 (Cytoplasmic) | H3K4me3 (Nuclear) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median IRS (+/−SD) | % | p (NPAR) | ρ | Median IRS (+/−SD) | % | p (NPAR) | ρ | Median IRS (+/−SD) | % | p (NPAR) | ρ | |
| Histology | ||||||||||||
| SCC | 4 (+/−3.45) | 40.10% | 0.013 | - | 0 (+/−2.67) | 57.40% | 0.296 | - | 8 (+/−3.56) | 32.20% | 0.603 | - |
| Adeno-Ca | 8 (+/−3.78) | 31.30% | 0 (+/−3.35) | 52.10% | 8 (+/−3.58) | 27.10% | ||||||
| Grade | ||||||||||||
| G1 | 8 (+/−3.51) | 31.00% | 0.004 | −0.209 (p = 0.001) | 0 (+/−2.94) | 57.10% | 0.197 | 0.082 (p > 0.05) | 8 (+/−3.66) | 28.60% | 0.917 | −0.017 (p > 0.05) |
| G2 | 4 (+/−3.57) | 35.00% | 0 (+/−3.08) | 52.40% | 8 (+/−3.59) | 31.50% | ||||||
| G3 | 4 (+/−3.26) | 41.00% | 0 (+/−2.24) | 62.80% | 8 (+/−3.55) | 33.30% | ||||||
| pN | ||||||||||||
| N− | 4 (+/−3.55) | 86.10% | 0.001 | −0.236 (p = 0.000) | 0 (+/−3.02) | 57.00% | 0.981 | −0.001 (p > 0.05) | 8 (+/−3.50) | 32.50% | 0.695 | 0.025 (p > 0.05) |
| N+ | 4 (+/−3.33) | 66.00% | 0 (+/−2.43) | 55.70% | 8 (+/−3.69) | 28.90% | ||||||
| pT | ||||||||||||
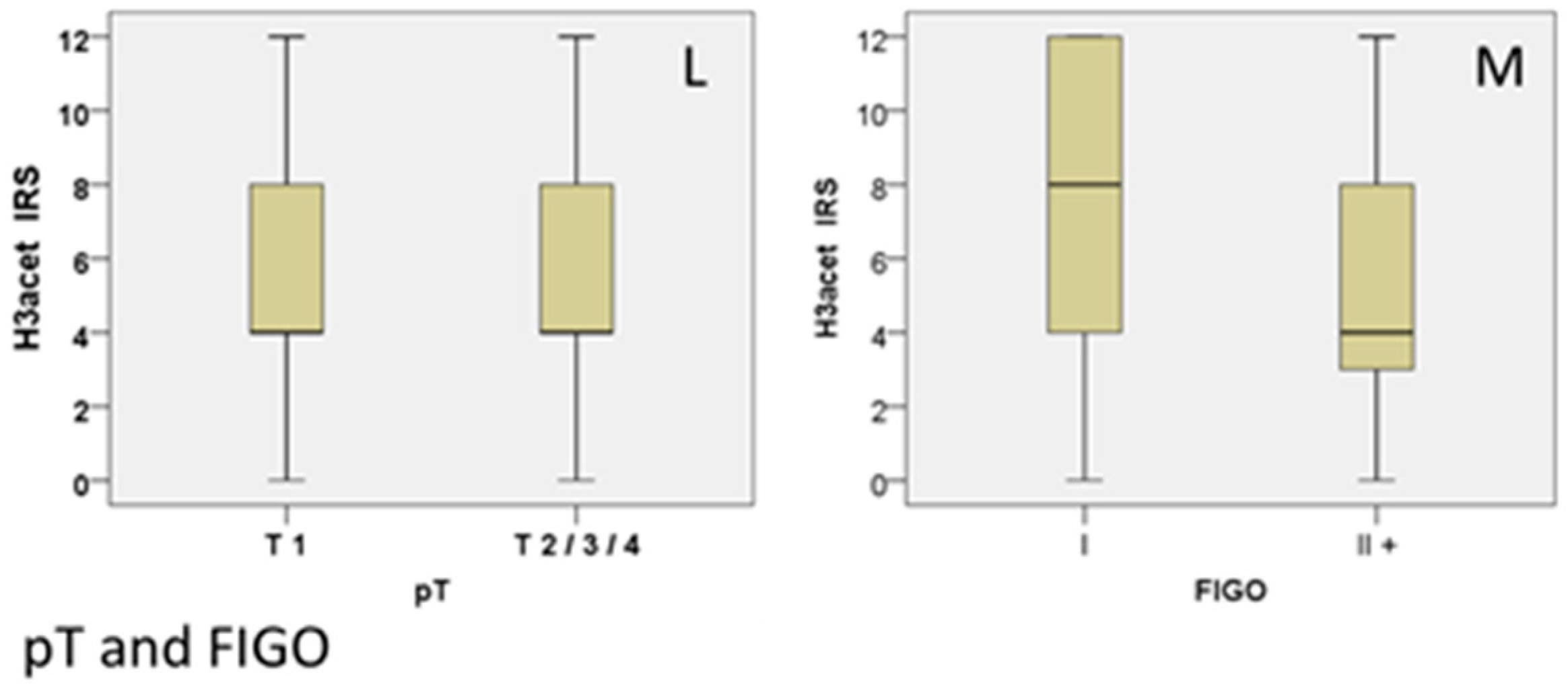
| T1 | 4 (+/−3.52) | 30.90% | 0.035 | −0.149 (p = 0.019) | 0 (+/−2.34) | 69.10% | 0.002 | 0.191 (p = 0.003) | 8 (+/−3.49) | 30.00% | 0.171 | 0.081 (p > 0.05) |
| T2/3/4 | 4 (+/−3.49) | 40.90% | 2 (+/−2.93) | 3.60% | 8 (+/−3.60) | 32.10% | ||||||
| FIGO | ||||||||||||
| I | 8 (+/−3.91) | 26.60% | 0.016 | −0.192 (p = 0.016) | 0 (+/−2.45) | 64.10% | 0.324 | 0.070 (p = 0.384) | 8 (+/−3.37) | 32.80% | 0.862 | −0.005 (p = 0.948) |
| II+ | 4 (+/−3.44) | 23.90% | 2 (+/−2.66) | 5.40% | 8 (+/−3.67) | 30.40% | ||||||
| p16 | - | - | - | 0.047 (p > 0.05) | - | - | - | 0.009 (p > 0.05) | - | - | - | 0.144 (p = 0.027) |
| Variable | Significance | Hazard Ratio of Exp(B) | Lower 95% CI of Exp(B) | Upper 95% CI of Exp(B) |
|---|---|---|---|---|
| Histology | 0.040 | 1.893 | 1.030 | 3.479 |
| pT | 0.751 | 0.910 | 0.509 | 1.626 |
| pN | 0.003 | 2.447 | 1.367 | 4.380 |
| FIGO | 0.012 | 3.181 | 1.287 | 7.863 |
| Grading | 0.198 | 1.360 | 0.852 | 2.170 |
| Age at surgery | 0.000 | 1.049 | 1.026 | 1.072 |
| H3K9ac | 0.027 | 1.900 | 1.076 | 3.356 |
| H3K4me3 nucleus | 0.708 | 1.216 | 0.436 | 3.389 |
| H3K4me3 cytoplasm | 0.159 | 1.503 | 0.853 | 2.651 |
| Variable | Significance | Hazard Ratio of Exp(B) | Lower 95% CI of Exp(B) | Upper 95% CI of Exp(B) |
|---|---|---|---|---|
| Histology | 0.753 | 1.156 | 0.469 | 2.851 |
| pT | 0.760 | 0.890 | 0.423 | 1.875 |
| pN | 0.843 | 1.082 | 0.495 | 2.368 |
| FIGO | 0.044 | 3.085 | 1.031 | 9.235 |
| Grading | 0.521 | 1.228 | 0.656 | 2.299 |
| Age at surgery | 0.157 | 1.021 | 0.992 | 1.052 |
| H3K9ac | 0.763 | 0.890 | 0.417 | 1.900 |
| H3K4me3 nucleus | 0.476 | 0.681 | 0.236 | 1.963 |
| H3K4me3 cytoplasm | 0.030 | 2.278 | 1.084 | 4.790 |
| Histone H3 Acetyl K9 1 | Histone H3 Tri Methyl K4 2 |
|---|---|
| Blocking solution 3: 5 min | Blocking solution 3: 5 min |
| primary antibody 1: 1:200 in PBS 5, incubation: 16 h, 4 °C | primary antibody 2: 1:500 in PBS 5, incubation: 16 h, 4 °C |
| PostBlock 3: 20 min | PostBlock 3: 20 min |
| HRP Polymer 3: 30 min | HRP Polymer 3: 30 min |
| Chromogen: DAB 4 (0.5 min) | Chromogen: DAB 4 (1 min) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, S.; Zhu, J.; Mayr, D.; Kuhn, C.; Schulze, S.; Hofmann, S.; Dannecker, C.; Jeschke, U.; Kost, B.P. Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. Int. J. Mol. Sci. 2017, 18, 477. https://doi.org/10.3390/ijms18030477
Beyer S, Zhu J, Mayr D, Kuhn C, Schulze S, Hofmann S, Dannecker C, Jeschke U, Kost BP. Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. International Journal of Molecular Sciences. 2017; 18(3):477. https://doi.org/10.3390/ijms18030477
Chicago/Turabian StyleBeyer, Susanne, Junyan Zhu, Doris Mayr, Christina Kuhn, Sandra Schulze, Simone Hofmann, Christian Dannecker, Udo Jeschke, and Bernd P. Kost. 2017. "Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer" International Journal of Molecular Sciences 18, no. 3: 477. https://doi.org/10.3390/ijms18030477
APA StyleBeyer, S., Zhu, J., Mayr, D., Kuhn, C., Schulze, S., Hofmann, S., Dannecker, C., Jeschke, U., & Kost, B. P. (2017). Histone H3 Acetyl K9 and Histone H3 Tri Methyl K4 as Prognostic Markers for Patients with Cervical Cancer. International Journal of Molecular Sciences, 18(3), 477. https://doi.org/10.3390/ijms18030477








