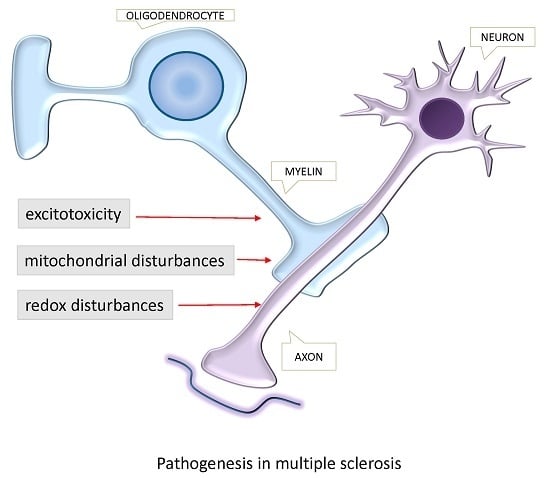
Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis
Abstract
:1. Introduction
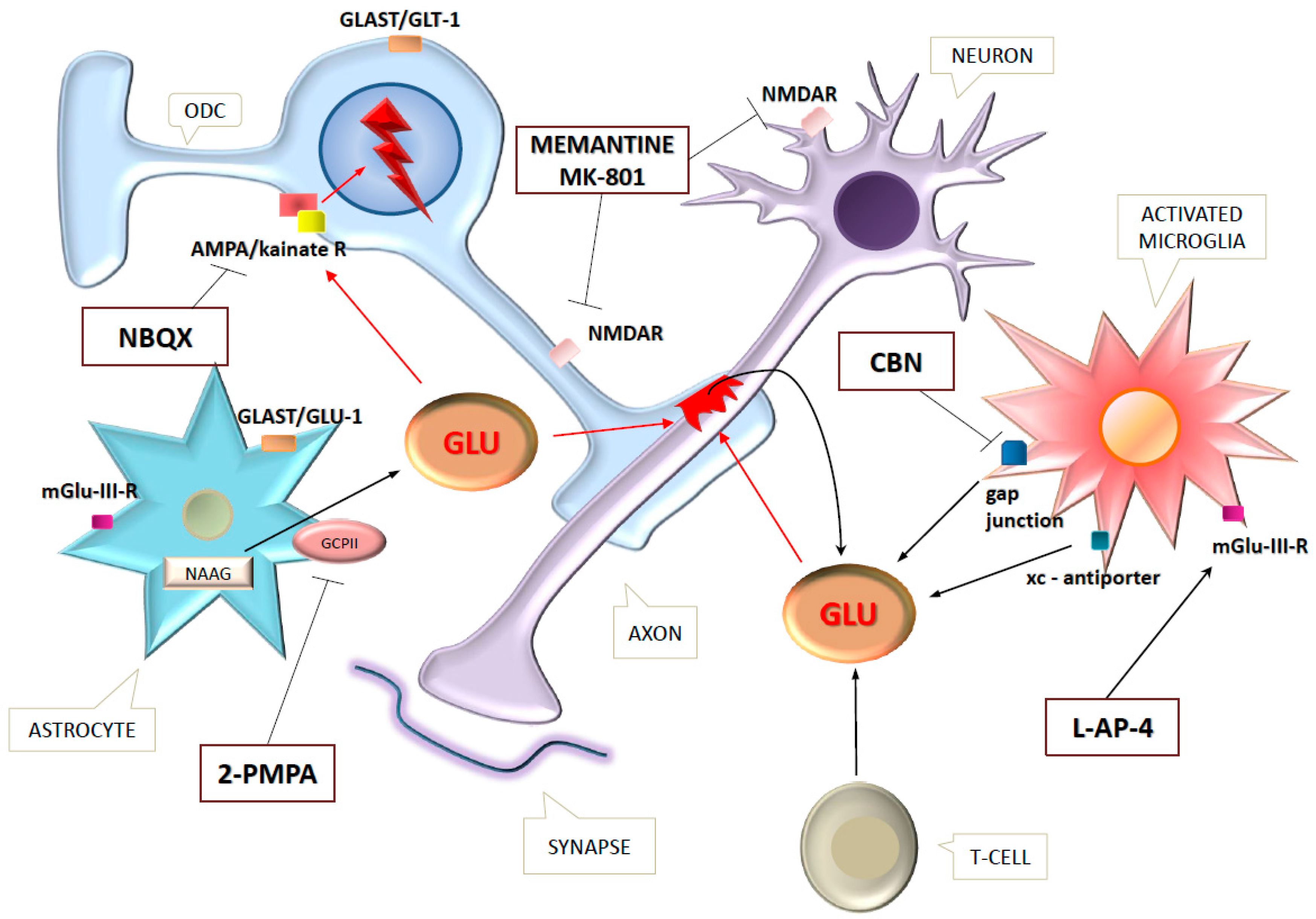
2. Excitotoxicity in Multiple Sclerosis
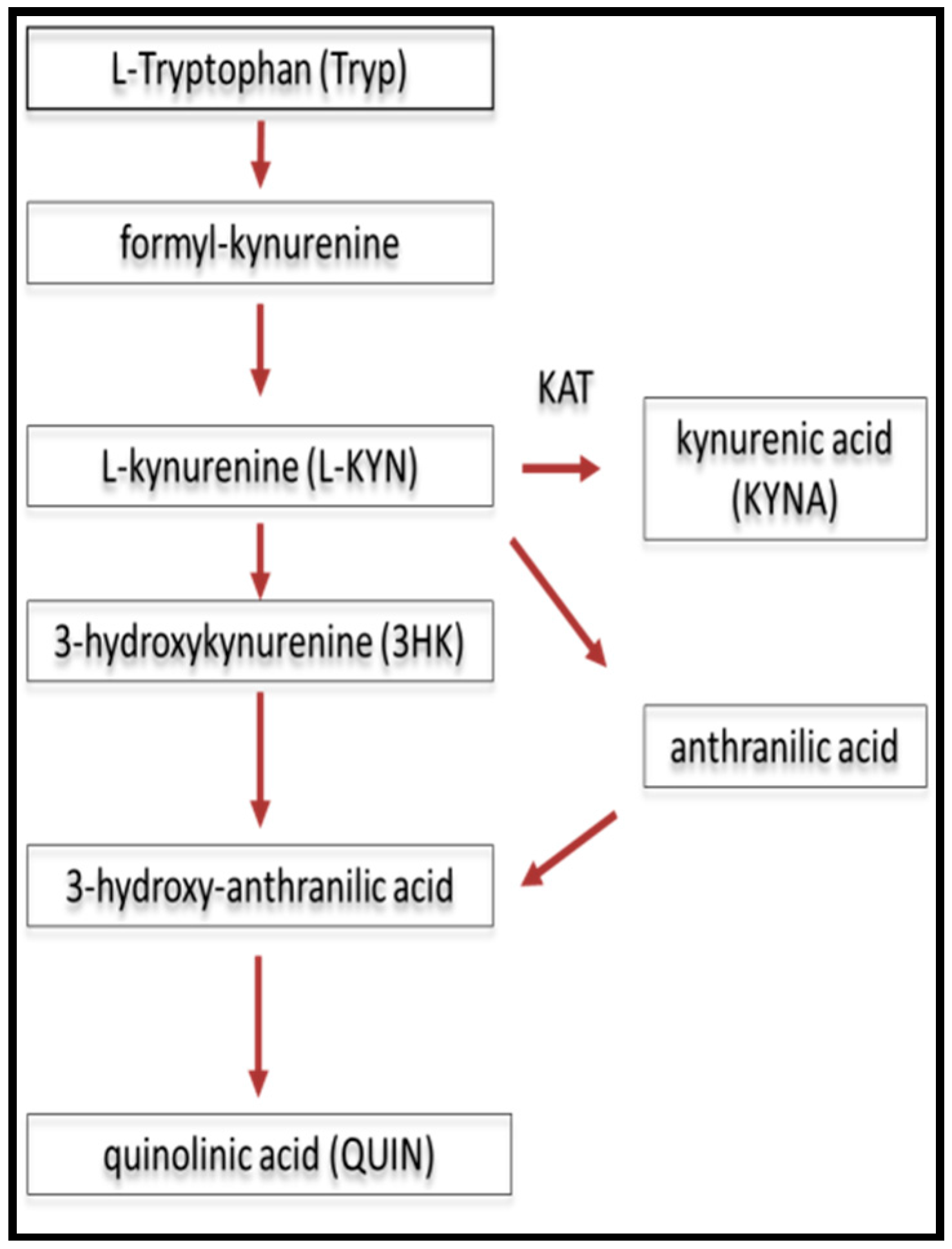
2.1. The Role of Kynurenines in Glutamate Excitotoxicity in MS/EAE
2.2. Blood-Brain Barrier Dysfunction
3. Mitochondrial Disturbances in EAE and MS
4. Redox Disturbances in EAE and MS
5. Biomarkers in Tissue Damage of MS
6. Therapeutic Trials
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| ATP | adenosine triphosphate |
| BBB | blood-brain barrier |
| CHI3L1 | chitinase 3-like 1 protein |
| CIS | clinically isolated syndrome |
| CNS | central nervous system |
| CNTF | ciliary neurotrophic factor |
| CSF | cerebrospinal fluid |
| Cu/Zn SOD | copper/zinc superoxide dismutase |
| CyPD | Cyclophylin6 D |
| DNA | deoxyribonucleic acid |
| DHODH | dihydroorotate dehydrogenase |
| EAE | experimental autoimmune encephalomyelitis |
| EAAC | excitatory amino acid carrier |
| EAAT | excitatory amino acid transporter |
| GA | glatiramer-acetate |
| GFAP | glial fibrillary glial fibrillary acidic protein |
| Glu | glutamate |
| GluR | glutamatergic receptor |
| GLAST | Glu-aspartate transporter |
| GLT-1 | glial transporter-1 |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| HHV-6 | human herpesvirus 6 |
| IL | interleukin |
| IsoP | isoprostanes |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| KP | kynurenine pathway |
| KYNA | kynurenic acid |
| LHON | Leber’s hereditary optic neuropathy |
| MBP | myelin basic protein |
| MDA | malondialdehyde |
| mGluR | metabotropic glutamate receptor |
| MnSOD | manganese superoxide dismutase |
| MOG | myelin-oligodendrocyte glycoprotein |
| MRI | magnetic resonance imaging |
| mtDNA | mitochondrial DNA |
| N-CAM | neuronal cell adhesion molecule |
| NFH | neurofilament heavy chain |
| NFL | neurofilament light chain |
| NMDA | N-methyl-d-aspartate |
| NMDAR | N-methyl-d-aspartate receptor |
| NO | nitric-oxide |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| OCB | oligoclonal band |
| ODC | oligodendrocyte |
| QUIN | quinolinic acid |
| RNA | ribonucleic acid |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RRMS | relapsing-remitting multiple sclerosis |
| S100B | S100 calcium-binding protein B |
| SH | sulfhydryl |
| SOD | superoxide dismutase |
| SPMS | secondary progressive multiple sclerosis |
| TCA | tricarboxylic acid |
| TNF-α | tumor necrosis-alpha |
| TRY | tryptophan |
| WM | white matter |
References
- Peterson, L.K.; Fujinami, R.S. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2007, 184, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K.; Zamponi, G.W.; van Minnen, J.; Geurts, J.J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012, 13, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.; Zivkovic, N.; Stojanovic, I. Multiple sclerosis and glutamate excitotoxicity. Rev. Neurosci. 2013, 24, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, I.R.; Kostic, M.; Ljubisavljevic, S. The role of glutamate and its receptors in multiple sclerosis. J. Neural Transm. 2014, 121, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Pitt, D.; Raine, C.S. Multiple sclerosis: Altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann. Neurol. 2001, 50, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis: Where do we stand? Continuum 2013, 19, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Schul, E.; Geurts, J.; van der Valk, P.; Drukarch, B.; van Dam, A.M. Pathological differences between white and grey matter multiple sclerosis lesions. Ann. N. Y. Acad. Sci. 2015, 1351, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; Ramasamy, D.; Munschauer, F.; Weinstock-Guttman, B.; Zivadinov, R. Memory impairment in multiple sclerosis: Correlation with deep grey matter and mesial temporal atrophy. J. Neurol. Neurosurg. Psychiatry 2009, 80, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Bö, L.; Mörk, S.; Chang, A.; Trapp, B.D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 2001, 50, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Brink, B.P.; Veerhuis, R.; Breij, E.C.; van der Valk, P.; Dijkstra, C.D.; Bö, L. The pathology of multiple sclerosis is location-dependent: No significant complement activation is detected in purely cortical lesions. J. Neuropathol. Exp. Neurol. 2005, 64, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Brink, B.P.; de Vries, H.E.; van der Valk, P.; Bø, L. The blood-brain barrier in cortical multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2007, 66, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Muzio, L.; Rossi, S.; Cavasinni, F.; de Chiara, V.; Bergami, A.; Musella, A.; D’Amelio, M.; Cavallucci, V.; Martorana, A.; et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci. 2009, 29, 3442–3452. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Nissim, I. Acidosis and astrocyte amino acid metabolism. Neurochem. Int. 2000, 36, 329–339. [Google Scholar] [CrossRef]
- Daikhin, Y.; Yudkoff, M. Compartmentation of brain glutamate metabolism in neurons and glia. J. Nutr. 2000, 130, 1026S–1031S. [Google Scholar] [PubMed]
- Tansey, F.A.; Farooq, M.; Cammer, W. Glutamine synthetase in oligodendrocytes and astrocytes: New biochemical and immunocytochemical evidence. J. Neurochem. 1991, 56, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Nagelmeier, I.E.; Wilson, H.C.; Raine, C.S. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology 2003, 61, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; O’Driscoll, C.M.; Bressler, J.; Kaufmann, W.; Rojas, C.J.; Slusher, B.S. Small molecule glutaminase inhibitors block glutamate release from stimulated microglia. Biochem. Biophys. Res. Commun. 2014, 443, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Sharpe, L.G. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science 1969, 166, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.F.; Pleines, U.E.; Morganti-Kossmann, M.C.; Kossmann, T.; Lowitzsch, K.; Kempski, O.S. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur. J. Clin. Investig. 1997, 27, 1038–1043. [Google Scholar] [CrossRef]
- Sarchielli, P.; Greco, L.; Floridi, A.; Gallai, V. Excitatory amino acids and multiple sclerosis: Evidence from cerebrospinal fluid. Arch. Neurol. 2003, 60, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Sailasuta, N.; Hurd, R.; Nelson, S.; Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005, 128, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Cianfoni, A.; Niku, S.; Imbesi, S.G. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am. J. Neuroradiol. 2007, 28, 272–277. [Google Scholar] [PubMed]
- Matute, C.; Alberdi, E.; Domercq, M.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Sánchez-Gómez, M.V. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001, 24, 224–230. [Google Scholar] [CrossRef]
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Bing, S.J.; Ahn, G.; Kim, J.; Cho, J.; Kim, A.; Herath, K.; Yu, H.S.; Jo, S.A.; Cho, I.H.; et al. Blocking glutamate carboxypeptidase II inhibits glutamate excitotoxicity and regulates immune responses in experimental autoimmune encephalomyelitis. FEBS J. 2016, 283, 3438–3456. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Bolton, C. Modulation of blood-brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-d-aspartate receptor antagonist memantine. J. Pharmacol. Exp. Ther. 2002, 302, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Groom, A.; Zhu, B.; Turski, L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 2000, 6, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Barkhof, F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008, 7, 841–851. [Google Scholar] [CrossRef]
- Olney, J.W. Glutamate, a neurotoxic transmitter. J. Child. Neurol 1989, 4, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, G.; Gentile, A.; Musella, A.; Fresegna, D.; de Vito, F.; Bullitta, S.; Sepman, H.; Marfia, G.A.; Centonze, D. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat. Rev. Neurol. 2015, 11, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Piani, D.; Frei, K.; Do, K.Q.; Cuénod, M.; Fontana, A. Murine brain macrophages induced nmda receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 1991, 133, 159–162. [Google Scholar] [CrossRef]
- Yawata, I.; Takeuchi, H.; Doi, Y.; Liang, J.; Mizuno, T.; Suzumura, A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci. 2008, 82, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Piani, D.; Fontana, A. Involvement of the cystine transport system xc− in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J. Immunol. 1994, 152, 3578–3585. [Google Scholar] [PubMed]
- Pampliega, O.; Domercq, M.; Soria, F.N.; Villoslada, P.; Rodríguez-Antigüedad, A.; Matute, C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J. Neuroinflamm. 2011, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, P.; Carmignoto, G.; Pasti, L.; Vesce, S.; Rossi, D.; Rizzini, B.L.; Pozzan, T.; Volterra, A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 1998, 391, 281–285. [Google Scholar] [PubMed]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; de Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J.; et al. CXCR4-activated astrocyte glutamate release via TNFα: Amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Kukley, M.; Capetillo-Zarate, E.; Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007, 10, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, J.; Uddin, A.; Dove, R.; Patel, B.; Turski, L.; Nishizawa, Y.; Smith, T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008, 18, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Rodriguez, M. Indictment of the microglia as the villain in multiple sclerosis. Neurology 1997, 48, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, A.; Bell, K.P.; Norenberg, M.D. Glutamine synthetase: Glial localization in brain. Science 1977, 195, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Belliveau, M.J.; Rosenberg, P.A.; Volpe, J.J. Vulnerability of oligodendroglia to glutamate: Pharmacology, mechanisms, and prevention. J. Neurosci. 1993, 13, 1441–1453. [Google Scholar] [PubMed]
- Olmos, G.; Lladó, J. Tumor necrosis factor α: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Magnus, T.; Jung, S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: A mechanism mediated by tumor necrosis factor-α. FASEB J. 2005, 19, 1878–1880. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Huang, Y.; Zhao, L.; Li, Y.; Sun, L.; Zhou, Y.; Qian, G.; Zheng, J.C. Il-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 2013, 125, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Vesce, S.; Rossi, D.; Brambilla, L.; Volterra, A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007, 82, 57–71. [Google Scholar] [PubMed]
- Stys, P.K.; Waxman, S.G.; Ransom, B.R. Ionic mechanisms of anoxic injury in mammalian cns white matter: Role of Na+ channels and Na+-Ca2+ exchanger. J. Neurosci. 1992, 12, 430–439. [Google Scholar] [PubMed]
- Sulkowski, G.; Dąbrowska-Bouta, B.; Salińska, E.; Strużyńska, L. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS ONE 2014, 9, e113954. [Google Scholar] [CrossRef] [PubMed]
- Domercq, M.; Etxebarria, E.; Pérez-Samartín, A.; Matute, C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia 2005, 52, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Illarramendi, A.; Domercq, M.; Pérez-Cerdá, F.; Ravid, R.; Matute, C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol. Dis. 2006, 21, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mitosek-Szewczyk, K.; Sulkowski, G.; Stelmasiak, Z.; Struzyńska, L. Expression of glutamate transporters GLT-1 and GLAST in different regions of rat brain during the course of experimental autoimmune encephalomyelitis. Neuroscience 2008, 155, 45–52. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.M.; Kabakov, A.Y.; Goldhoff, P.E.; Volpe, J.J.; Rosenberg, P.A. Regulation of glutamate transport in developing rat oligodendrocytes. J. Neurosci. 2009, 29, 7898–7908. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Wolswijk, G.; Bö, L.; van der Valk, P.; Polman, C.H.; Troost, D.; Aronica, E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 2003, 126, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Hwang, S.I.; Roy, D.B.; Lundgren, D.H.; Price, J.V.; Ousman, S.S.; Fernald, G.H.; Gerlitz, B.; Robinson, W.H.; Baranzini, S.E.; et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008, 451, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Lee, F.H.; D’Souza, C.; Su, P.; Zhang, S.; Jia, Z.; Zhang, L.; Wong, A.H.; Liu, F. Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis. Ann. Clin. Transl. Neurol. 2015, 2, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Káradóttir, R.; Cavelier, P.; Bergersen, L.H.; Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005, 438, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Livesey, M.R.; Magnani, D.; Cleary, E.M.; Vasistha, N.A.; James, O.T.; Selvaraj, B.T.; Burr, K.; Story, D.; Shaw, C.E.; Kind, P.C.; et al. Maturation and electrophysiological properties of human pluripotent stem cell-derived oligodendrocytes. Stem Cells 2016, 34, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Sánchez-Gómez, M.V.; Martínez-Millán, L.; Miledi, R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc. Natl. Acad. Sci. USA 1997, 94, 8830–8835. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Pitt, D.; Raine, C.S. Glutamate excitotoxicity—A mechanism for axonal damage and oligodendrocyte death in multiple sclerosis? J. Neural Transm. Suppl. 2000, 60, 375–385. [Google Scholar]
- Ouardouz, M.; Coderre, E.; Zamponi, G.W.; Hameed, S.; Yin, X.; Trapp, B.D.; Stys, P.K. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 2009, 65, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wosik, K.; Ruffini, F.; Almazan, G.; Olivier, A.; Nalbantoglu, J.; Antel, J.P. Resistance of human adult oligodendrocytes to ampa/kainate receptor-mediated glutamate injury. Brain 2004, 127, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, P.; Horiuchi, M.; Feldman, D.; Hahn, A.; Itoh, A.; See, J.; Jia, Z.P.; Itoh, T.; Pleasure, D. GluR2-free α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors intensify demyelination in experimental autoimmune encephalomyelitis. J. Neurochem. 2007, 102, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Macrez, R.; Stys, P.K.; Vivien, D.; Lipton, S.A.; Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol. 2016, 15, 1089–1102. [Google Scholar] [CrossRef]
- Matute, C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc. Natl. Acad. Sci. USA 1998, 95, 10229–10234. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sánchez-Gómez, M.V.; Torre, I.; Domercq, M.; Pérez-Samartín, A.; Pérez-Cerdá, F.; Matute, C. Activation of kainate receptors sensitizes oligodendrocytes to complement attack. J. Neurosci. 2006, 26, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Degos, V.; Peineau, S.; Nijboer, C.; Kaindl, A.M.; Sigaut, S.; Favrais, G.; Plaisant, F.; Teissier, N.; Gouadon, E.; Lombet, A.; et al. G protein-coupled receptor kinase 2 and group I metabotropic glutamate receptors mediate inflammation-induced sensitization to excitotoxic neurodegeneration. Ann. Neurol. 2013, 73, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Rajda, C.; Majláth, Z.; Pukoli, D.; Vécsei, L. Kynurenines and multiple sclerosis: The dialogue between the immune system and the central nervous system. Int. J. Mol. Sci. 2015, 16, 18270–18282. [Google Scholar] [CrossRef] [PubMed]
- Bohár, Z.; Toldi, J.; Fülöp, F.; Vécsei, L. Changing the face of kynurenines and neurotoxicity: Therapeutic considerations. Int. J. Mol. Sci 2015, 16, 9772–9793. [Google Scholar] [CrossRef] [PubMed]
- Majláth, Z.; Toldi, J.; Fülöp, F.; Vécsei, L. Excitotoxic mechanisms in non-motor dysfunctions and levodopa-induced dyskinesia in Parkinson’s disease: The role of the interaction between the dopaminergic and the kynurenine system. Curr. Med. Chem. 2016, 23, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Majláth, Z.; Fülöp, F.; Toldi, J.; Vécsei, L. Brain aging and disorders of the central nervous system: Kynurenines and drug metabolism. Curr. Drug Metab. 2016, 17, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Curr. Alzheimer Res. 2015, 12, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Majlath, Z.; Szok, D.; Csati, A.; Toldi, J.; Fulop, F.; Vecsei, L. Novel kynurenic acid analogues in the treatment of migraine and neurodegenerative disorders: Preclinical studies and pharmaceutical design. Curr. Pharm. Des. 2015, 21, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Robotka, H.; Rózsa, E.; Agoston, M.; Szénási, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vécsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Robotka, H.; Sas, K.; Agoston, M.; Rózsa, E.; Szénási, G.; Gigler, G.; Vécsei, L.; Toldi, J. Neuroprotection achieved in the ischaemic rat cortex with l-kynurenine sulphate. Life Sci. 2008, 82, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Rozsa, E.; Robotka, H.; Nagy, D.; Farkas, T.; Sas, K.; Vecsei, L.; Toldi, J. The pentylenetetrazole-induced activity in the hippocampus can be inhibited by the conversion of l-kynurenine to kynurenic acid: An in vitro study. Brain Res. Bull. 2008, 76, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on ampa receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Füvesi, J.; Rajda, C.; Bencsik, K.; Toldi, J.; Vécsei, L. The role of kynurenines in the pathomechanism of amyotrophic lateral sclerosis and multiple sclerosis: Therapeutic implications. J. Neural Transm. 2012, 119, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, G.; Brew, B.J.; Jones, S.P.; Adams, S.; Lim, C.K.; Guillemin, G.J. Quinolinic acid toxicity on oligodendroglial cells: Relevance for multiple sclerosis and therapeutic strategies. J. Neuroinflamm. 2014, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Cammer, W. Oligodendrocyte killing by quinolinic acid in vitro. Brain Res. 2001, 896, 157–160. [Google Scholar] [CrossRef]
- Sharp, C.D.; Hines, I.; Houghton, J.; Warren, A.; Jackson, T.H.; Jawahar, A.; Nanda, A.; Elrod, J.W.; Long, A.; Chi, A.; et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochim. Biophys. Acta 2010, 1802, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.; Lassmann, H.; Turnbull, D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008, 34, 577–589. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003, 348, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromol. Med. 2008, 10, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxid. Redox Signal. 2007, 9, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial oxidative damage in aging and Alzheimer’s disease: Implications for mitochondrially targeted antioxidant therapeutics. J. Biomed. Biotechnol. 2006, 2006, 31372. [Google Scholar] [CrossRef] [PubMed]
- Ghafourifar, P.; Mousavizadeh, K.; Parihar, M.S.; Nazarewicz, R.R.; Parihar, A.; Zenebe, W.J. Mitochondria in multiple sclerosis. Front. Biosci. 2008, 13, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Bourdette, D.; Forte, M. Mitochondrial dysfunction and neurodegeneration in multiple sclerosis. Front. Physiol. 2013, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Szalárdy, L.; Zádori, D.; Klivényi, P.; Toldi, J.; Vécsei, L. Electron transport disturbances and neurodegeneration: From albert Szent-Györgyi’s concept (Szeged) till novel approaches to boost mitochondrial bioenergetics. Oxid. Med. Cell. Longev. 2015, 2015, 498401. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Yankovskaya, V.; Horsefield, R.; Törnroth, S.; Luna-Chavez, C.; Miyoshi, H.; Léger, C.; Byrne, B.; Cecchini, G.; Iwata, S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 2003, 299, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Senoo-Matsuda, N.; Hartman, P.S.; Akatsuka, A.; Yoshimura, S.; Ishii, N. A complex II defect affects mitochondrial structure, leading to ced-3- and ced-4-dependent apoptosis and aging. J. Biol. Chem. 2003, 278, 22031–22036. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; Manfredi, G. Neuronal degeneration and mitochondrial dysfunction. J. Clin Investig. 2003, 111, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Mahad, D.J.; Lassmann, H.; van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014, 20, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Selak, M.; O’Connor, J.; Croul, S.; Lorenzana, C.; Butunoi, C.; Kalman, B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 2000, 177, 95–103. [Google Scholar] [CrossRef]
- Kalman, B.; Leist, T.P. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromol. Med. 2003, 3, 147–158. [Google Scholar] [CrossRef]
- Nikić, I.; Merkler, D.; Sorbara, C.; Brinkoetter, M.; Kreutzfeldt, M.; Bareyre, F.M.; Brück, W.; Bishop, D.; Misgeld, T.; Kerschensteiner, M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011, 17, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, C.D.; Wagner, N.E.; Ladwig, A.; Nikić, I.; Merkler, D.; Kleele, T.; Marinković, P.; Naumann, R.; Godinho, L.; Bareyre, F.M.; et al. Pervasive axonal transport deficits in multiple sclerosis models. Neuron 2014, 84, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.J.; Ziabreva, I.; Campbell, G.; Lax, N.; White, K.; Hanson, P.S.; Lassmann, H.; Turnbull, D.M. Mitochondrial changes within axons in multiple sclerosis. Brain 2009, 132, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007, 68, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; McDonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Gold, B.G.; Marracci, G.; Chaudhary, P.; Basso, E.; Johnsen, D.; Yu, X.; Fowlkes, J.; Rahder, M.; Stem, K.; et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7558–7563. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.E.; Sweeney, M.G.; Miller, D.H.; Mumford, C.J.; Kellar-Wood, H.; Menard, D.; McDonald, W.I.; Compston, D.A. Occurrence of a multiple sclerosis-like illness in women who have a leber’s hereditary optic neuropathy mitochondrial DNA mutation. Brain 1992, 115 Pt 4, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Kalman, B.; Alder, H. Is the mitochondrial DNA involved in determining susceptibility to multiple sclerosis? Acta Neurol. Scand. 1998, 98, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Mojon, D.S.; Fujihara, K.; Hirano, M.; Miller, C.; Lincoff, N.S.; Jacobs, L.D.; Greenberg, S.J. Leber’s hereditary optic neuropathy mitochondrial DNA mutations in familial multiple sclerosis. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. Nadph oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Geurts, J.J.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Mitochondrial dysfunction: A potential link between neuroinflammation and neurodegeneration? Mitochondrion 2010, 10, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Fischer, M.T.; Wimmer, I.; Höftberger, R.; Gerlach, S.; Haider, L.; Zrzavy, T.; Hametner, S.; Mahad, D.; Binder, C.J.; Krumbholz, M.; et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 2013, 136, 1799–1815. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Mastrolia, V.; Rezaei Haddad, A.; Mosley, A.; Mullali, G.; Schiza, D.; Sajic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci. Rep. 2016, 6, 33249. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lewin, A.S.; Sun, L.; Hauswirth, W.W.; Guy, J. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J. Biol. Chem. 2006, 281, 31950–31962. [Google Scholar] [CrossRef] [PubMed]
- Redford, E.J.; Kapoor, R.; Smith, K.J. Nitric oxide donors reversibly block axonal conduction: Demyelinated axons are especially susceptible. Brain 1997, 120, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Shrager, P.; Custer, A.W.; Kazarinova, K.; Rasband, M.N.; Mattson, D. Nerve conduction block by nitric oxide that is mediated by the axonal environment. J. Neurophysiol. 1998, 79, 529–536. [Google Scholar] [PubMed]
- Cassina, A.M.; Hodara, R.; Souza, J.M.; Thomson, L.; Castro, L.; Ischiropoulos, H.; Freeman, B.A.; Radi, R. Cytochrome C nitration by peroxynitrite. J. Biol. Chem. 2000, 275, 21409–21415. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 2004, 1658, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Maruyama, W.; Kato, Y.; Yi, H.; Shamoto-Nagai, M.; Tanaka, M.; Sato, Y.; Naoi, M. Selective nitration of mitochondrial complex I by peroxynitrite: Involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J. Neural Transm. 2002, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Karg, E.; Klivényi, P.; Németh, I.; Bencsik, K.; Pintér, S.; Vécsei, L. Nonenzymatic antioxidants of blood in multiple sclerosis. J. Neurol. 1999, 246, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Cheng, S.C.; Webb, A.; Ingold, K.U. Vitamin E in young and old human red blood cells. Biochim. Biophys. Acta 1986, 860, 84–90. [Google Scholar] [CrossRef]
- Pollack, M.; Leeuwenburgh, C. Molecular Mechanisms of Oxidative Stress in Aging: Free Radicals, Aging, Antioxidants and Disease; Online-Datei; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Gonsette, R.E. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ljubisavljevic, S.; Stojanovic, I.; Pavlovic, D.; Sokolovic, D.; Stevanovic, I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox Rep. 2011, 16, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Morales Pantoja, I.E.; Hu, C.L.; Perrone-Bizzozero, N.I.; Zheng, J.; Bizzozero, O.A. Nrf2-dysregulation correlates with reduced synthesis and low glutathione levels in experimental autoimmune encephalomyelitis. J. Neurochem. 2016, 139, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Ren, H.; Zhang, L.; Sun, X.; Wang, W.; Zhang, S.; Zhao, J.; Ming, L. α-Tocopherol ameliorates experimental autoimmune encephalomyelitis through the regulation of th1 cells. Iran. J. Basic Med. Sci. 2016, 19, 561–566. [Google Scholar] [PubMed]
- Liu, Y.; Liu, J.; Tetzlaff, W.; Paty, D.W.; Cynader, M.S. Biliverdin reductase, a major physiologic cytoprotectant, suppresses experimental autoimmune encephalomyelitis. Free Radic. Biol. Med. 2006, 40, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Brown, D.A.; Scofield, B.A.; Yu, I.C.; Chang, F.L.; Wang, P.Y.; Yen, J.H. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2016, 57, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Karg, E.; Klivenyi, P.; Bencsik, K.; Turi, S.; Vecsei, L. α-Tocopherol and NADPH in the erythrocytes and plasma of multiple sclerosis patients. Effect of interferon-β-1B treatment. Eur. Neurol. 2003, 50, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Løken-Amsrud, K.I.; Myhr, K.M.; Bakke, S.J.; Beiske, A.G.; Bjerve, K.S.; Bjørnarå, B.T.; Hovdal, H.; Lilleås, F.; Midgard, R.; Pedersen, T.; et al. α-Tocopherol and mRi outcomes in multiple sclerosis—Association and prediction. PLoS ONE 2013, 8, e54417. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Ugur Cevik, M.; Evliyaoglu, O.; Uzar, E.; Tamam, Y.; Arıkanoglu, A.; Yucel, Y.; Varol, S.; Onder, H.; Taşdemir, N. Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol. Belg. 2012, 112, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tasset, I.; Agüera, E.; Sánchez-López, F.; Feijóo, M.; Giraldo, A.I.; Cruz, A.H.; Gascón, F.; Túnez, I. Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin. Biochem. 2012, 45, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Macías-Islas, M.A.; Pacheco-Moisés, F.P.; Cruz-Ramos, J.A.; Sustersik, S.; Barba, E.A.; Aguayo, A. Oxidative stress is increased in serum from mexican patients with relapsing-remitting multiple sclerosis. Dis. Markers 2009, 26, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Mitosek-Szewczyk, K.; Gordon-Krajcer, W.; Walendzik, P.; Stelmasiak, Z. Free radical peroxidation products in cerebrospinal fluid and serum of patients with multiple sclerosis after glucocorticoid therapy. Folia Neuropathol. 2010, 48, 116–122. [Google Scholar] [PubMed]
- Kemp, K.; Redondo, J.; Hares, K.; Rice, C.; Scolding, N.; Wilkins, A. Oxidative injury in multiple sclerosis cerebellar grey matter. Brain Res. 2016, 1642, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Mostert, J.; Arutjunyan, A.V.; Stepanov, M.; Teelken, A.; Heersema, D.; de Keyser, J. Plasma lipid peroxidation and progression of disability in multiple sclerosis. Eur. J. Neurol. 2007, 14, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Sowa, M.; Sowa, P.; Pierzchala, K.; Polaniak, R.; Labuz-Roszak, B. Antioxidative enzymes activity and malondialdehyde concentration during mitoxantrone therapy in multiple sclerosis patients. J. Physiol. Pharmacol. 2012, 63, 683–690. [Google Scholar] [PubMed]
- Choi, I.Y.; Lee, P.; Hughes, A.J.; Denney, D.R.; Lynch, S.G. Longitudinal changes of cerebral glutathione (GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis. Mult. Scler. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sorto-Gomez, T.E.; Ortiz, G.G.; Pacheco-Moises, F.P.; Torres-Sanchez, E.D.; Ramirez-Ramirez, V.; Macias-Islas, M.A.; de la Rosa, A.C.; Velázquez-Brizuela, I.E. Effect of fish oil on glutathione redox system in multiple sclerosis. Am. J. Neurodegener. Dis. 2016, 5, 145–151. [Google Scholar] [PubMed]
- Ghabaee, M.; Jabedari, B.; Al-E-Eshagh, N.; Ghaffarpour, M.; Asadi, F. Serum and cerebrospinal fluid antioxidant activity and lipid peroxidation in guillain-barre syndrome and multiple sclerosis patients. Int. J. Neurosci. 2010, 120, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Klivenyi, P.; Karg, E.; Rozsa, C.; Horvath, R.; Komoly, S.; Nemeth, I.; Turi, S.; Vecsei, L. α-Tocopherol/lipid ratio in blood is decreased in patients with leber’s hereditary optic neuropathy and asymptomatic carriers of the 11778 MTDNA mutation. J. Neurol. Neurosurg. Psychiatry 2001, 70, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Karg, E.; Németh, I.; Horányi, M.; Pintér, S.; Vécsei, L.; Hollán, S. Diminished blood levels of reduced glutathione and α-tocopherol in two triosephosphate isomerase-deficient brothers. Blood Cells Mol. Dis. 2000, 26, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Ibrahim, W.; Wei, Z.; Chan, A.C. Vitamin E regulates mitochondrial hydrogen peroxide generation. Free Radic. Biol. Med. 1999, 27, 580–587. [Google Scholar] [CrossRef]
- Lass, A.; Sohal, R.S. Electron transport-linked ubiquinone-dependent recycling of α-tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem. Biophys. 1998, 352, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Besler, H.T.; Comoğlu, S.; Okçu, Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr. Neurosci. 2002, 5, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Sørensen, P.S.; Hansen, J.C. High dose antioxidant supplementation to MS patients. Effects on glutathione peroxidase, clinical safety, and absorption of selenium. Biol. Trace Elem. Res. 1990, 24, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, B.; Batocchi, A.P.; Amorini, A.M.; Nociti, V.; D’Urso, S.; Longo, S.; Gullotta, S.; Picardi, M.; Lazzarino, G. Serum metabolic profile in multiple sclerosis patients. Mult. Scler. Int. 2011, 2011, 167156. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Montalban, X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol. 2014, 13, 113–126. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Karussis, D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: A critical review. J. Autoimmun. 2014, 48–49, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.J.; Kornak, J.; Chu, P.; Sampat, M.; Okuda, D.T.; Cree, B.A.; Nelson, S.J.; Hauser, S.L.; Pelletier, D. In vivo evidence of glutamate toxicity in multiple sclerosis. Ann. Neurol. 2014, 76, 269–278. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, E.L.; Tam, R.; Zhao, Y.; Vavasour, I.M.; Li, D.K.; Oger, J.; Freedman, M.S.; Kolind, S.H.; Traboulsee, A.L. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler. 2016, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Karlík, M.; Valkovič, P.; Hančinová, V.; Krížová, L.; Tóthová, L.; Celec, P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin. Biochem. 2015, 48, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Laranga, J.; Peltonen, I.; Keskitalo, S.; Duran-Torres, G.; Natarajan, R.; Männistö, P.T.; Nurmi, A.; Vartiainen, N.; Airas, L.; Elovaara, I.; et al. Alteration of prolyl oligopeptidase and activated α-2-macroglobulin in multiple sclerosis subtypes and in the clinically isolated syndrome. Biochem. Pharmacol. 2013, 85, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, O.G.; Haines, J.D.; Katz Sand, I.; Adula, K.P.; Huynh, J.L.; McGraw, C.A.; Zhang, F.; Varghese, M.; Sotirchos, E.; Bhargava, P.; et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014, 137, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Horiuchi, H.; Mizuno, T.; Takeuchi, H.; Suzumura, A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 2015, 63, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Ljubisavljevic, S.; Stojanovic, I.; Vojinovic, S.; Stojanov, D.; Stojanovic, S.; Cvetkovic, T.; Savic, D.; Pavlovic, D. The patients with clinically isolated syndrome and relapsing remitting multiple sclerosis show different levels of advanced protein oxidation products and total thiol content in plasma and CSF. Neurochem. Int. 2013, 62, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Kallaur, A.P.; Reiche, E.M.; Oliveira, S.R.; Simão, A.N.; Pereira, W.L.; Alfieri, D.F.; Flauzino, T.; Proença, C.M.; Lozovoy, M.A.; Kaimen-Maciel, D.R.; et al. Genetic, immune-inflammatory, and oxidative stress biomarkers as predictors for disability and disease progression in multiple sclerosis. Mol. Neurobiol. 2017, 54, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Greco, A.; Stromillo, M.L.; Prosperini, L.; Puopolo, M.; Cefaro, L.A.; Pantano, P.; de Stefano, N.; Minghetti, L.; Pozzilli, C. Isoprostanes in clinically isolated syndrome and early multiple sclerosis as biomarkers of tissue damage and predictors of clinical course. Mult. Scler. 2013, 19, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Sombekke, M.; van Winsen, L.; Killestein, J.; Barkhof, F.; Polman, C.H.; Dijkstra, C.D.; Blankenstein, M.A.; Pratico, D. Increased plasma 8,12-iso-iPF2α-VI levels in relapsing multiple sclerosis patients are not predictive of disease progression. Mult. Scler. 2012, 18, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Polachini, C.R.; Spanevello, R.M.; Zanini, D.; Baldissarelli, J.; Pereira, L.B.; Schetinger, M.R.; da Cruz, I.B.; Assmann, C.E.; Bagatini, M.D.; Morsch, V.M. Evaluation of δ-aminolevulinic dehydratase activity, oxidative stress biomarkers, and vitamin D levels in patients with multiple sclerosis. Neurotox. Res. 2016, 29, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, A.; Koudriavtseva, T.; Bucaj, E.; Coccia, R.; Foppoli, C.; Giorgi, A.; Schininà, M.E.; Di Domenico, F.; de Marco, F.; Perluigi, M. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: The spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS ONE 2013, 8, e65184. [Google Scholar] [CrossRef] [PubMed]
- Füvesi, J.; Hanrieder, J.; Bencsik, K.; Rajda, C.; Kovács, S.K.; Kaizer, L.; Beniczky, S.; Vécsei, L.; Bergquist, J. Proteomic analysis of cerebrospinal fluid in a fulminant case of multiple sclerosis. Int. J. Mol. Sci. 2012, 13, 7676–7693. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, B.; Hecker, M.; Zettl, U.K. Molecular biomarkers in cerebrospinal fluid of multiple sclerosis patients. Autoimmun. Rev. 2015, 14, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.C.; Zhang, S.; Xu, Y.M.; Zhang, G.X.; Zhu, L. Matrine regulates glutamate-related excitotoxic factors in experimental autoimmune encephalomyelitis. Neurosci. Lett. 2014, 560, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Shijie, J.; Takeuchi, H.; Yawata, I.; Harada, Y.; Sonobe, Y.; Doi, Y.; Liang, J.; Hua, L.; Yasuoka, S.; Zhou, Y.; et al. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J. Exp. Med. 2009, 217, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Krissansen, G.W. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain 2004, 127, 1313–1331. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Lee, S.C.; Schwarcz, R. Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 2000, 390, 267–274. [Google Scholar] [CrossRef]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Copeland, C.S.; Neale, S.A.; Bruno, V.; Battaglia, G.; Salt, T.E.; Nicoletti, F. Cinnabarinic acid and xanthurenic acid: Two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology 2017, 112 Pt B, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Zappulla, C.; Notartomaso, S.; Busceti, C.; Bessede, A.; Scarselli, P.; Vacca, C.; Gargaro, M.; Volpi, C.; Allegrucci, M.; et al. Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice. Neuropharmacology 2014, 81, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L. Kynurenines and the nervous system: Therapeutic perspectives. J. Neural Transm. 2012, 119, 107. [Google Scholar] [CrossRef] [PubMed]
- Zádori, D.; Klivényi, P.; Toldi, J.; Fülöp, F.; Vécsei, L. Kynurenines in Parkinson’s disease: Therapeutic perspectives. J. Neural Transm. 2012, 119, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.; Gollan, R.; Ellwardt, E.; Birkenstock, J.; Robohm, K.; Siffrin, V.; Zipp, F. In vivo and in vitro effects of multiple sclerosis immunomodulatory therapeutics on glutamatergic excitotoxicity. J. Neurochem. 2016, 136, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Besong, G.; Battaglia, G.; D’Onofrio, M.; di Marco, R.; Ngomba, R.T.; Storto, M.; Castiglione, M.; Mangano, K.; Busceti, C.L.; Nicoletti, F.R.; et al. Activation of group III metabotropic glutamate receptors inhibits the production of rantes in glial cell cultures. J. Neurosci. 2002, 22, 5403–5411. [Google Scholar] [PubMed]
- Villoslada, P.; Arrondo, G.; Sepulcre, J.; Alegre, M.; Artieda, J. Memantine induces reversible neurologic impairment in patients with MS. Neurology 2009, 72, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lewin, A.S.; Sun, L.; Hauswirth, W.W.; Guy, J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Su, K.G.; Savino, C.; Marracci, G.; Chaudhary, P.; Yu, X.; Morris, B.; Galipeau, D.; Giorgio, M.; Forte, M.; Bourdette, D. Genetic inactivation of the p66 isoform of shca is neuroprotective in a murine model of multiple sclerosis. Eur. J. Neurosci. 2012, 35, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Desai, R.A.; Bloomfield, P.S.; McIntosh, P.R.; Chapple, K.J.; Linington, C.; Fairless, R.; Diem, R.; Kasti, M.; Murphy, M.P.; et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013, 74, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Manczak, M.; Shirendeb, U.P.; Reddy, P.H. MITOQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim. Biophys. Acta 2013, 1832, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Mora, O.; Yubero, P.; Rodriguez de la Concepción, M.; Iglesias, R.; Giralt, M.; Villarroya, F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1α gene transcription: An autoregulatory loop controls PGC-1α expression in adipocytes via peroxisome proliferator-activated receptor-γ coactivation. Endocrinology 2006, 147, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Q.; Lu, Y.F.; Gong, Q.H.; Shi, J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008, 600, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Shindler, K.S.; Ventura, E.; Dutt, M.; Elliott, P.; Fitzgerald, D.C.; Rostami, A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 2010, 30, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Demir, S.; Wiese, S.; Kruse, N.; Siglienti, I.; Gerhardt, E.; Neumann, H.; Sendtner, M.; Lühder, F.; et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: Therapeutic implications in a model of multiple sclerosis. Brain 2010, 133, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Geyer, E.; Flach, A.C.; Jung, K.; Gold, R.; Flügel, A.; Linker, R.A.; Lühder, F. Central nervous system rather than immune cell-derived bdnf mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 2012, 123, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Anderson, J.M.; Phuah, C.L.; Fox, E.J.; Selmaj, K.; Margolin, D.; Lake, S.L.; Palmer, J.; Thompson, S.J.; Wilkins, A.; et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 2010, 133, 2232–2247. [Google Scholar] [CrossRef] [PubMed]
- Thöne, J.; Ellrichmann, G.; Seubert, S.; Peruga, I.; Lee, D.H.; Conrad, R.; Hayardeny, L.; Comi, G.; Wiese, S.; Linker, R.A.; et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am. J. Pathol. 2012, 180, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Gold, R.; Linker, R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: Therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012, 13, 11783–11803. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Licht-Mayer, S.; Wimmer, I.; Traffehn, S.; Metz, I.; Brück, W.; Bauer, J.; Bradl, M.; Lassmann, H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. 2015, 130, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Talla, V.; Yu, H.; Chou, T.H.; Porciatti, V.; Chiodo, V.; Boye, S.L.; Hauswirth, W.W.; Lewin, A.S.; Guy, J. NADH-dehydrogenase type-2 suppresses irreversible visual loss and neurodegeneration in the EAE animal model of ms. Mol. Ther. 2013, 21, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.M.; Sun, M.; Kemp, K.; Gray, E.; Wilkins, A.; Scolding, N.J. Mitochondrial sirtuins—A new therapeutic target for repair and protection in multiple sclerosis. Eur. J. Neurosci. 2012, 35, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. d-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Sedel, F.; Papeix, C.; Bellanger, A.; Touitou, V.; Lebrun-Frenay, C.; Galanaud, D.; Gout, O.; Lyon-Caen, O.; Tourbah, A. High doses of biotin in chronic progressive multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2015, 4, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Gold, R. Multiple sclerosis: TOWER confirms the efficacy of oral teriflunomide in MS. Nat. Rev. Neurol. 2014, 10, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, Ø.; Løken-Amsrud, K.I.; Wergeland, S.; Myhr, K.M.; Holmøy, T. Fat-soluble vitamins as disease modulators in multiple sclerosis. Acta Neurol. Scand. Suppl. 2013. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, H.; de Graaf, R.A.; Mason, G.F.; Pelletier, D.; Juchem, C. Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T. J. Magn. Reson. Imaging 2017, 45, 187–198. [Google Scholar] [CrossRef] [PubMed]
| Patient Groups | Glutamate Levels in CSF (µM) | Glutamine Levels in CSF (µM) |
|---|---|---|
| Controls, n = 20 | 1.3 ± 0.1 | 574 ± 25 |
| Facial palsy, n = 5 | 1.0 ± 0.1 | 570 ± 54 |
| MS (non-active disease), n = 14 | 1.2 ± 0.1 | 467 ± 47 |
| MS (active disease), n = 21 | 3.3 ± 0.3 * | 528 ± 22 |
| Meningitis, n = 14 | 2.8 ± 0.2 * | 587 ± 35 |
| Myelopathy, n = 15 | 3.1 ± 0.3 * | 597 ± 54 |
| Stroke, n = 8 | 2.2 ± 0.2 * | 655 ± 31 |
| NPH, n = 6 | 1.7 ± 0.2 * | 615 ± 48 |
| Epilepsy, n = 4 | 5.0 ± 1.8 * | 629 ± 84 |
| Groups | Glutamate Level (Mean ± SEM, mg/dL) | Significance |
|---|---|---|
| Control subjects, n = 20 | 0.050 ± 0.017 | NA |
| RRMS patients (stable phase), n = 25 | 0.080 ± 0.031 | Vs. control subjects, p < 0.007 Vs. patients with SPMS, p = 0.09 Vs. control subjects, p = 0.013 Vs. patients without Gd+ lesion, p < 0.001 Vs. control subjects, p = 0.08 Vs. patients with RRMS assessed during relapse, p < 0.001 |
| with Gd+ lesion on MRI, n = 14 | 1.103 ± 0.024 | |
| without Gd+ lesion on MRI, n = 11 | 0.053 ± 0.017 | |
| RRMS patients (active disorder- sample gathered after 72 h of onset) n = 30 | 0.103 ± 0.033 | Vs. control subjects, p < 0.001 Vs. patients with RRMS during a stable phase, p < 0.001 |
| SPMS subjects n = 25 | 0.073 ± 0.024 | Vs. control subjects, p < 0.01 Vs. patients during stable phase, p = 0.13 Vs. patients with RRMS during relapse, p < 0.003 Vs. control subjects, p = 0.16 Vs. patients with SPMS with at least 1 point increase in EDSS for the last 6 month, p < 0.001 Vs. control subjects, p < 0.001 Vs. patients with RRMS during relapse, p = 0.04 |
| SPMS patients with no EDSS score increasing for the past 6 months, n = 13 | 0.062 ± 0.024 | |
| SPMS patients, whose EDSS score increased at least 1 point for the past 6 months, n = 12 | 0.103 ± 0.014 |
| 1. Increased Glu-expression |
| a. Activated microglia/ma, leukocytes [5,35]—emission channels are: |
|
| b. Astrocytes [4]—causes |
| c. Demyelinated axons [41]: |
|
| 2. Decreased Glu-reuptake (dysfunction of EAATs) |
| 3. Defects of enzymes involved in Glu homeostasis: |
|
| 4. Glutamate receptor overexpression [4,42] |
|
| Transporter (Human) | Transporter (Mammals) | Occurrence (Cell) |
|---|---|---|
| EAAT1 | GLAST | Astrocyte, ODC, microglia |
| EAAT2 | GLT-1 | Astrocyte, ODC |
| EAAT3 | EAAC1 | Neuron (somatodendritic), astrocyte (low) |
| EAAT4 | EAAT4 | Purkinje cell |
| EAAT5 | EAAT5 | Müller cell (retina) |
| Groups | Subtypes | Localization |
|---|---|---|
| Group I. | mGlu1, mGlu5 | Neurons: postsynaptic (excitatory effect) Normal case: somatodendritic OPC In MS/EAE: WM, axons |
| Group II. | mGlu2, mGlu3 | Neurons: presynaptic (inhibitor) In MS/EAE: microglia, astrocyte overexpression |
| Group III. | mGlu4, mGlu6, mGlu7, mGlu8 | Neurons: presynaptic (inhibitor) In MS/EAE: microglia, astrocyte overexpression |
| Enzymatic Antioxidants | Source | Properties |
| Zn/Cu-SOD | nucleus and cytosol | inhibitor of lipid peroxidation |
| Mn-SOD | mitochondria | inhibitor of lipid peroxidation |
| catalase | peroxisome | inhibitor of lipid peroxidation |
| glutathione peroxidase | mitochondria | inhibitor of lipid peroxidation |
| glucose-6-phosphate dehydrogenase | mitochondria | inhibitor of lipid peroxidation |
| Nonenzymatic Antioxidants | Source | Properties |
| α-tocopherol | intravasal, cell membrane | inhibitor of lipid peroxidation hydrophobic scavenger inhibits the propagation of the chain reaction |
| carotenoids | intravasal, cell membrane | |
| glutathione | intravasal, mitochondrial, nuclear | inhibitor of lipid peroxidation hydrophilic scavenger prevents the initiation of radical formation |
| ascorbic acid | intravasal | inhibitor of lipid peroxidation |
| ceruloplasmin | intravasal | inhibitor of lipid peroxidation |
| transferrin | intravasal | inhibitor of lipid peroxidation |
| uric acid | intravasal | inhibitor of lipid peroxidation |
| Retinol | intravasal | inhibitor of lipid peroxidation |
| SH groups | intravasal | inhibitor of lipid peroxidation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajda, C.; Pukoli, D.; Bende, Z.; Majláth, Z.; Vécsei, L. Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 353. https://doi.org/10.3390/ijms18020353
Rajda C, Pukoli D, Bende Z, Majláth Z, Vécsei L. Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis. International Journal of Molecular Sciences. 2017; 18(2):353. https://doi.org/10.3390/ijms18020353
Chicago/Turabian StyleRajda, Cecilia, Dániel Pukoli, Zsuzsanna Bende, Zsófia Majláth, and László Vécsei. 2017. "Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis" International Journal of Molecular Sciences 18, no. 2: 353. https://doi.org/10.3390/ijms18020353
APA StyleRajda, C., Pukoli, D., Bende, Z., Majláth, Z., & Vécsei, L. (2017). Excitotoxins, Mitochondrial and Redox Disturbances in Multiple Sclerosis. International Journal of Molecular Sciences, 18(2), 353. https://doi.org/10.3390/ijms18020353