Cisplatin, Oxaliplatin, and Kiteplatin Subcellular Effects Compared in a Plant Model
Abstract
:1. Introduction
2. Results
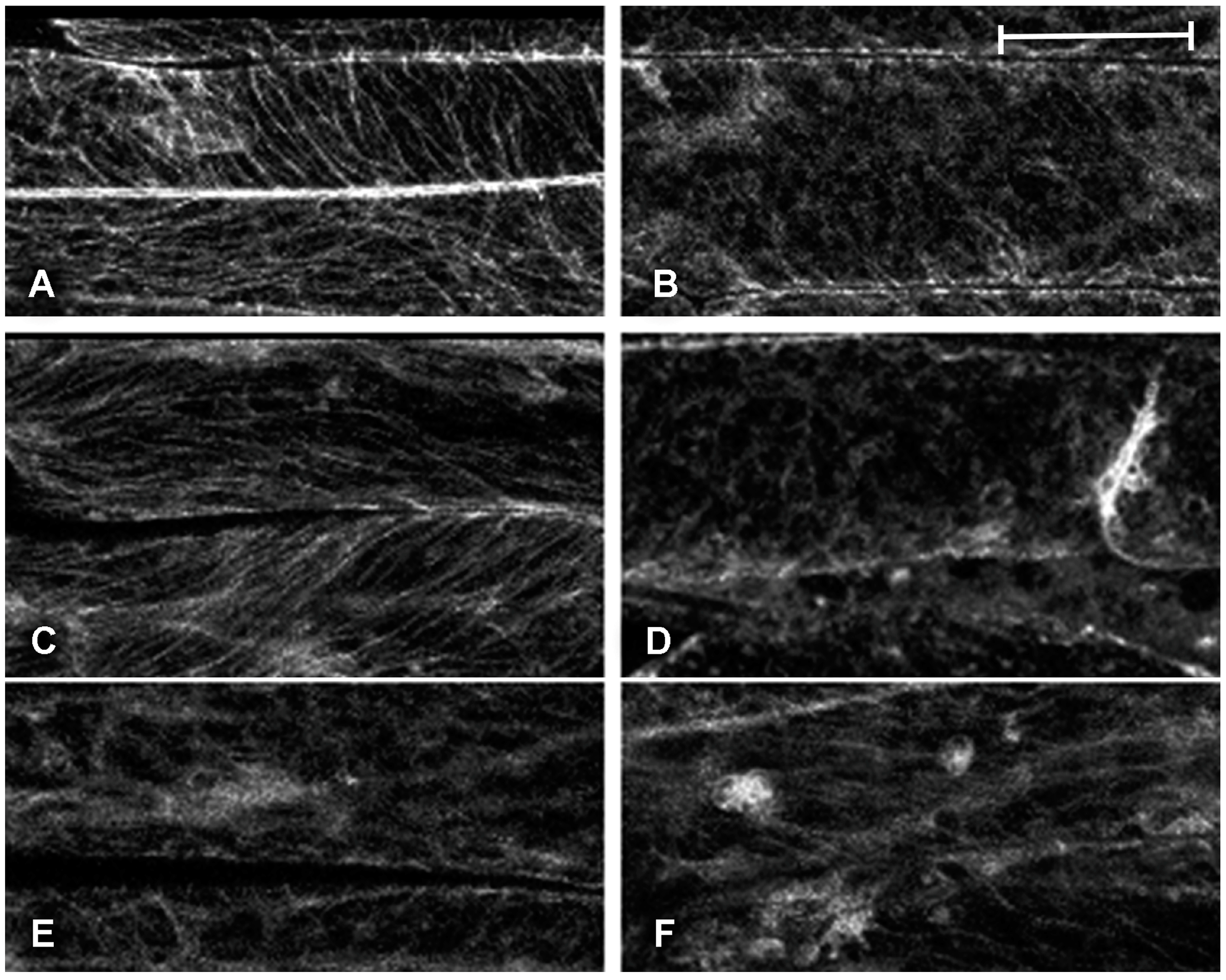
2.1. Effect of Platinum Compounds on Plant Cell Cytoskeleton
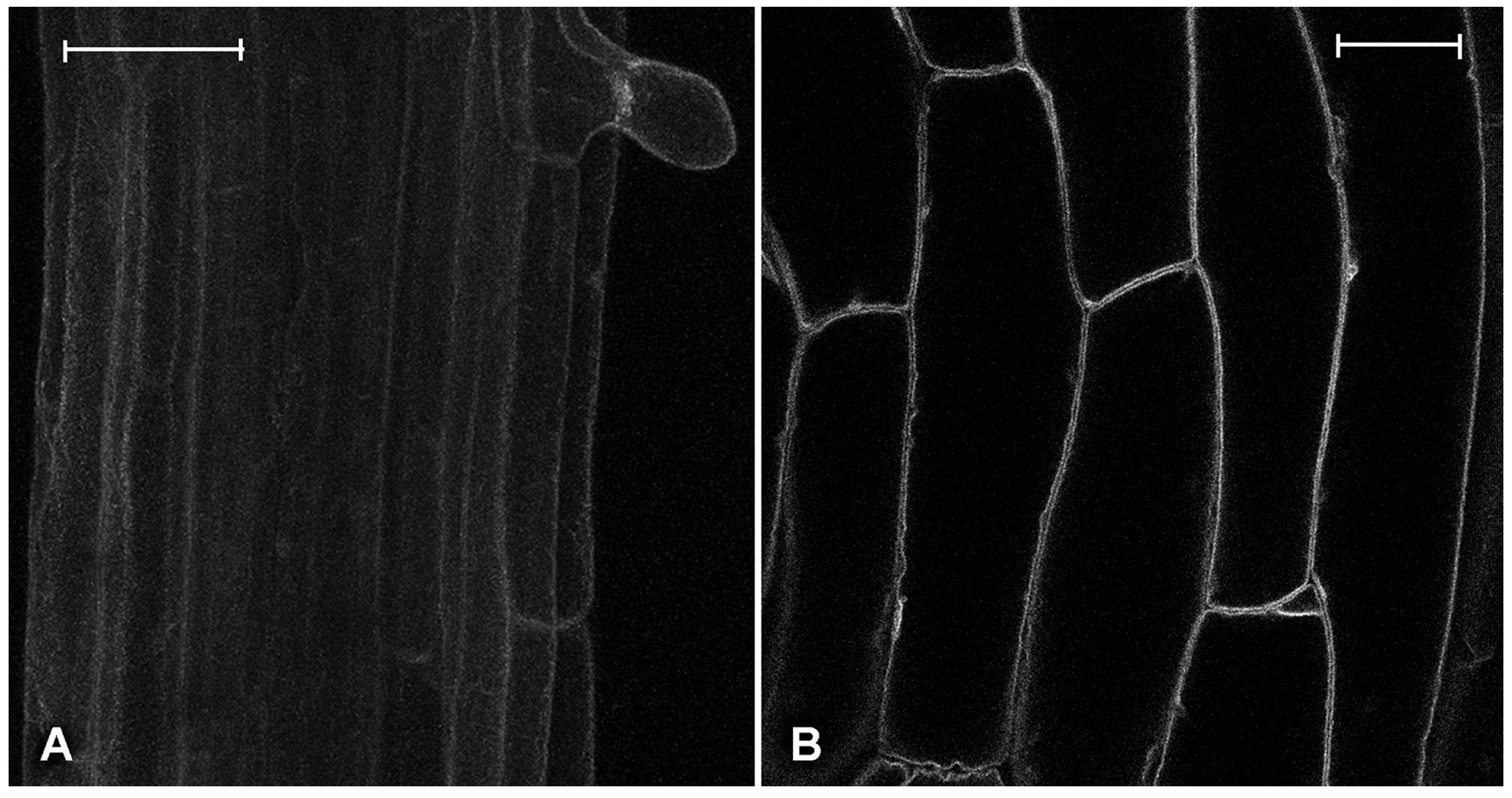
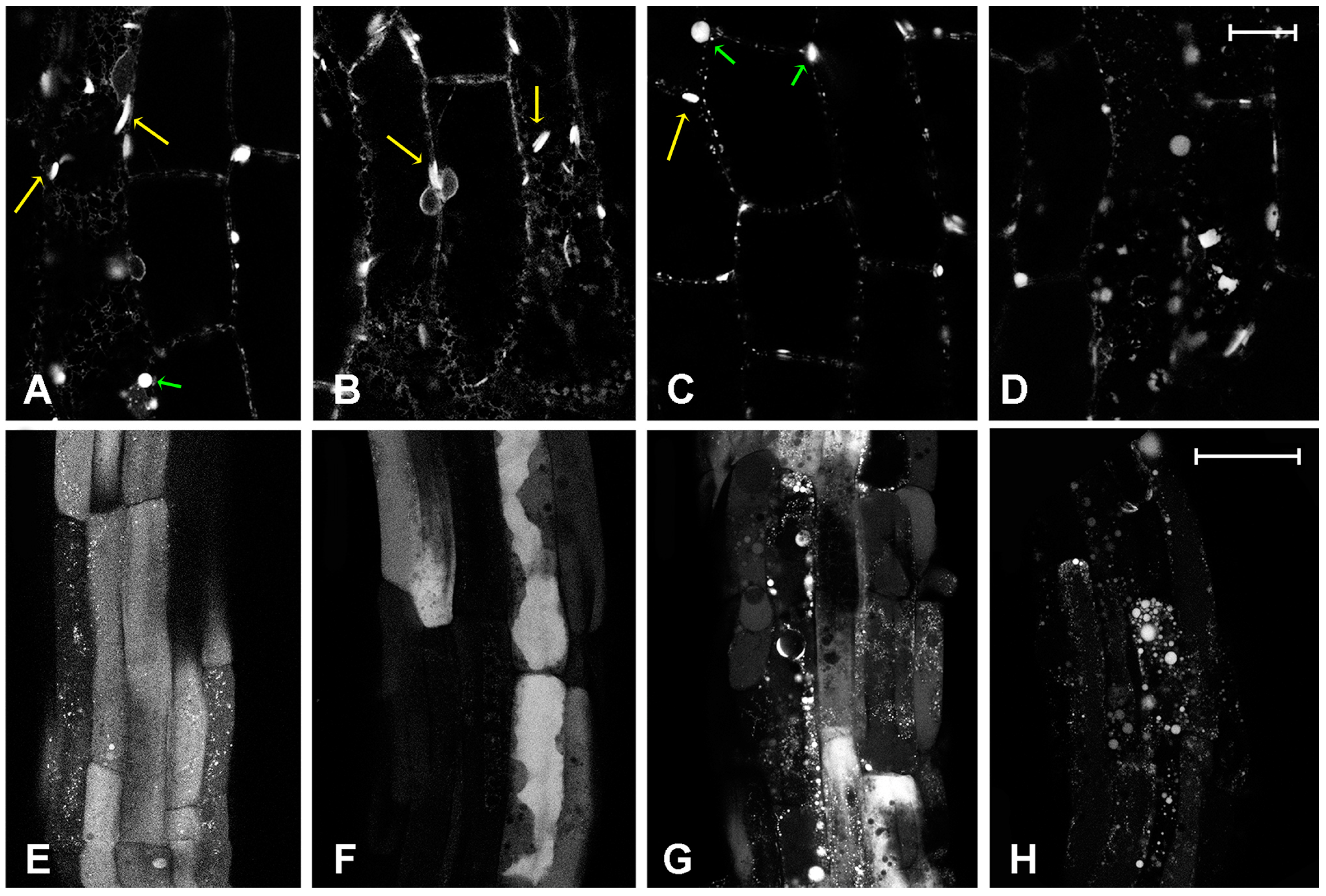
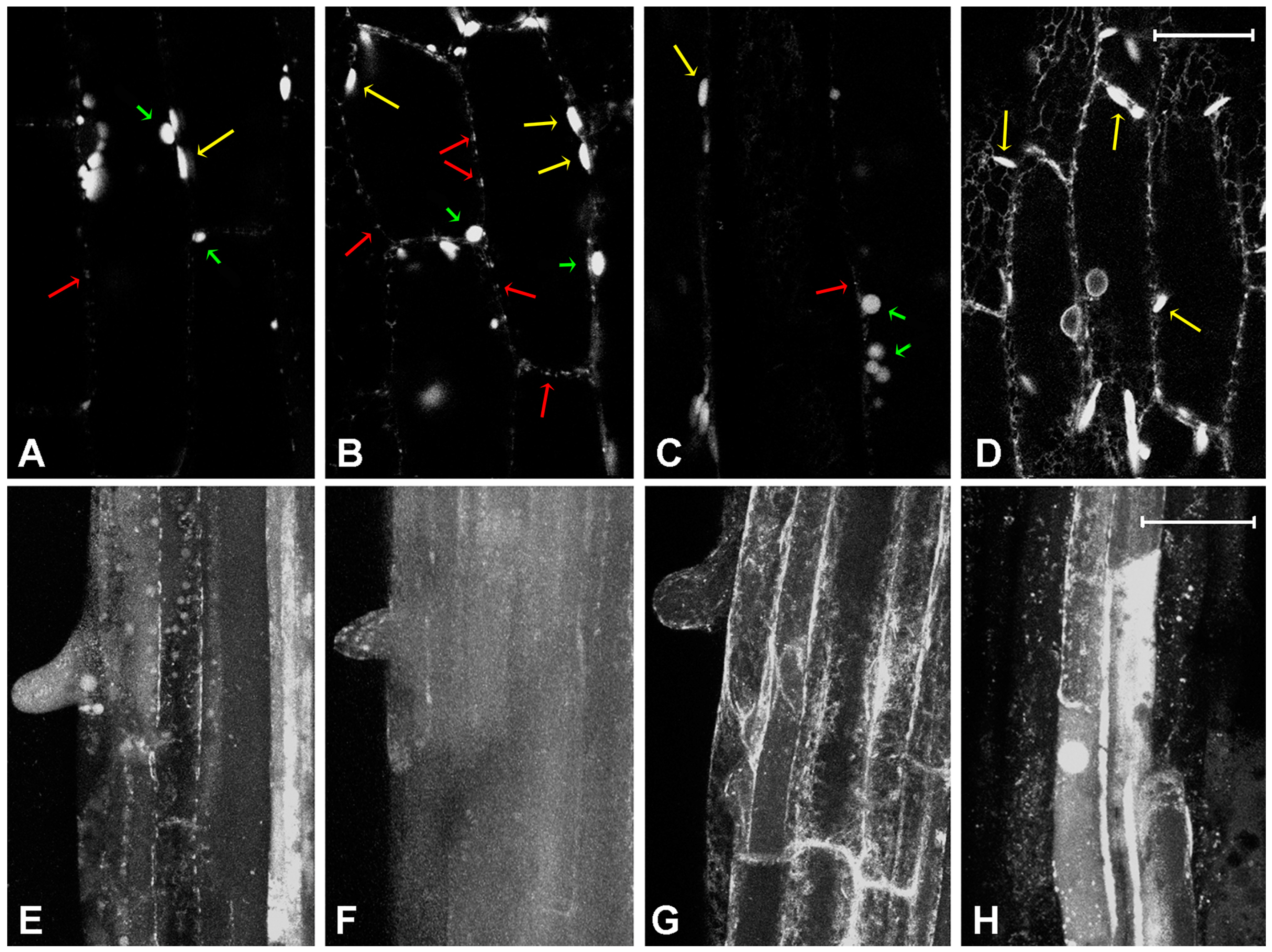
2.2. Effect of Platinum Compounds on Plant Cells’ Central Vacuolar and Golgi Mediated Transport
2.3. Effect of Platinum Compounds on Plant Cell Golgi-Independent Vacuolar Transport
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Transgenic Plants and Confocal Microscopy
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DACH | Diaminocyclohexane |
| Atg | Autophagy related proteins |
| GFP | Green fluorescent protein |
| COPII | Coat protein complex that initiates the budding process from the rough endoplasmic reticulum to the Golgi apparatus |
| ER | Endoplasmic reticulum |
| PI3K | Phosphatidylinositol 3-kinase |
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug; Lippert, B. (Ed.) Verlag Helvetica Chimica Acta: Zürich, Switzerland, 1999.
- Reedijk, J. Platinum Anticancer Coordination Compounds: Study of DNA Binding Inspires New Drug Design. Eur. J. Inorg. Chem. 2009, 2009, 1303–1312. [Google Scholar] [CrossRef]
- Wong, E.; Giandomenico, C.M. Current Status of Platinum-Based Antitumor Drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.C.; Lippard, S.J. Inhibition of transcription by platinum antitumor compounds. Met. Integr. Biometal Sci. 2009, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.A.; Alonso, C.; Pérez, J.M. Biochemical Modulation of Cisplatin Mechanisms of Action: Enhancement of Antitumor Activity and Circumvention of Drug Resistance. Chem. Rev. 2003, 103, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hagen, K.D.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; Reedijk, J.; van Wezel, G.P. Phenanthroline Derivatives with Improved Selectivity as DNA-Targeting Anticancer or Antimicrobial Drugs. ChemMedChem 2008, 3, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Hoeschele, J.D.; Showalter, H.D.H.; Kraker, A.J.; Elliott, W.L.; Roberts, B.J.; Kampf, J.W. Synthesis, Structural Characterization, and Antitumor Properties of a Novel Class of Large-Ring Platinum(II) Chelate Complexes Incorporating the cis-1,4-Diaminocyclohexane Ligand in a Unique Locked Boat Conformation. J. Med. Chem. 1994, 37, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, S.; Takahashi, I.; Siddik, Z.H.; Khokhar, A.R. Synthesis, characterization, and antitumor activity of a series of novel cisplatin analogs with cis-1,4-diaminocyclohexane as nonleaving amine group. J. Inorg. Biochem. 1996, 61, 291–301. [Google Scholar] [CrossRef]
- Margiotta, N.; Marzano, C.; Gandin, V.; Osella, D.; Ravera, M.; Gabano, E.; Platts, J.A.; Petruzzella, E.; Hoeschele, J.D.; Natile, G. Revisiting [PtCl2(cis-1,4-DACH)]: An Underestimated Antitumor Drug with Potential Application to the Treatment of Oxaliplatin-Refractory Colorectal Cancer. J. Med. Chem. 2012, 55, 7182–7192. [Google Scholar] [CrossRef] [PubMed]
- Kasparkova, J.; Suchankova, T.; Halamikova, A.; Zerzankova, L.; Vrana, O.; Margiotta, N.; Natile, G.; Brabec, V. Cytotoxicity, cellular uptake, glutathione and DNA interactions of an antitumor large-ring PtII chelate complex incorporating the cis-1,4-diaminocyclohexane carrier ligand. Biochem. Pharmacol. 2010, 79, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Malina, J.; Margiotta, N.; Natile, G.; Kasparkova, J. Thermodynamic and Mechanistic Insights into Translesion DNA Synthesis Catalyzed by Y-Family DNA Polymerase Across a Bulky Double-Base Lesion of an Antitumor Platinum Drug. Chemistry 2012, 18, 15439–15448. [Google Scholar] [CrossRef] [PubMed]
- Mutter, S.T.; Margiotta, N.; Papadia, P.; Platts, J.A. Computational evidence for structural consequences of kiteplatin damage on DNA. J. Biol. Inorg. Chem. 2014, 20, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Savino, S.; Marzano, C.; Pacifico, C.; Hoeschele, J.D.; Gandin, V.; Natile, G. Cytotoxicity-boosting of kiteplatin by Pt(IV) prodrugs with axial benzoate ligands. J. Inorg. Biochem. 2016, 160, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tulub, A.A.; Stefanov, V.E. Cisplatin stops tubulin assembly into microtubules. A new insight into the mechanism of antitumor activity of platinum complexes. Int. J. Biol. Macromol. 2001, 28, 191–198. [Google Scholar] [CrossRef]
- Herzog, C.; Yang, C.; Holmes, A.; Kaushal, G.P. zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am. J. Physiol. Renal Physiol. 2012, 303, F1239–F1250. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gao, J.; Lu, Z.; Gong, J.; Li, Y.; Dong, B.; Li, Z.; Zhang, X.; Shen, L. Combination of microtubule associated protein-tau and β-tubulin III predicts chemosensitivity of paclitaxel in patients with advanced gastric cancer. Eur. J. Cancer 2014, 50, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Chao, A.; Tsai, C.L.; Chuang, W.C.; Huang, W.P.; Chen, G.C.; Lin, C.Y.; Wang, T.H.; Wang, H.S.; Lai, C.H. Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis. 2014, 5, e1510. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Jaramillo, M.C.; Zhang, Z.; Zheng, Y.; Yao, M.; Zhang, D.D.; Yi, X. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol. Med. Rep. 2015, 11, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Shi, X.Y.; Zhang, Y.; Zhu, Y.; Zhu, U.; Tian, W.; Zhu, B.K.; Wei, Z.L. YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. Onco Targets Ther. 2016, 9, 1105–1114. [Google Scholar] [PubMed]
- Le Bars, R.; Marion, J.; Satiat-Jeunemaitre, B.; Bianchi, M.W. Folding into an autophagosome: ATG5 sheds light on how plants do it. Autophagy 2014, 10, 1861–1863. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Galili, G.; Genschik, P.; Fernie, A.R.; Avin-Wittenberg, T. Autophagy in Plants—What’s New on the Menu? Trends Plant Sci. 2016, 21, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chung, K.P.; Jiang, L. Origin of the Autophagosomal Membrane in Plants. Front. Plant Sci. 2016, 7, 1655. [Google Scholar] [CrossRef] [PubMed]
- Kulich, I.; Žárský, V. Autophagy-Related Direct Membrane Import from ER/Cytoplasm into the Vacuole or Apoplast: A Hidden Gateway also for Secondary Metabolites and Phytohormones? Int. J. Mol. Sci. 2014, 15, 7462–7474. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C. ER and vacuoles: Never been closer. Front. Plant Sci. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Cui, Y.; Gao, C.; Jiang, L. Endocytic and autophagic pathways crosstalk in plants. Curr. Opin. Plant Biol. 2015, 28, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhuang, X.; Cui, Y.; Fu, X.; He, Y.; Zhao, Q.; Zeng, Y.; Shen, J.; Luo, M.; Jiang, L. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. USA 2015, 112, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; de Domenico, S.; Maffia, M.; Piro, G.; Di Sansebastiano, G.P. Transgenic plants as low-cost platform for chemotherapeutic drugs screening. Int. J. Mol. Sci. 2015, 16, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Matsuyama, T.; Hashimoto, T. Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma 1999, 206, 201–206. [Google Scholar] [CrossRef]
- Fluckiger, R.; de Caroli, M.; Piro, G.; Dalessandro, G.; Neuhaus, J.M.; di Sansebastiano, G.P. Vacuolar system distribution in Arabidopsis tissues, visualized using GFP fusion proteins. J. Exp. Bot. 2003, 54, 1577–1584. [Google Scholar] [CrossRef]
- Ranaldo, R.; Margiotta, N.; Intini, F.P.; Pacifico, C.; Natile, G. Conformer Distribution in (cis-1,4-DACH)bis(guanosine-5′-phosphate)platinum(II) Adducts: A Reliable Model for DNA Adducts of Antitumoral Cisplatin. Inorg. Chem. 2008, 47, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.-T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early Effects of Salinity on Water Transport in Arabidopsis Roots. Molecular and Cellular Features of Aquaporin Expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Santiskulvong, C.; Bentolila, L.A.; Rao, J.; Dorigo, O.; Gimzewski, J.K. Correlative nanomechanical profiling with super-resolution F-actin imaging reveals novel insights into mechanisms of cisplatin resistance in ovarian cancer cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, E.; Faraco, M.; Neuhaus, J.-M.; Montefusco, A.; Dalessandro, G.; Piro, G.; di Sansebastiano, G.-P. Two glycosylated vacuolar GFPs are new markers for ER-to-vacuole sorting. Plant Physiol. Biochem. 2013, 73, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, R.; Hayashi, Y.; Yamada, K.; Shimada, T.; Nishimura, M.; Hara-Nishimura, I. The ER Body, a Novel Endoplasmic Reticulum-Derived Structure in Arabidopsis. Plant Cell Physiol. 2003, 44, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Occhialini, A.; Gouzerh, G.; Di Sansebastiano, G.P.; Neuhaus, J.M. Dimerization of the Vacuolar Receptors AtRMR1 and -2 from Arabidopsis thaliana Contributes to Their Localization in the trans-Golgi Network. Int. J. Mol. Sci. 2016, 17, 1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hussey, P.J. Interactions between plant endomembrane systems and the actin cytoskeleton. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Gamez, G.; Menke, C.; Hernandez, A.; Thorburn, J.; Gidan, F.; Staskiewicz, L.; Morgan, S.; Cummings, C.; Maycotte, P.; et al. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy 2014, 10, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, N.; Singh, R. ATGs: Scaffolds for MAPK/ERK signaling. Autophagy 2014, 10, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, E.; di Sansebastiano, G.P.; Neuhaus, J.M. Contribution of chitinase A’s C-terminal vacuolar sorting determinant to the study of soluble protein compartmentation. Int. J. Mol. Sci. 2014, 15, 11030–11039. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.U.; Rojo, E.; Kovaleva, V.; Venkataraman, S.; Dombrowski, J.E.; Matsuoka, K.; Raikhel, N.V. The plant vacuolar sorting receptor AtELP is involved in transport of NH2-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 2000, 149, 1335–1344. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.P.; Renna, L.; Gigante, M.; Caroli, M.; Piro, G.; Dalessandro, G. Green fluorescent protein reveals variability in vacuoles of three plant species. Biol. Plant. 2007, 51, 49–55. [Google Scholar] [CrossRef]
- Di Sansebastiano, G.P.; Ul Rehman, R.; Neuhaus, J.M. Rat β-glucuronidase as a reporter protein for the analysis of the plant secretory pathway. Plant Biosyst. 2007, 141, 329–336. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.D.; Cao, L.; Zhang, Q.; Hu, X.Y.; Zhang, Y. Effect of PI3K-mediated autophagy in human osteosarcoma MG63 cells on sensitivity to chemotherapy with cisplatin. Asian Pac. J. Trop. Med. 2015, 8, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Friedhuber, A.M.; Chandolu, V.; Manchun, S.; Donkor, O.; Sriamornsak, P.; Dass, C.R. Nucleotropic doxorubicin nanoparticles decrease cancer cell viability, destroy mitochondria, induce autophagy and enhance tumour necrosis. J. Pharm. Pharmacol. 2015, 67, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.C. A rapid method for the synthesis of cis-[Pt(NH3)2Cl2]. Indian J. Chem. 1970, 8, 193–194. [Google Scholar]
- Kidani, Y.; Inagaki, K.; Iigo, M.; Hoshi, A.; Kuretani, K. Antitumor activity of 1,2-diaminocyclohexaneplatinum complexes against Sarcoma-180 ascites form. J. Med. Chem. 1978, 21, 1315–1318. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadia, P.; Barozzi, F.; Hoeschele, J.D.; Piro, G.; Margiotta, N.; Di Sansebastiano, G.-P. Cisplatin, Oxaliplatin, and Kiteplatin Subcellular Effects Compared in a Plant Model. Int. J. Mol. Sci. 2017, 18, 306. https://doi.org/10.3390/ijms18020306
Papadia P, Barozzi F, Hoeschele JD, Piro G, Margiotta N, Di Sansebastiano G-P. Cisplatin, Oxaliplatin, and Kiteplatin Subcellular Effects Compared in a Plant Model. International Journal of Molecular Sciences. 2017; 18(2):306. https://doi.org/10.3390/ijms18020306
Chicago/Turabian StylePapadia, Paride, Fabrizio Barozzi, James D. Hoeschele, Gabriella Piro, Nicola Margiotta, and Gian-Pietro Di Sansebastiano. 2017. "Cisplatin, Oxaliplatin, and Kiteplatin Subcellular Effects Compared in a Plant Model" International Journal of Molecular Sciences 18, no. 2: 306. https://doi.org/10.3390/ijms18020306
APA StylePapadia, P., Barozzi, F., Hoeschele, J. D., Piro, G., Margiotta, N., & Di Sansebastiano, G.-P. (2017). Cisplatin, Oxaliplatin, and Kiteplatin Subcellular Effects Compared in a Plant Model. International Journal of Molecular Sciences, 18(2), 306. https://doi.org/10.3390/ijms18020306







