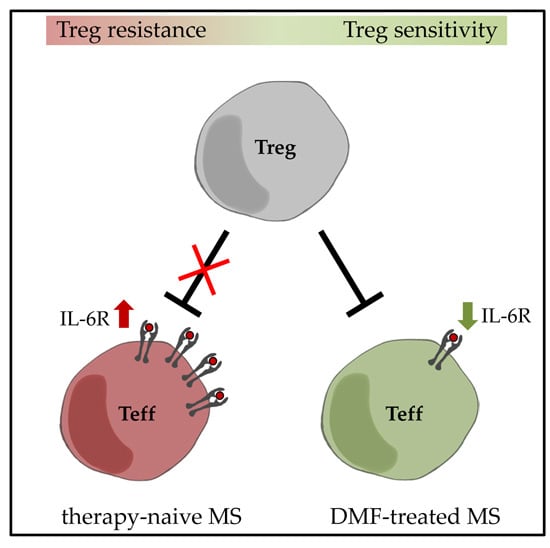
Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells
Abstract
:1. Introduction
2. Results
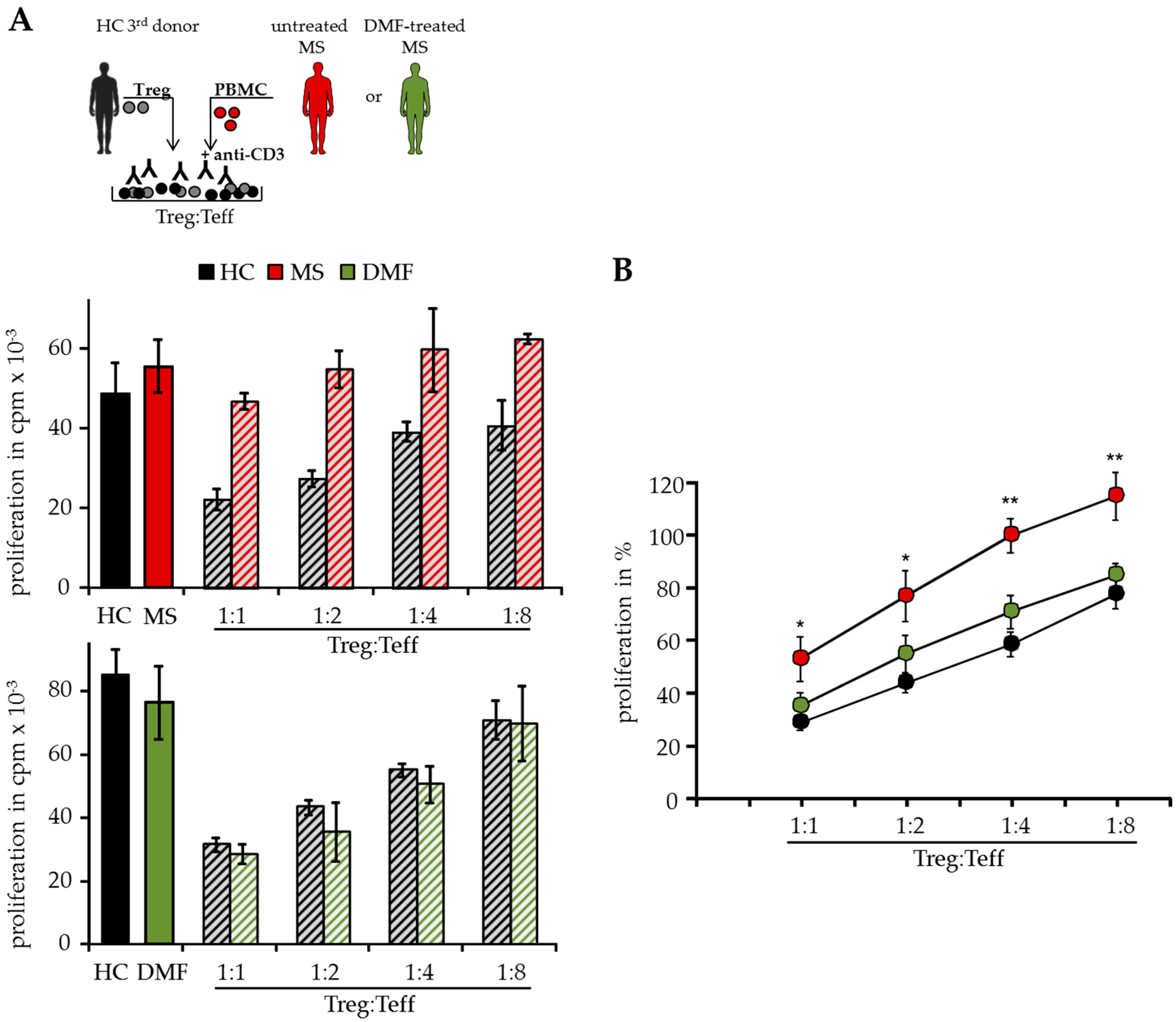
2.1. DMF Restores Treg Resistance of MS T Effector Cells In Vitro
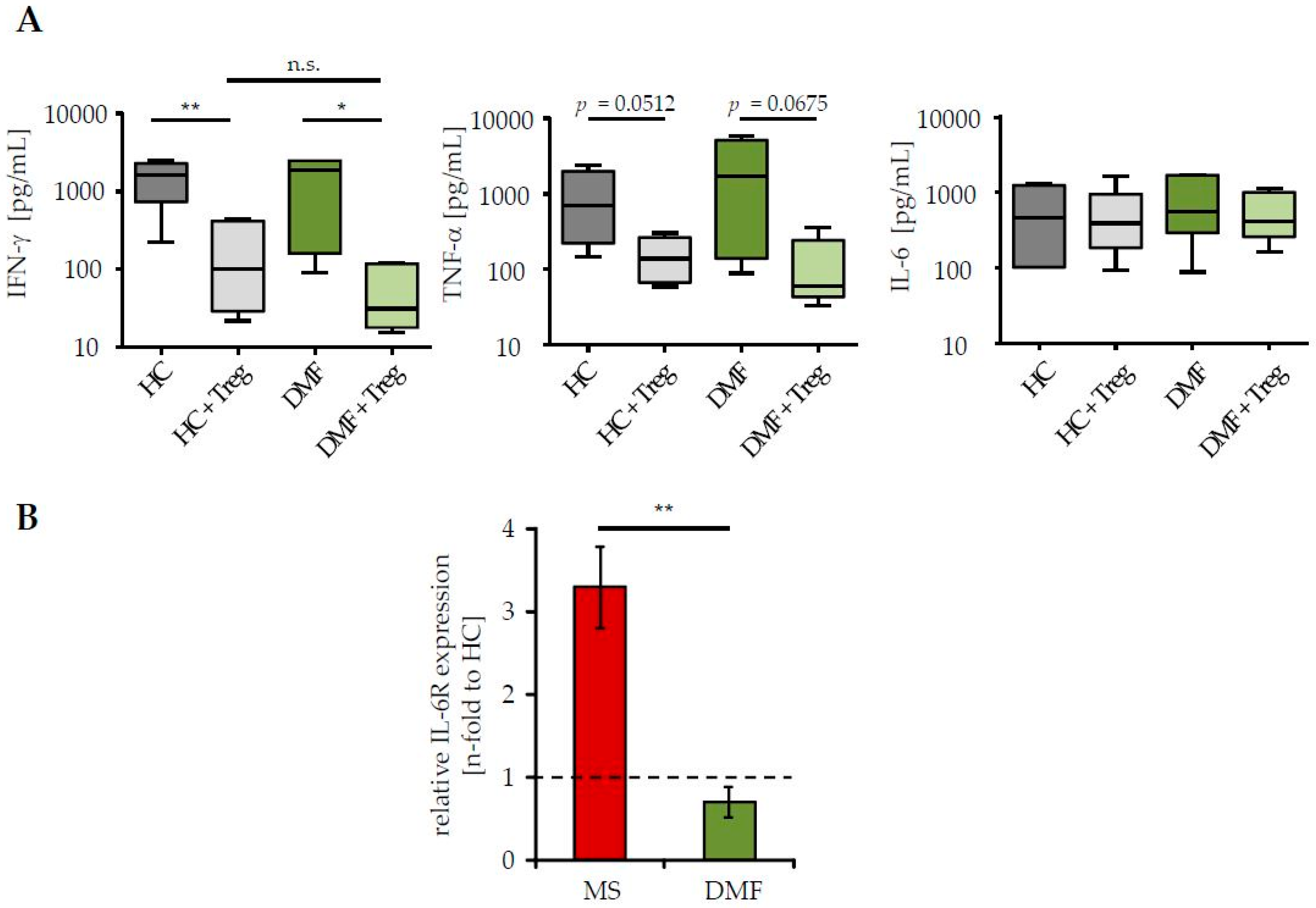
2.2. Restored Treg Sensitivity of Teff from DMF-Treated MS Patients Correlates with a Downregulated IL-6R Expression
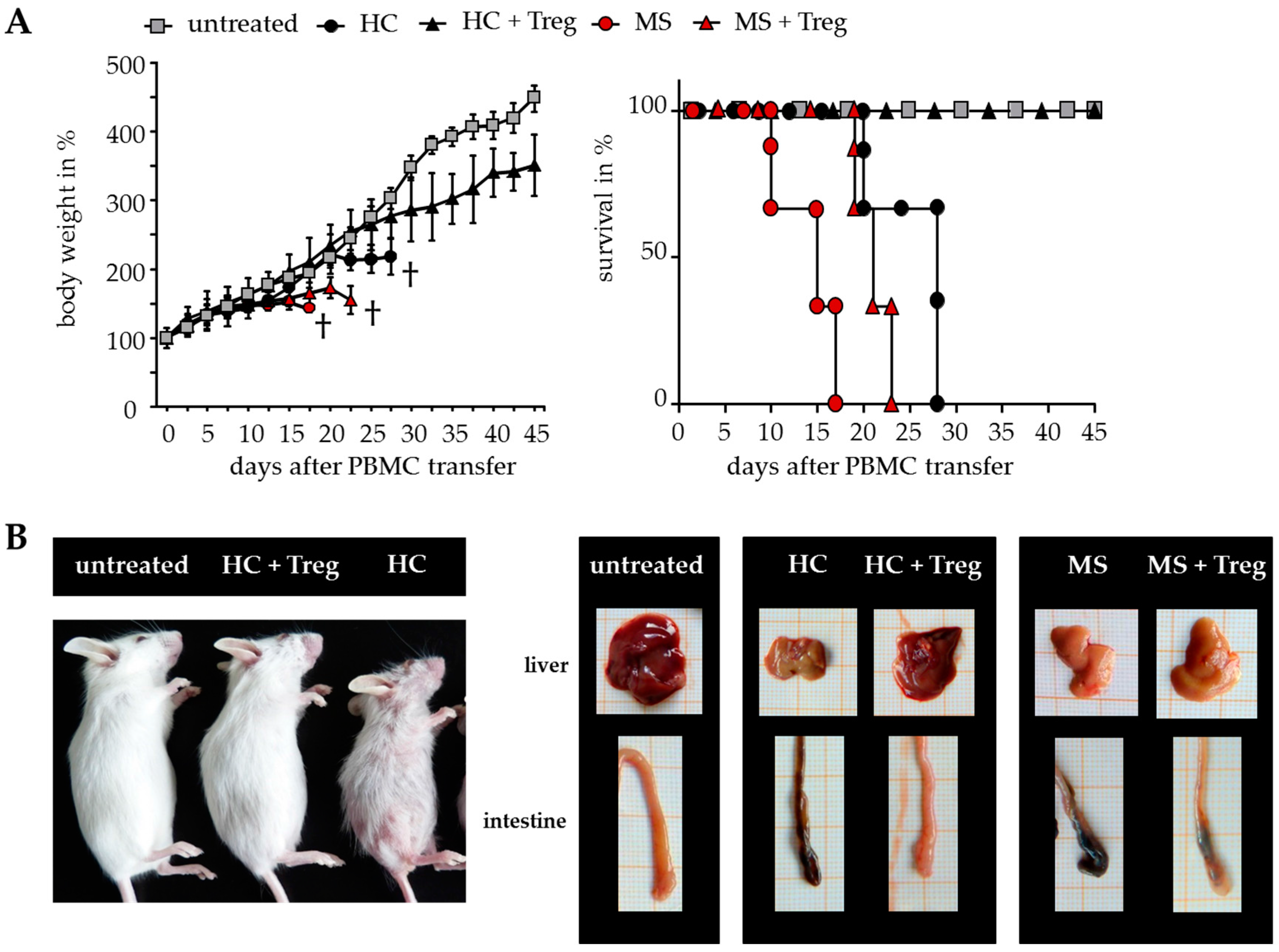
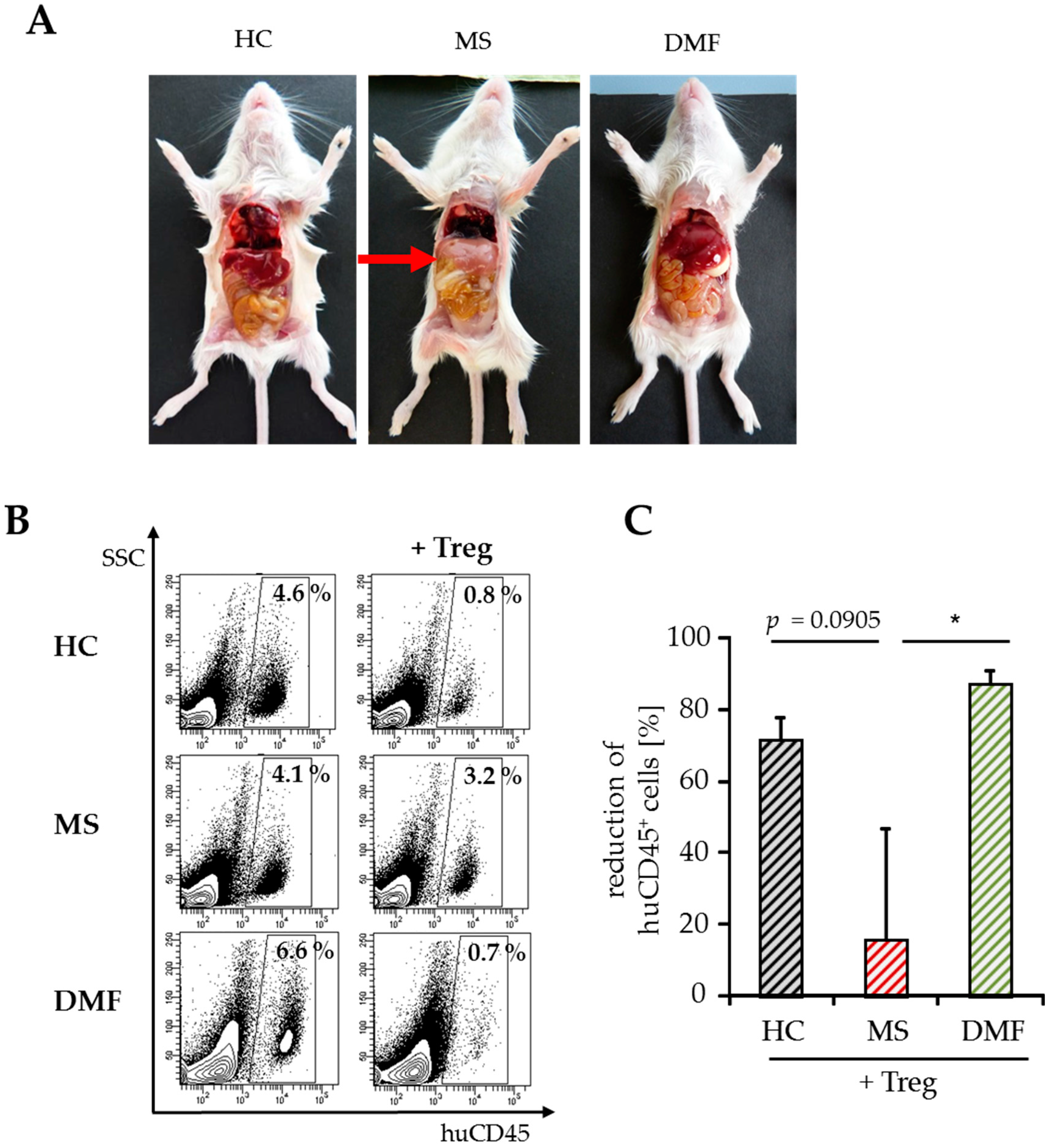
2.3. Improved Responsiveness of T Cells from DMF-Treated MS Patients to Treg-Mediated Suppression In Vivo
2.4. Treg-Reduced Amount of Activated CD4+ T Cells from DMF-Treated MS Patients In Vivo
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Transfer of Human Immune Cells
4.3. Culture Medium and Antibodies
4.4. Flow Cytometry
4.5. Cell Isolation from Spleen
4.6. Isolation of T Cell Subsets
4.7. Cytokine Analysis
4.8. Suppressor Assays
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004, 199, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Reddy, J.; Gao, W.; Bettelli, E.; Awasthi, A.; Petersen, T.R.; Backstrom, B.T.; Sobel, R.A.; Wucherpfennig, K.W.; Strom, T.B.; et al. Myelin-specific regulatory T cells accumulate in the cns but fail to control autoimmune inflammation. Nat. Med. 2007, 13, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Long, S.A.; Cerosaletti, K.; Ni, C.T.; Samuels, P.; Kita, M.; Buckner, J.H. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci. Transl. Med. 2013, 5, 170ra115. [Google Scholar] [CrossRef] [PubMed]
- Trinschek, B.; Luessi, F.; Haas, J.; Wildemann, B.; Zipp, F.; Wiendl, H.; Becker, C.; Jonuleit, H. Kinetics of IL-6 production defines T effector cell responsiveness to regulatory T cells in multiple sclerosis. PLoS ONE 2013, 8, e77634. [Google Scholar] [CrossRef]
- Palace, J.; Duddy, M.; Bregenzer, T.; Lawton, M.; Zhu, F.; Boggild, M.; Piske, B.; Robertson, N.P.; Oger, J.; Tremlett, H.; et al. Effectiveness and cost-effectiveness of interferon β and glatiramer acetate in the UK multiple sclerosis risk sharing scheme at 6 years: A clinical cohort study with natural history comparator. Lancet Neurol. 2015, 14, 497–505. [Google Scholar] [CrossRef]
- Paty, D.W.; Li, D.K.; UBC MS/MRI study group and the IFNB multiple sclerosis study group. Interferon β-1b is effective in relapsing-remitting multiple sclerosis. II. Mri analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993, 43, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A.; Ransohoff, R.M.; Lee, J.C.; Peppler, R.; Yu, M.; Mathisen, P.M.; Tuohy, V.K. In vivo effects of interferon β-1a on immunosuppressive cytokines in multiple sclerosis. Neurology 1998, 50, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Trinschek, B.; Luessi, F.; Gross, C.C.; Wiendl, H.; Jonuleit, H. Interferon-β therapy of multiple sclerosis patients improves the responsiveness of T cells for immune suppression by regulatory T cells. Int. J. Mol. Sci. 2015, 16, 16330–16346. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S.; Ross, C.; Clemmesen, K.M.; Bendtzen, K.; Frederiksen, J.L.; Jensen, K.; Kristensen, O.; Petersen, T.; Rasmussen, S.; Ravnborg, M.; et al. Clinical importance of neutralising antibodies against interferon β in patients with relapsing-remitting multiple sclerosis. Lancet 2003, 362, 1184–1191. [Google Scholar] [CrossRef]
- Jacobs, L.D.; Cookfair, D.L.; Rudick, R.A.; Herndon, R.M.; Richert, J.R.; Salazar, A.M.; Fischer, J.S.; Goodkin, D.E.; Granger, C.V.; Simon, J.H.; et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann. Neurol. 1996, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; D’Souza, M.; Kappos, L.; Yaldizli, O. Dimethyl fumarate for multiple sclerosis. Expert. Opin. Investig. Drug 2010, 19, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Al-Khamis, F.A. The use of immune modulating drugs for the treatment of multiple sclerosis. Neurosciences 2016, 21, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.K.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Bruck, J.; Kellerer, C.; Deng, C.; Peng, H.; Rothfuss, O.; Hussain, R.Z.; Gocke, A.R.; Respa, A.; Glocova, I.; et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011, 208, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guerau-de-Arellano, M.; Mehta, V.B.; Yang, Y.; Huss, D.J.; Papenfuss, T.L.; Lovett-Racke, A.E.; Racke, M.K. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J. Biol. Chem. 2012, 287, 28017–28026. [Google Scholar] [CrossRef] [PubMed]
- Loewe, R.; Holnthoner, W.; Groger, M.; Pillinger, M.; Gruber, F.; Mechtcheriakova, D.; Hofer, E.; Wolff, K.; Petzelbauer, P. Dimethylfumarate inhibits tnf-induced nuclear entry of NF-κB/P65 in human endothelial cells. J. Immunol. 2002, 168, 4781–4787. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Klinsing, S.; Posevitz-Fejfar, A.; Wiendl, H.; Klotz, L. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e183. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Gold, R. Dimethyl fumarate for treatment of multiple sclerosis: Mechanism of action, effectiveness, and side effects. Curr. Neurol. Neurosci. Rep. 2013, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Gorochov, G.; Ehrenstein, M.; Musset, L.; Sakaguchi, S.; Amoura, Z. Human FOXP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 2011, 10, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, E.J.; Mijnheer, G.; Duurland, C.L.; Klein, M.; Meerding, J.; van Loosdregt, J.; de Jager, W.; Sawitzki, B.; Coffer, P.J.; Vastert, B.; et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/C-AKT hyperactivation in effector cells. Blood 2011, 118, 3538–3548. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Imitola, J.; Chitnis, T.; Khoury, S.J. Cytokines in multiple sclerosis: From bench to bedside. Pharmacol. Ther. 2005, 106, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Haug, M.; Schepp, C.; Kuemmerle-Deschner, J.; Hansmann, S.; Rieber, N.; Tzaribachev, N.; Hospach, T.; Maier, J.; Dannecker, G.E.; et al. Impaired suppression of synovial fluid CD4+CD25− T cells from patients with juvenile idiopathic arthritis by CD4+CD25+ treg cells. Arthritis Rheum. 2011, 63, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, S.; Miao, R.; Kan, W. Trail is associated with impaired regulation of CD4+CD25− T cells by regulatory T cells in patients with rheumatoid arthritis. J. Clin. Immunol. 2011, 31, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.M.; Tremble, J.; Dayan, C.; Beyan, H.; Leslie, R.D.; Peakman, M.; Tree, T.I. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008, 154, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.A.; Levine, A.D.; Massari, J.V.; Sugiyama, H.; McCormick, T.S.; Cooper, K.D. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009, 183, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Valencia, X.; Stephens, G.; Goldbach-Mansky, R.; Wilson, M.; Shevach, E.M.; Lipsky, P.E. Tnf downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 2006, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Noronha, A.; Toscas, A.; Jensen, M.A. Interferon β decreases T cell activation and interferon gamma production in multiple sclerosis. J. Neuroimmunol. 1993, 46, 145–153. [Google Scholar] [CrossRef]
- Treumer, F.; Zhu, K.; Glaser, R.; Mrowietz, U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J. Investig. Dermatol. 2003, 121, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.C.; Listopad, J.J.; Rentzsch, C.U.; Igney, F.H.; von Bonin, A.; Hennekes, H.H.; Asadullah, K.; Docke, W.D. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J. Investig. Dermatol. 2007, 127, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Neurath, M.F. IL-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, P.; Lombardo, F.; Moscato, G.; Mosti, S.; Meucci, G. T cell interleukin-6 receptor binding in interferon-β-1b-treated multiple sclerosis patients. Eur. J. Neurol. 2000, 7, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Christophi, G.P.; Christophi, J.A.; Gruber, R.C.; Mihai, C.; Mejico, L.J.; Massa, P.T.; Jubelt, B. Quantitative differences in the immunomodulatory effects of rebif and avonex in IFN-β 1a treated multiple sclerosis patients. J. Neurol. Sci. 2011, 307, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflamm. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.A.; Guyton, M.K.; Haque, A.; Vandenbark, A.; Tyor, W.R.; Ray, S.K.; Banik, N.L. Increased calpain correlates with th1 cytokine profile in pbmcs from ms patients. J. Neuroimm. 2007, 190, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sharief, M.K.; Hentges, R. Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 1991, 325, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hollifield, R.D.; Harbige, L.S.; Pham-Dinh, D.; Sharief, M.K. Evidence for cytokine dysregulation in multiple sclerosis: Peripheral blood mononuclear cell production of pro-inflammatory and anti-inflammatory cytokines during relapse and remission. Autoimmunity 2003, 36, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ockenfels, H.M.; Schultewolter, T.; Ockenfels, G.; Funk, R.; Goos, M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br. J. Dermatol. 1998, 139, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Kubach, J.; Wijdenes, J.; Knop, J.; Jonuleit, H. CD4-mediated functional activation of human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 2007, 37, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.A.; Stahl, H.F.; Becker, C.; Correll, A.; Schneider, F.J.; Tuettenberg, A.; Jonuleit, H. Soluble garp has potent antiinflammatory and immunomodulatory impact on human CD4+ T cells. Blood 2013, 122, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Kirberg, J.; Berns, A.; von Boehmer, H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 1997, 186, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Taube, C.; Bopp, T.; Becker, C.; Michel, K.; Kubach, J.; Reuter, S.; Dehzad, N.; Neurath, M.F.; Reifenberg, K.; et al. Protection from graft-versus-host disease by HIV-1 envelope protein gp120-mediated activation of human CD4+CD25+ regulatory T cells. Blood 2009, 114, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, N.; Ding, X.; Blank, G.; Dennehy, K.M.; Kerkau, T.; Hunig, T. Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood 2008, 112, 4328–4336. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Schmitt, E.; Stassen, M.; Tuettenberg, A.; Knop, J.; Enk, A.H. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001, 193, 1285–1294. [Google Scholar] [CrossRef] [PubMed]

| Sex | Age (year) | Disease Course | Disease Duration (year) | Treatment | Level of Disability (EDSS) | State |
|---|---|---|---|---|---|---|
| M | 28.90 | RRMS | 7.87 | Tecfidera | 0.00 | Remission |
| M | 30.16 | RRMS | 0.72 | Tecfidera | 1.00 | Relapse |
| W | 45.74 | RRMS | 22.89 | Tecfidera | 5.00 | Remission |
| W | 45.89 | RRMS | 2.78 | Tecfidera | 1.00 | Remission |
| W | 33.30 | RRMS | 3.67 | Tecfidera | 4.50 | Remission |
| W | 43.11 | RRMS | 21.94 | Tecfidera | 2.50 | Remission |
| M | 32.55 | RRMS | 5.69 | Tecfidera | 0.00 | Remission |
| W | 42.45 | RRMS | 3.05 | Tecfidera | 0.00 | Remission |
| M | 23.28 | RRMS | 0.80 | Tecfidera | 2.00 | Remission |
| W | 50.99 | RRMS | 14.00 | Tecfidera | 1.00 | Remission |
| W | 30.93 | RRMS | 4.35 | Tecfidera | 4.00 | Remission |
| M | 19.13 | RRMS | 1.62 | Tecfidera | 0.00 | Remission |
| W | 54.45 | RRMS | 2.54 | Tecfidera | 1.00 | Remission |
| M | 25.98 | RRMS | 2.40 | Tecfidera | 0.00 | Remission |
| M | 31.62 | RRMS | 8.26 | Tecfidera | 1.00 | Relapse |
| W | 51.70 | RRMS | 20.09 | Tecfidera | 2.00 | Remission |
| W | 26.15 | RRMS | 11.10 | Tecfidera | 2.00 | Remission |
| W | 22.25 | RRMS | 3.70 | Tecfidera | 0.00 | Remission |
| W | 29.85 | RRMS | 1.55 | Tecfidera | 0.00 | Remission |
| W | 35.88 | RRMS | 3.26 | Tecfidera | 1.00 | Remission |
| M | 26.15 | RRMS | 3.07 | Tecfidera | 3.00 | Remission |
| W | 20.05 | RRMS | 1.16 | Tecfidera | 0.00 | Remission |
| M | 37.88 | RRMS | 6.17 | Tecfidera | 1.00 | Remission |
| W | 36.32 | RRMS | 11.43 | Tecfidera | 3.00 | Remission |
| M | 49.81 | RRMS | 18.44 | Tecfidera | 6.00 | Remission |
| W | 63.94 | RRMS | 0.15 | untreated | 2.00 | Relapse |
| M | 57.96 | RRMS | 0.42 | untreated | 1.00 | Remission |
| W | 56.00 | RRMS | 6.80 | untreated | 2.00 | Remission |
| W | 51.96 | RRMS | 1.33 | untreated | 1.50 | Remission |
| M | 23.20 | RRMS | 0.72 | untreated | 2.00 | Remission |
| W | 41.11 | RRMS | 0.57 | untreated | 3.00 | Remission |
| W | 27.56 | RRMS | 4.59 | untreated | 3.00 | Relapse |
| W | 63.75 | PPMS | 3.03 | untreated | 3.00 | Remission |
| W | 18.81 | RRMS | 9.22 | untreated | 1.00 | Relapse |
| M | 41.22 | RRMS | 8.98 | untreated | 2.00 | Relapse |
| W | 41.66 | CIS | 1.19 | untreated | 2.00 | Remission |
| W | 21.54 | RRMS | 5.04 | untreated | 1.00 | Relapse |
| W | 24.22 | RRMS | 0.29 | untreated | 1.00 | Remission |
| W | 48.59 | CIS | 1.14 | untreated | 2.00 | Remission |
| W | 41.74 | CIS | 1.27 | untreated | 2.00 | Remission |
| W | 37.41 | RRMS | 6.10 | untreated | 1.00 | Remission |
| M | 45.38 | RRMS | 24.10 | untreated | 3.00 | Remission |
| W | 40.66 | RRMS | 0.53 | untreated | 1.00 | Remission |
| W | 51.08 | RRMS | 5.29 | untreated | 3.50 | Remission |
| W | 37.54 | RRMS | 13.18 | untreated | 1.00 | Relapse |
| M | 34.71 | RRMS | 18.21 | untreated | 1.00 | Remission |
| W | 56.07 | RRMS | 15.23 | untreated | 3.00 | Relapse |
| M | 45.20 | RRMS | 15.25 | untreated | 1.00 | Remission |
| W | 51.85 | RRMS | 2.47 | untreated | 1.00 | Remission |
| M | 46.15 | RRMS | 5.44 | untreated | 1.00 | Relapse |
| W | 48.54 | RRMS | 5.45 | untreated | 1.00 | Remission |
| W | 45.04 | RRMS | 12.52 | untreated | 2.00 | Remission |
| HC | HC + Treg | MS | MS + Treg | DMF | DMF + Treg | |
|---|---|---|---|---|---|---|
| spleen cells ×106 | 52.41 ± 14.34 | 32.09 ± 4.85 | 41.67 ± 6.47 | 34.70 ± 9.95 | 57.38 ± 10.09 | 59.75 ± 9.65 |
| huCD45+ cells in % | 5.77 ± 3.89 | 1.77 ± 0.28 | 3.87 ± 0.52 | 2.80 ± 0.86 | 7.41 ± 3.06 | 0.79 ± 0.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlöder, J.; Berges, C.; Luessi, F.; Jonuleit, H. Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. Int. J. Mol. Sci. 2017, 18, 271. https://doi.org/10.3390/ijms18020271
Schlöder J, Berges C, Luessi F, Jonuleit H. Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. International Journal of Molecular Sciences. 2017; 18(2):271. https://doi.org/10.3390/ijms18020271
Chicago/Turabian StyleSchlöder, Janine, Carsten Berges, Felix Luessi, and Helmut Jonuleit. 2017. "Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells" International Journal of Molecular Sciences 18, no. 2: 271. https://doi.org/10.3390/ijms18020271
APA StyleSchlöder, J., Berges, C., Luessi, F., & Jonuleit, H. (2017). Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. International Journal of Molecular Sciences, 18(2), 271. https://doi.org/10.3390/ijms18020271