Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p38MAPK and MEK1/2/ERK1/2 Signalling Pathways
Abstract
1. Introduction
2. Results
2.1. In Vivo and In Vitro Regulation of PRL Transcript by Leptin
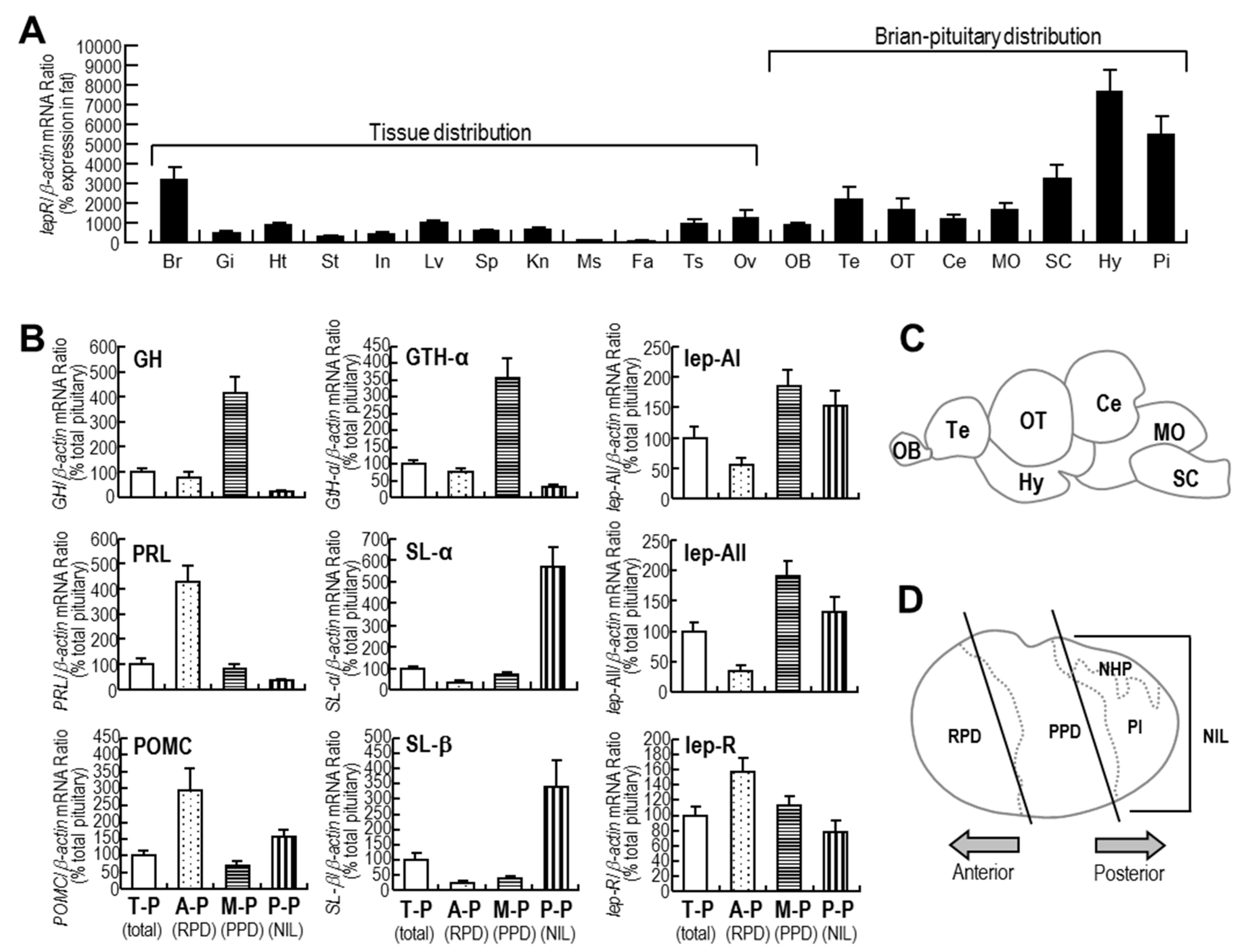
2.2. Tissue Expression Profiles of lepR and Expression Profiles of Major Hormones in Different Parts of the Pituitary
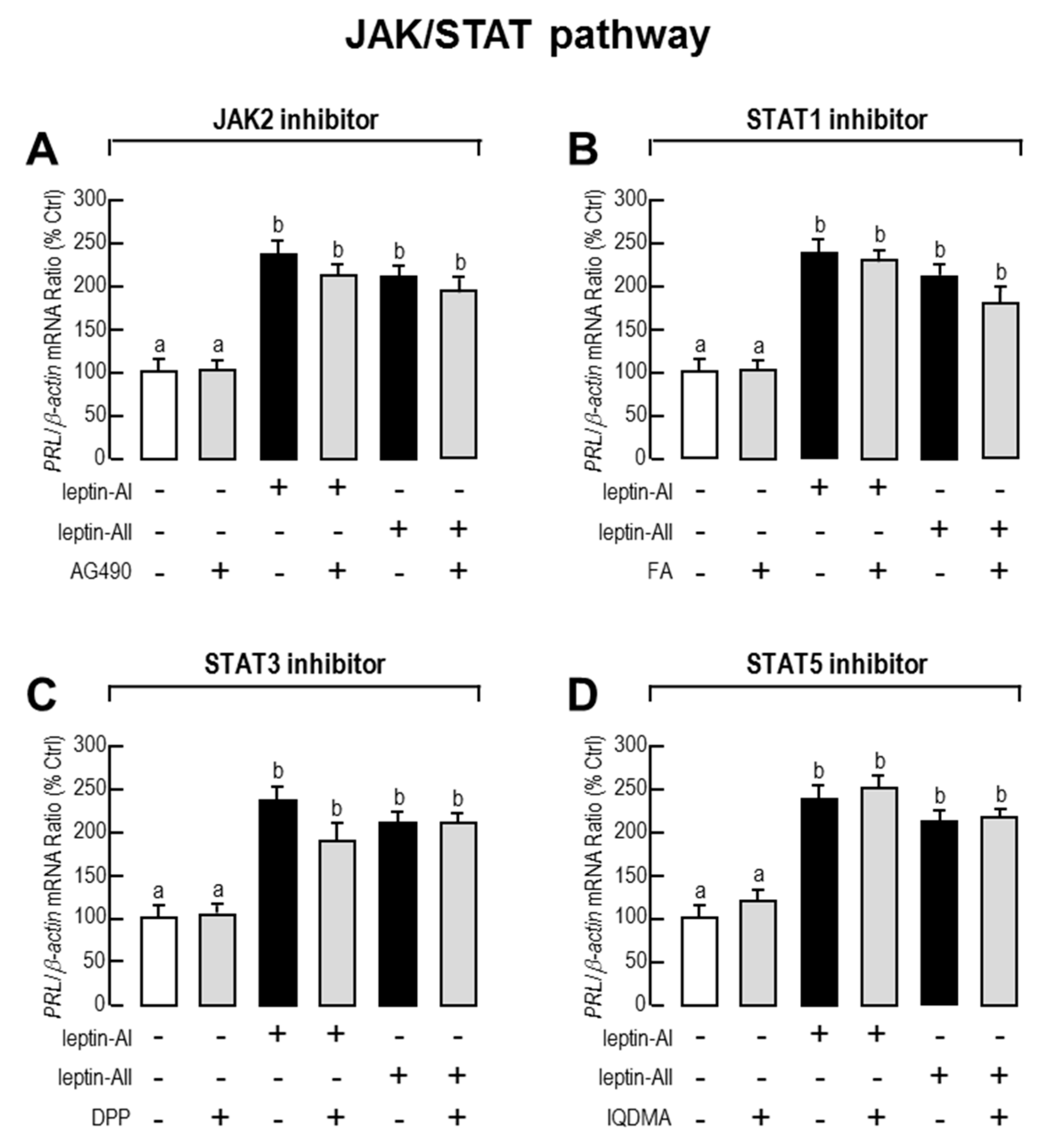
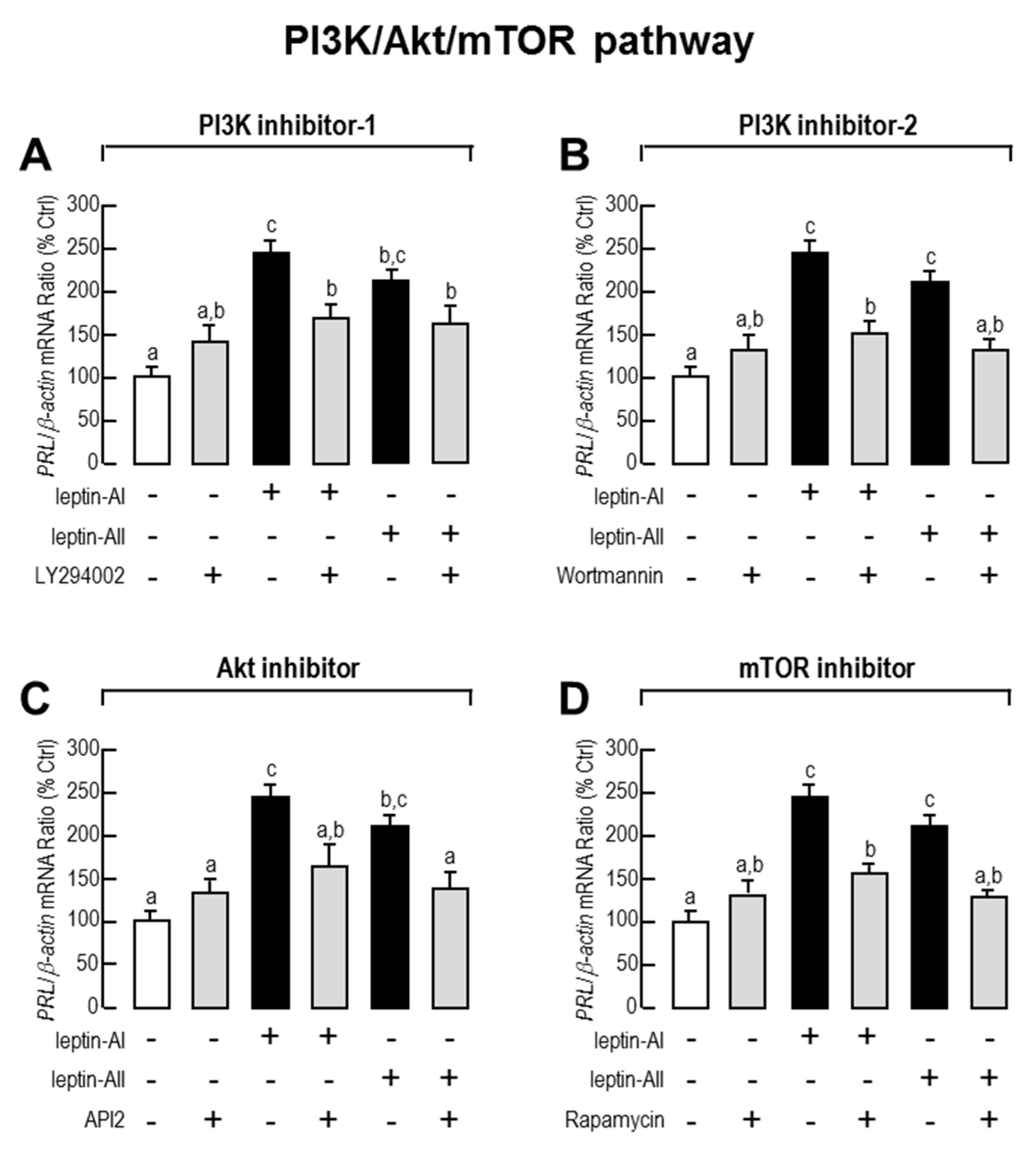
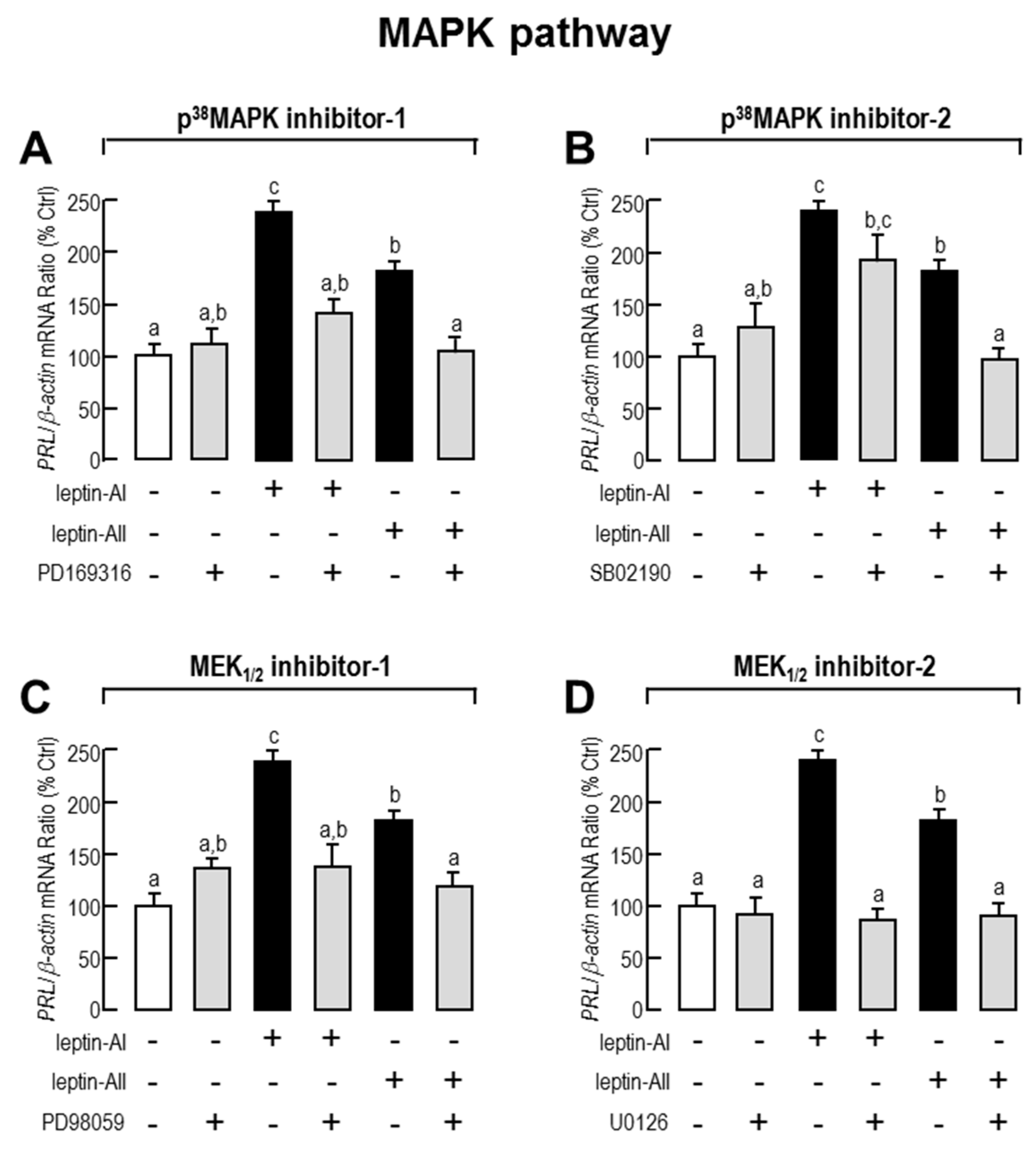
2.3. Signal Pathways Involved in Pituitary PRL mRNA Expression Regulated by Leptin
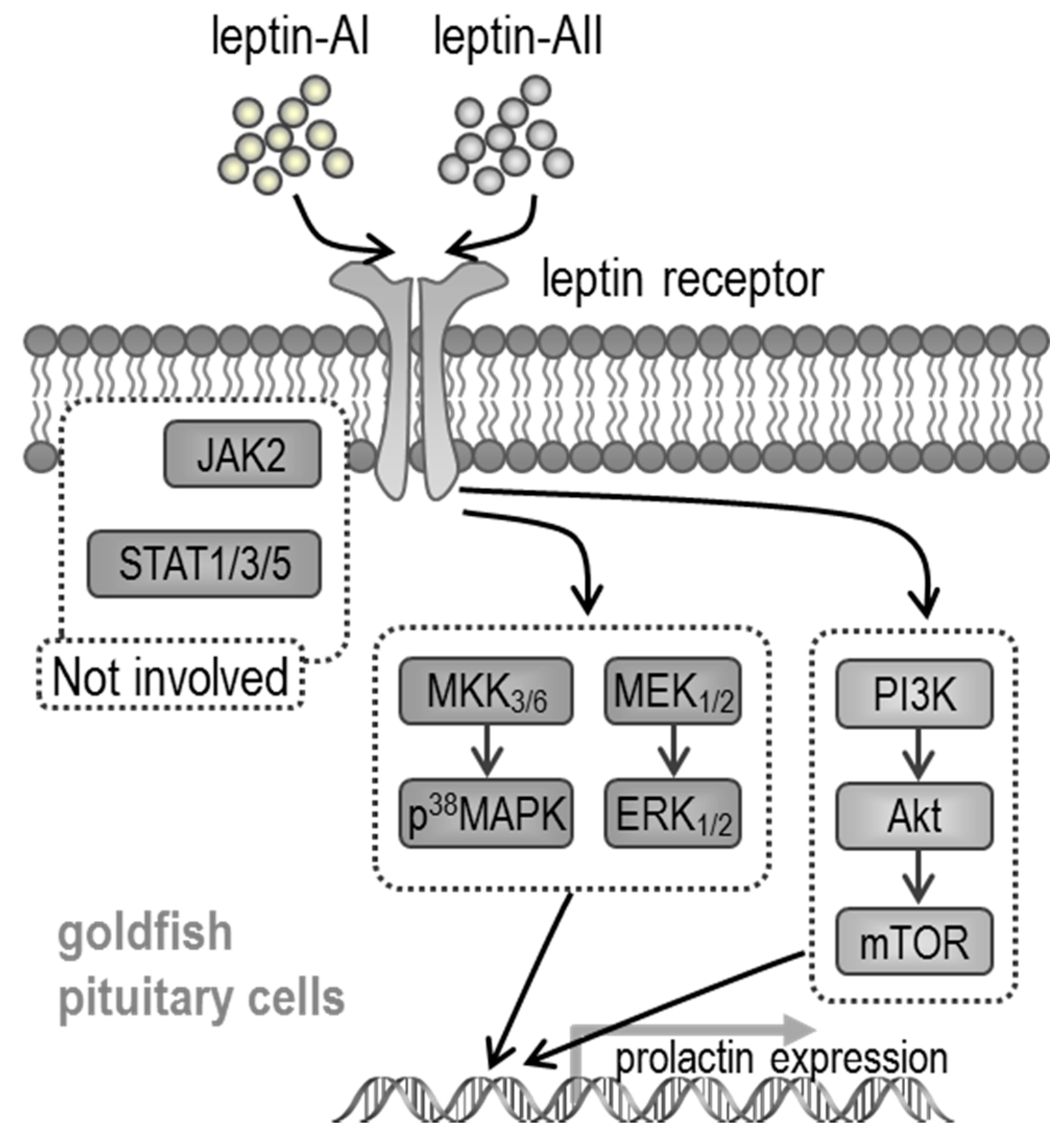
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Test Substances
4.3. Tissue Distribution of lepR mRNA and Expression Profiles of lepR and Major Hormones in Different Regions of the Pituitary
4.4. Intraperitoneal Injection and In Vivo Sample Collection
4.5 Isolation, Primary Culture, and Static Incubation of Goldfish Pituitary Cells
4.6 Measurement of Transcriptional Expression of Target Genes by Quantitative PCR
4.7. Data Transformation and Statistical Analysis
Reference
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homolog. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Campfield, L.A.; Smith, F.J.; Guisez, Y.; Devos, R.; Burn, P. Recombinant Mouse Ob Protein—Evidence for a Peripheral Signal Linking Adiposity and Central Neural Networks. Science 1995, 269, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-Reducing Effects of the Plasma-Protein Encoded by the Obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the Obese Gene-Product on Body-Weight Regulation in Ob/Ob Mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Cravo, R.M.; Frazao, R.; Elias, C.F. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology 2011, 93, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gogga, P.; Karbowska, J.; Meissner, W.; Kochan, Z. [Role of leptin in the regulation of lipid and carbohydrate metabolism]. Postepy Hig. Med. Dosw. 2011, 65, 255–262. [Google Scholar]
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 2012, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Jin, L.; Tsumanuma, I.; Vidal, S.; Kovacs, K.; Horvath, E.; Scheithauer, B.W.; Couce, M.E.; Burguera, B. Leptin and leptin receptor in anterior pituitary function. Pituitary 2001, 4, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Huising, M.O.; Geven, E.J.W.; Kruiswijk, C.P.; Nabuurs, S.B.; Stolte, E.H.; Spanings, F.A.T.; Verburg-Van Kemenade, B.M.L.; Flik, G. Increased leptin expression in common Carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology 2006, 147, 5786–5797. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Murashita, K. Genomic characterization of multiple leptin genes and a leptin receptor gene in the Japanese medaka, Oryzias latipes. Gen. Comp. Endocrinol. 2009, 161, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Bernier, N.J.; Nabuurs, S.B.; Flik, G.; Huising, M.O. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J. Endocrinol. 2009, 201, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ronnestad, I.; Nilsen, T.O.; Murashita, K.; Angotzi, A.R.; Moen, A.G.G.; Stefansson, S.O.; Kling, P.; Bjornsson, B.T.; Kurokawa, T. Leptin and leptin receptor genes in Atlantic salmon: Cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen. Comp. Endocrinol. 2010, 168, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Baltzegar, D.A.; Reading, B.J.; Douros, J.D.; Borski, R.J. Role for leptin in promoting glucose mobilization during acute hyperosmotic stress in teleost fishes. J. Endocrinol. 2014, 220, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Shpilman, M.; Hollander-Cohen, L.; Ventura, T.; Gertler, A.; Levavi-Sivan, B. Production, gene structure and characterization of two orthologs of leptin and a leptin receptor in tilapia. Gen. Comp. Endocrinol. 2014, 207, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, S.; Ren, C.; Hu, C.; Tang, D.; Yan, A. Two isoforms of leptin in the White-clouds Mountain minnow (Tanichthys albonubes): Differential regulation by estrogen despite similar response to fasting. Gen. Comp. Endocrinol. 2016, 225, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Flik, G. Leptin in teleostean fish, towards the origins of leptin physiology. J. Chem. Neuroanat. 2014, 61–62, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Copeland, D.L.; Duff, R.J.; Liu, Q.; Prokop, J.; Londraville, R.L. Leptin in teleost fishes: An argument for comparative study. Front. Physiol. 2011, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Vaisse, C.; Halaas, J.L.; Horvath, C.M.; Darnell, J.E., Jr.; Stoffel, M.; Friedman, J.M. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996, 14, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Pecori Giraldi, F.; Piccoletti, R. Leptin activates Stat3, Stat1 and AP-1 in mouse adipose tissue. Mol. Cell. Endocrinol. 2000, 168, 11–20. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.P.; Yin, P.H.; Hsu, Y.C.; Chang, Y.C.; Huang, S.Y.; Lee, J.J.; Chi, C.W. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol. Rep. 2011, 26, 1265–1271. [Google Scholar] [PubMed]
- Londraville, R.L.; Prokop, J.W.; Duff, R.J.; Liu, Q.; Tuttle, M. On the Molecular Evolution of Leptin, Leptin Receptor, and Endospanin. Front. Endocrinol. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Crown, A.; Clifton, D.K.; Steiner, R.A. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 2007, 86, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, S.; Burguera, B.G.; Couce, M.E.; Osamura, R.Y.; Kulig, E.; Lloyd, R.V. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 2000, 141, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Nagata, H.; Takekoshi, S.; Osamura, R.Y. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell. Tissue Res. 2001, 305, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Burguera, B.G.; Couce, M.E.; Scheithauer, B.W.; Lamsan, J.; Eberhardt, N.L.; Kulig, E.; Lloyd, R.V. Leptin and leptin receptor expression in normal and neoplastic human pituitary: Evidence of a regulatory role for leptin on pituitary cell proliferation. J. Clin. Endocrinol. Metab. 1999, 84, 2903–2911. [Google Scholar] [PubMed]
- Sone, M.; Osamura, R.Y. Leptin and the pituitary. Pituitary 2001, 4, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Douros, J.D.; Baltzegar, D.A.; Mankiewicz, J.; Taylor, J.; Yamaguchi, Y.; Lerner, D.T.; Seale, A.P.; Grau, E.G.; Breves, J.P.; Borski, R.J. Control of leptin by metabolic state and its regulatory interactions with pituitary growth hormone and hepatic growth hormone receptors and insulin like growth factors in the tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2017, 240, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Peyon, P.; Zanuy, S.; Carrillo, M. Action of leptin on in vitro luteinizing hormone release in the European sea bass (Dicentrarchus labrax). Biol. Reprod. 2001, 65, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Peyon, P.; de Celis, S.V.R.; Gomez-Requeni, P.; Zanuy, S.; Perez-Sanchez, J.; Carrillo, M. In vitro effect of leptin on somatolactin release in the European sea bass (Dicentrarchus labrax): Dependence on the reproductive status and interaction with NPY and GnRH. Gen. Comp. Endocrinol. 2003, 132, 284–292. [Google Scholar] [CrossRef]
- Weil, C.; Le Bail, P.Y.; Sabin, N.; Le Gac, F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 2003, 130, 2–12. [Google Scholar] [CrossRef]
- Chowdhury, I.; Chien, J.T.; Chatterjee, A.; Yu, J.Y.L. In vitro effects of mammalian leptin, neuropeptide-Y, beta-endorphin and galanin on transcript levels of thyrotropin beta and common alpha subunit mRNAs in the pituitary of bighead carp (aristichthys nobilis). Comp. Biochem. Phys. B 2004, 139, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Bernichtein, S.; Touraine, P.; Goffin, V. New concepts in prolactin biology. J. Endocrinol. 2010, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Kimura, M.; Walczewska, A.; Karanth, S.; McCann, S.M. Role of leptin in hypothalamic-pituitary function. Proc. Natl. Acad. Sci. USA 1997, 94, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, P.A.; Munno, A.; Gamberoni, M.; Viggiani, R.; De Ambrogi, M.; Tamanini, C.; Seren, E. Role of leptin on growth hormone and prolactin secretion by bovine pituitary explants. J. Dairy Sci. 2007, 90, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Hashizume, T.; Yamashita, T. Effects of leptin and leptin peptide amide on the release of luteinizing hormone, growth hormone and prolactin from cultured porcine anterior pituitary cells. Anim. Sci. J. 2006, 77, 47–52. [Google Scholar] [CrossRef]
- Douros, J.D.; Baltzegar, D.A.; Breves, J.P.; Lerner, D.T.; Seale, A.P.; Gordon Grau, E.; Borski, R.J. Prolactin is a major inhibitor of hepatic Leptin A synthesis and secretion: Studies utilizing a homologous Leptin A ELISA in the tilapia. Gen. Comp. Endocrinol. 2014, 207, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Strom, C.N.; Bailey, S.T.; Borski, R.J. Leptin stimulates pituitary prolactin release through an extracellular signal-regulated kinase-dependent pathway. J. Endocrinol. 2008, 196, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Canosa, L.F.; Chang, J.P.; Peter, R.E. Neuroendocrine control of growth hormone in fish. Gen. Comp. Endocrinol. 2007, 151, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.F.; Chen, T.; Chen, S.; Ren, C.H.; Hu, C.Q.; Cai, Y.M.; Liu, F.; Tang, D.S. Goldfish Leptin-AI and Leptin-AII: Function and Central Mechanism in Feeding Control. Int. J. Mol. Sci. 2016, 17, 783. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, A.B.; Nisembaum, L.G.; Isorna, E.; Delgado, M.J.; de Pedro, N. Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides 2012, 34, 329–335. [Google Scholar] [CrossRef] [PubMed]
- de Pedro, N.; Martinez-Alvarez, R.; Delgado, M.J. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J. Endocrinol. 2006, 188, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Vivas, Y.; Azpeleta, C.; Feliciano, A.; Velarde, E.; Isorna, E.; Delgado, M.J.; De Pedro, N. Time-dependent effects of leptin on food intake and locomotor activity in goldfish. Peptides 2011, 32, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Eykelbosh, A.J.; Peter, R.E. Role of leptin in the control of feeding of goldfish Carassius auratus: Interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003, 972, 90–109. [Google Scholar] [CrossRef]
- Yan, A.F.; Chen, T.; Chen, S.; Tang, D.S.; Liu, F.; Jiang, X.; Huang, W.; Ren, C.H.; Hu, C.Q. Signal transduction mechanism for glucagon-induced leptin gene expression in goldfish liver. Int. J. Biol. Sci. 2016, 12, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.P.; Yamaguchi, Y.; Johnstone, W.M., 3rd; Borski, R.J.; Lerner, D.T.; Grau, E.G. Endocrine regulation of prolactin cell function and modulation of osmoreception in the Mozambique tilapia. Gen. Comp. Endocrinol. 2013, 192, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Pickford, G.E.; Phillips, J.G. Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water. Science 1959, 130, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.Q.; Lou, Q.Y.; Dai, Z.R.; Dai, X.Y.; He, J.Y.; Hu, W.; Yin, Z. The basal function of teleost prolactin as a key regulator on ion uptake identified with zebrafish knockout models. Sci. Rep. 2016, 6, 18597. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.; Bernier, N.J.; Manuel, R.; de Gelder, S.; Metz, J.R.; Huising, M.O.; Flik, G. Recombinant human leptin attenuates stress axis activity in common carp (Cyprinus carpio L.). Gen. Comp. Endocrinol. 2012, 178, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [PubMed]
- Lin, C.Y.; Jiang, X.; Hu, G.F.; Ko, W.K.W.; Wong, A.O.L. Grass carp prolactin: Molecular cloning, tissue expression, intrapituitary autoregulation by prolactin and paracrine regulation by growth hormone and luteinizing hormone. Mol. Cell. Endocrinol. 2015, 399, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Nejigaki, Y.; Satoh, M.; Shimaura, C.; Tanaka, M.; Kawamoto, K.; Uchiyama, M.; Kawauchi, H.; Shioda, S.; Takahashi, A. Effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on prolactin and somatolactin release from the goldfish pituitary in vitro. Regul. Pept. 2008, 145, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Copeland, D.; Ball, H.; Duff, R.J.; Rockich, B.; Londraville, R.L. Expression of leptin receptor gene in developing and adult zebrafish. Gen. Comp. Endocrinol. 2010, 166, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Trombley, S.; Maugars, G.; Kling, P.; Bjornsson, B.T.; Schmitz, M. Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). Gen. Comp. Endocrinol. 2012, 175, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.P.; Bjornsson, B.T. Leptin Signaling in the Rainbow Trout Central Nervous System Is Modulated by a Truncated Leptin Receptor Isoform. Endocrinology 2014, 155, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Pasquier, J.; Dirks, R.; van den Thillart, G.; Tomkiewicz, J.; Rousseau, K.; Dufour, S.; Lafont, A.G. Duplicated Leptin Receptors in Two Species of Eel Bring New Insights into the Evolution of the Leptin System in Vertebrates. PLoS ONE 2015, 10, e0126008. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Murashita, K.; Suzuki, T.; Uji, S. Genomic characterization and tissue distribution of leptin receptor and leptin receptor overlapping transcript genes in the pufferfish, Takifugu rubripes. Gen. Comp. Endocrinol. 2008, 158, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.P.; Jonsson, E.; Bjornsson, B.T. Acute anorexigenic action of leptin in rainbow trout is mediated by the hypothalamic Pi3k pathway. J. Mol. Endocrinol. 2016, 56, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Mariano, G.; Stilo, R.; Terrazzano, G.; Coccia, E.; Vito, P.; Varricchio, E.; Paolucci, M. Effects of recombinant trout leptin in superoxide production and NF-kappaB/MAPK phosphorylation in blood leukocytes. Peptides 2013, 48, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Tan, X.Y.; Wei, C.C.; You, W.J.; Zhuo, M.Q.; Song, Y.F. Isolation and Expression Analysis of STAT Members from Synechogobius hasta and Their Roles in Leptin Affecting Lipid Metabolism. Int. J. Mol. Sci. 2016, 17, 406. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K. Citation-Classic—Physiological Salines for Fresh-Water Teleosts. Prog. Fish-Cult. 1963, 25, 135–140. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Q.; Chan, T.; Ko, W.K.; Wong, A.O. Goldfish kisspeptin: Molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. Gen. Comp. Endocrinol. 2010, 165, 60–71. [Google Scholar] [CrossRef] [PubMed]

| Gene Target/Accession No.(Primer Sequences, 5’–3’) | PCR Condition | Cycle | ||
|---|---|---|---|---|
| Denaturing | Annealing | Extension | ||
| PRL/ S82197 | ||||
| CACTCTCTCAGCACCTCTCTC | 94 °C | 58 °C | 72 °C | × 35 |
| CTCTTTGGTCTTGCTGTCAATG | 30 s | 30 s | 30 s | |
| lepR/ EU911005 | ||||
| TCATCAACCCAAACGACG | 94 °C | 56 °C | 72 °C | × 35 |
| GTGAACTCCTCTGAGCCATA | 30 s | 30 s | 30 s | |
| leptin-AI / FJ534535 | ||||
| TCCAAAGCTCCTCATAGG | 94 °C | 52 °C | 72 °C | × 35 |
| TGGTGGGTGGCGTTTTCC | 30 s | 30 s | 30 s | |
| leptin-AII / FJ854572 | ||||
| TATCGTGGACACCCTAACTAC | 94 °C | 52 °C | 72 °C | × 35 |
| GGTCTAAAGCCAAGAACCCTAA | 30 s | 30 s | 30 s | |
| GH/ EU157192 | ||||
| TTAACGACTTTGAGGACAGCCT | 94 °C | 58 °C | 72 °C | × 35 |
| CAGCTTCTCAGTGATCTGGTTG | 30 s | 30 s | 30 s | |
| GTH-α/ AY800267 | ||||
| GCTCCTGTCTATCAGTGTATG | 94 °C | 58 °C | 72 °C | × 35 |
| GCACCCGTTTAACTTCTTT | 30 s | 30 s | 30 s | |
| SL-α/ EU580712 | ||||
| ATATGTTTGTCCCGTACCCTCT | 94 °C | 56 °C | 72 °C | × 35 |
| TTTATCAGACACCCACTTGGTC | 30 s | 30 s | 30 s | |
| SL-β/ CAU72940 | ||||
| AGGGACCATGTGTTCTCCTAAA | 94 °C | 56 °C | 72 °C | × 35 |
| AGAACCAGTATACCCTGCTCCA | 30 s | 30 s | 30 s | |
| POMC/ AJ431209 | ||||
| AAGCGCTCCTACTCCATGGA | 94 °C | 60 °C | 72 °C | × 35 |
| CTCGTCCCAGGACTTCATGAA | 30 s | 30 s | 30 s | |
| β-actin/ AB039726 | ||||
| CTGGTATCGTGATGGACTCT | 94 °C | 56 °C | 72 °C | × 35 |
| AGCTCATAGCTCTTCTCCAG | 30 s | 30 s | 30 s | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.; Chen, Y.; Chen, S.; Li, S.; Zhang, Y.; Jia, J.; Yu, H.; Liu, L.; Liu, F.; Hu, C.; et al. Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p38MAPK and MEK1/2/ERK1/2 Signalling Pathways. Int. J. Mol. Sci. 2017, 18, 2781. https://doi.org/10.3390/ijms18122781
Yan A, Chen Y, Chen S, Li S, Zhang Y, Jia J, Yu H, Liu L, Liu F, Hu C, et al. Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p38MAPK and MEK1/2/ERK1/2 Signalling Pathways. International Journal of Molecular Sciences. 2017; 18(12):2781. https://doi.org/10.3390/ijms18122781
Chicago/Turabian StyleYan, Aifen, Yanfeng Chen, Shuang Chen, Shuisheng Li, Yong Zhang, Jirong Jia, Hui Yu, Lian Liu, Fang Liu, Chaoqun Hu, and et al. 2017. "Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p38MAPK and MEK1/2/ERK1/2 Signalling Pathways" International Journal of Molecular Sciences 18, no. 12: 2781. https://doi.org/10.3390/ijms18122781
APA StyleYan, A., Chen, Y., Chen, S., Li, S., Zhang, Y., Jia, J., Yu, H., Liu, L., Liu, F., Hu, C., Tang, D., & Chen, T. (2017). Leptin Stimulates Prolactin mRNA Expression in the Goldfish Pituitary through a Combination of the PI3K/Akt/mTOR, MKK3/6/p38MAPK and MEK1/2/ERK1/2 Signalling Pathways. International Journal of Molecular Sciences, 18(12), 2781. https://doi.org/10.3390/ijms18122781






