Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon
Abstract
:1. Introduction
2. Results and Interpretations
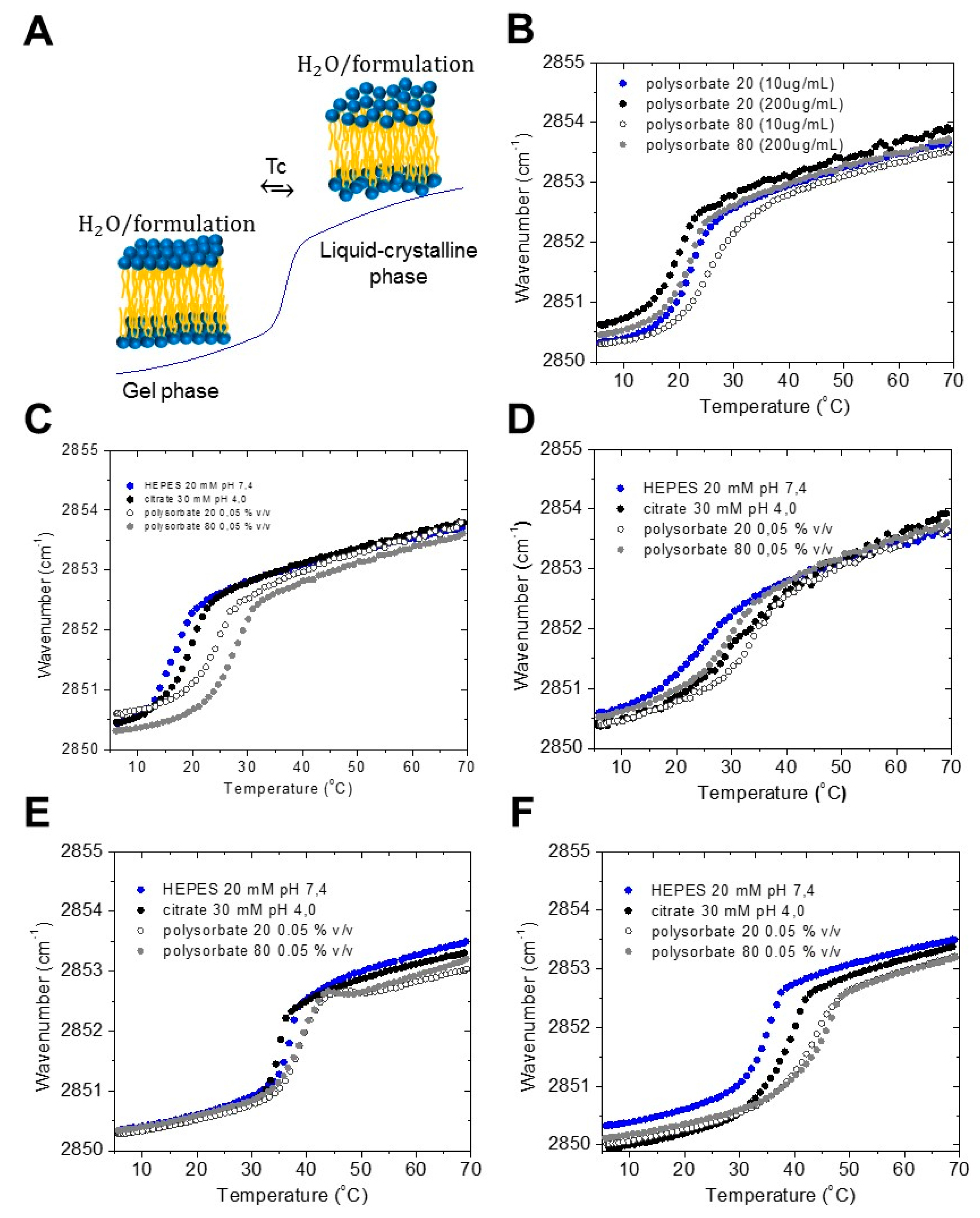
2.1. Gel to Liquid Crystalline Phase Transition of the Acyl Chains
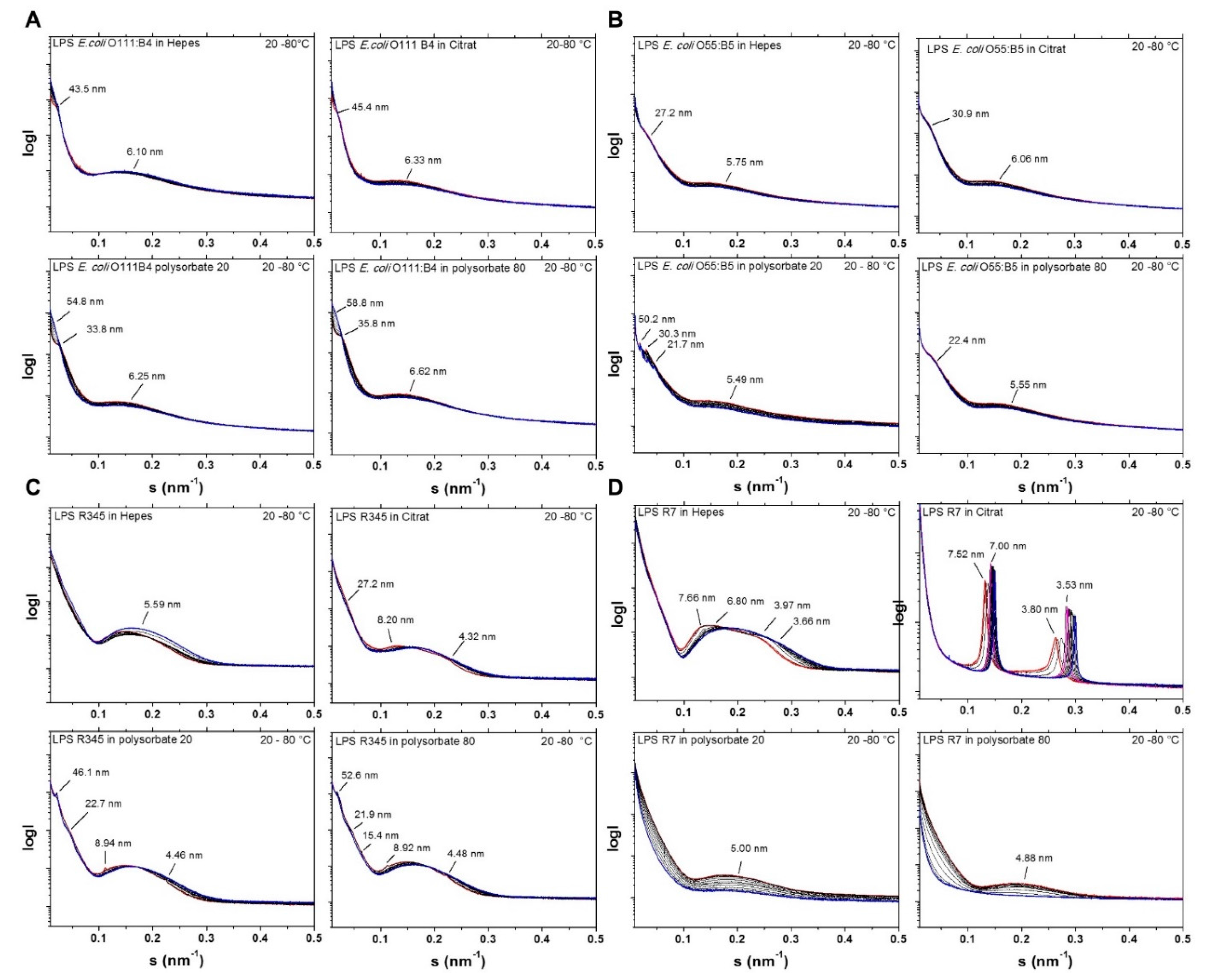
2.2. Aggregate Structures of LPS Preparations Used in This Study
2.3. Thermodynamics of Binding of the Synthetic Anti-LPS Peptide (SALP) Pep19-2.5 with the Different LPS Preparations
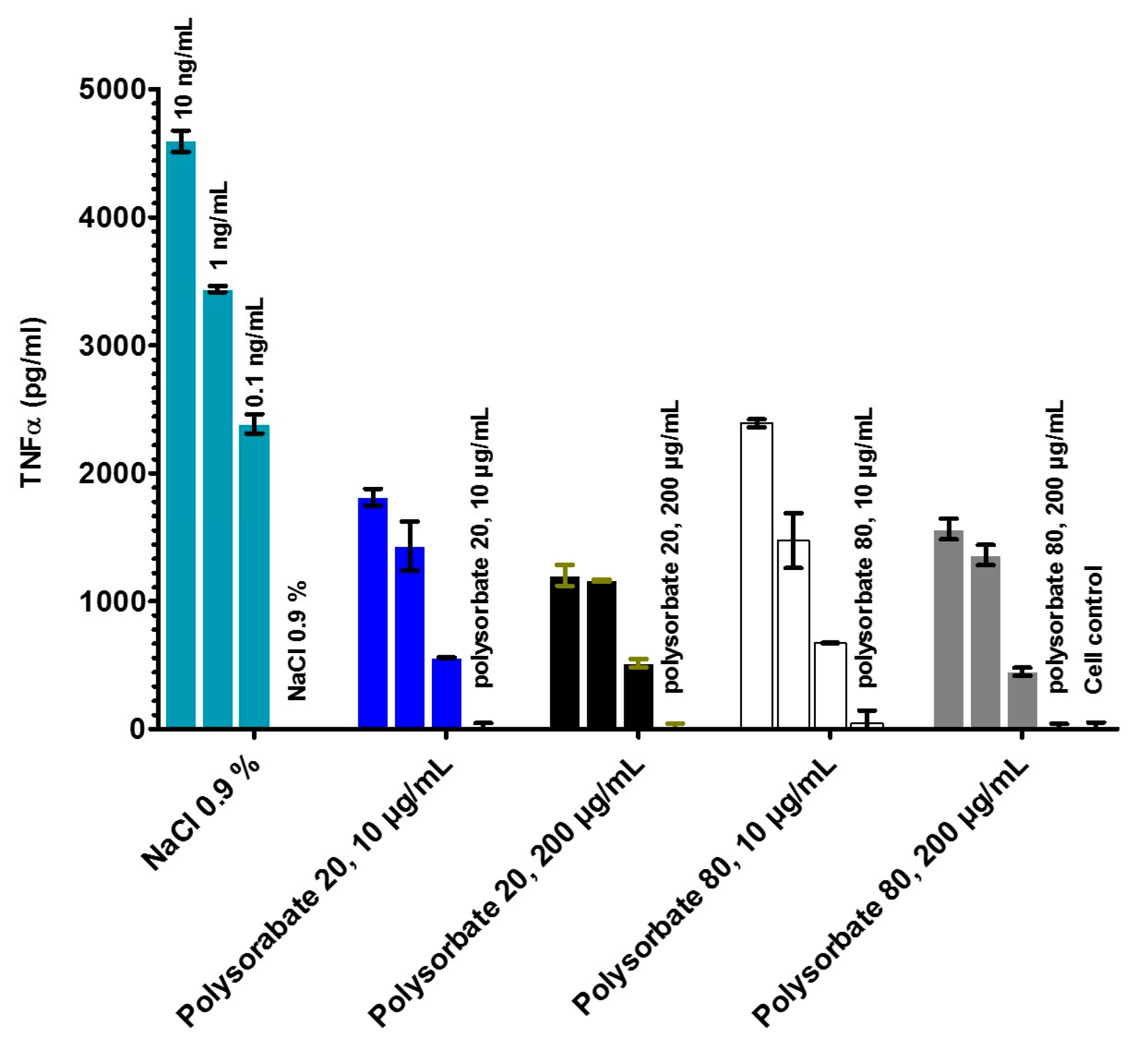
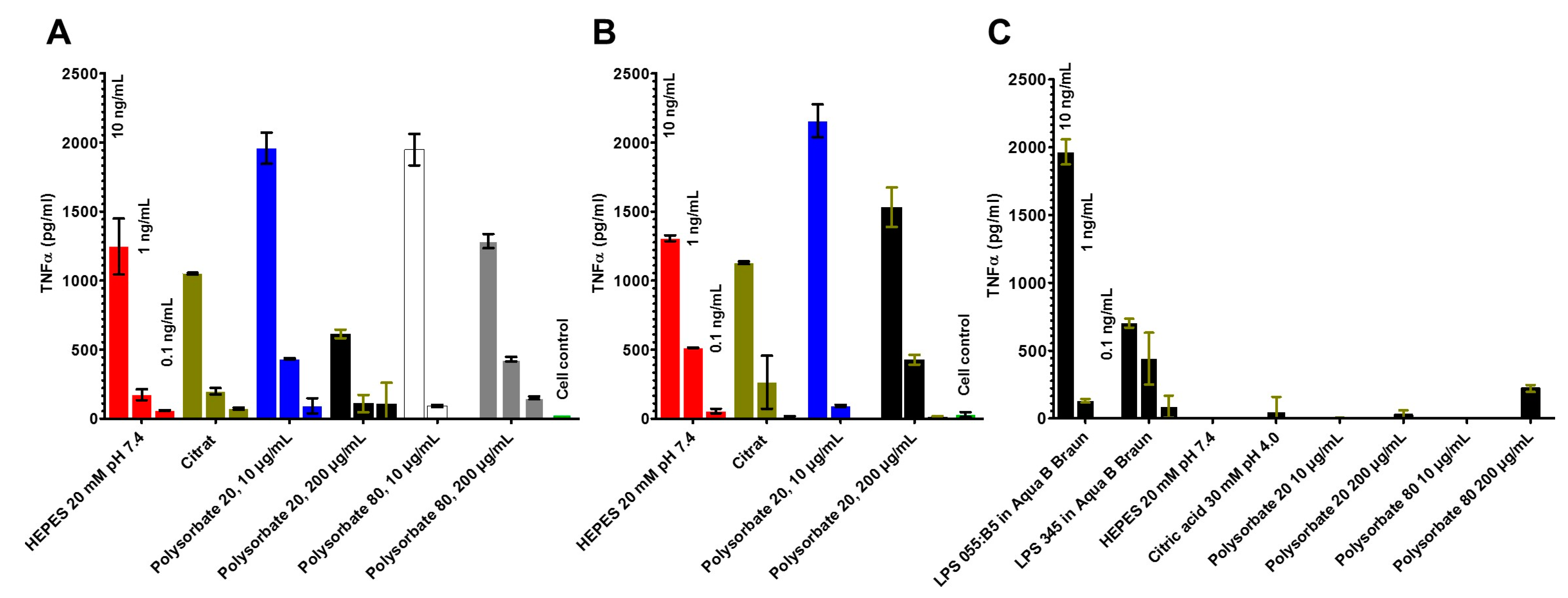
2.4. Stimulation of Immune Cells by the LPS in the Different Formulations (MAT)
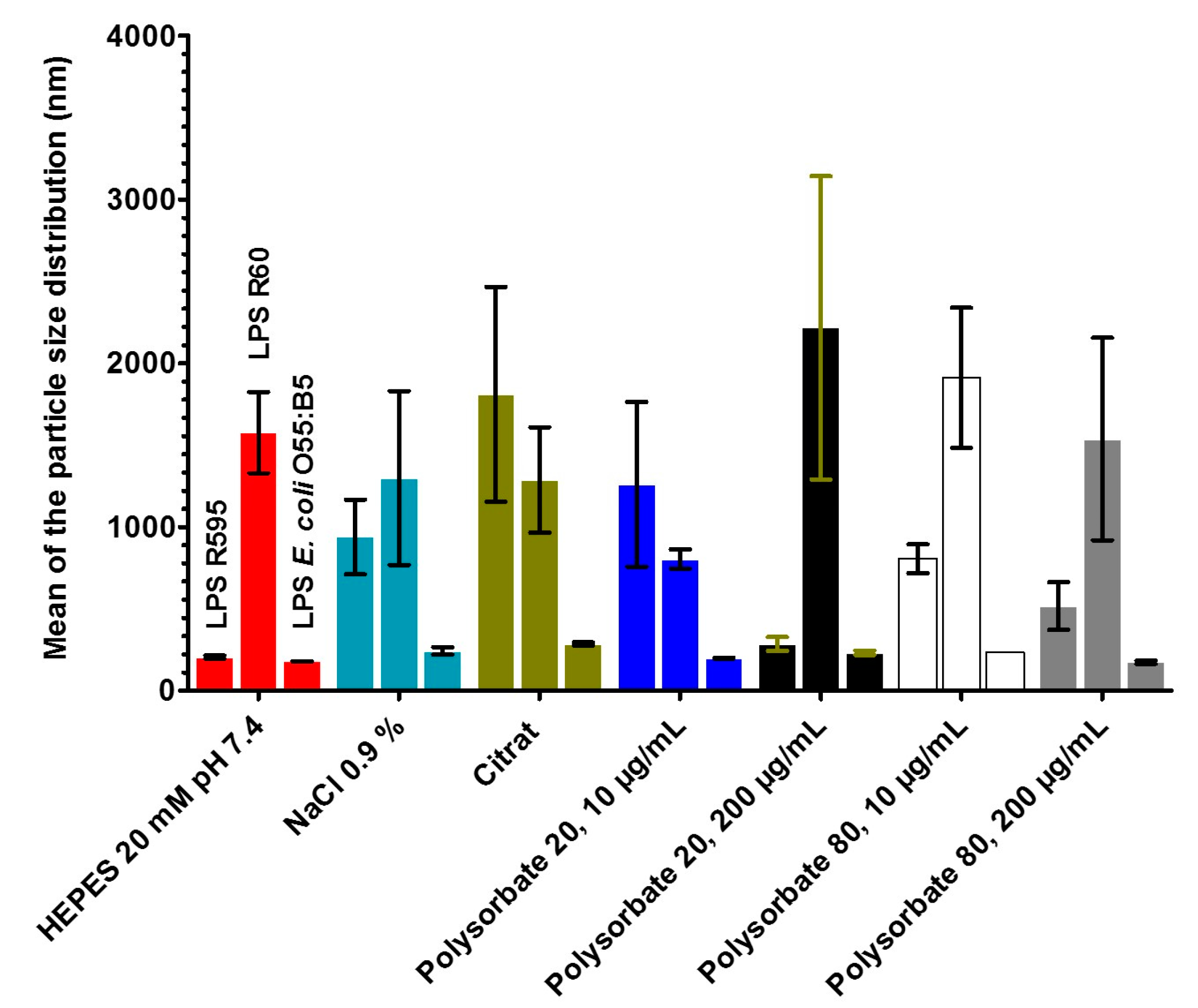
2.5. Measurements of the Size Distribution of LPS Aggregates by Zeta Sizer
2.5.1. Results for Deep Rough Mutant LPS R595
2.5.2. Results for Rough Mutant LPS Ra with Complete Core Oligosaccharide
2.6. Size Distribution in Relation to Cytokine Induction in Human Mononuclear Cells
3. Discussion
- Fluidity of their hydrocarbon chains;
- Aggregate size and structure;
- Head group conformation and orientation.
- (i)
- (i) gel to liquid crystalline phase transition of the hydrocarbon chains of LPS, and with that, the fluidity of the acyl chains, with Fourier-transform infrared spectroscopy (FTIR);
- (ii)
- three-dimensional aggregate structure of LPS by using synchrotron radiation small-angle X-ray scattering (SAXS);
- (iii)
- LPS aggregate sizes by dynamic light scattering and have related these data to the biological activities in the MAT;
- (iv)
- Furthermore, the interaction of LPS with a synthetic anti-LPS peptide Pep19-2.5 was monitored to find out whether differences in head group binding are observed.
4. Materials and Methods
4.1. Peptides, Reagents and LPS Formulations
4.2. Acyl Chain Melting Behavior by Fourier-Transform Infrared Spectroscopy
4.3. Aggregate Structure Determined by Small-Angle X-ray Scattering (SAXS)
4.4. Binding Affinity of LPS to Pep19-2.5 via Isothermal Titration Calorimetry (ITC)
4.5. Particle Size Measurements by Dynamic Light Scattering on a Zeta Sizer
4.6. Stimulation of Human Mononuclear Cells
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; di Padova, F. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [PubMed]
- Opal, S.M.; Yu, R.L. Antiendotoxin Strategies for the Prevention and Treatment of Septic Shock. Drugs 1998, 55, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Bolden, J.S.; Warburton, R.E.; Phelan, R.; Murphy, M.; Smith, K.R.; de Felippis, M.R.; Chen, D. Endotoxin recovery using limulus amebocyte lysate (LAL) assay. Biologicals 2016, 44, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Lindner, B.; Kusumoto, S.; Fukase, K.; Schromm, A.B.; Seydel, U. Aggregates are the biologically active units of endotoxin. J. Biol. Chem. 2004, 279, 26307–26313. [Google Scholar] [CrossRef] [PubMed]
- Gioannini, T.L.; Teghanemt, A.; Zhang, D.; Levis, E.N.; Weiss, J.P. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J. Endotoxin Res. 2005, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.S.; Ho, B.; Ding, J.L. High-affinity LPS binding domain(s) in recombinant factor C of a horseshoe crab neutralizes LPS-induced lethality. FASEB J. 2000, 14, 859–870. [Google Scholar] [PubMed]
- Brandenburg, K.; Howe, J.; Gutsmann, T.; Garidel, P. The Expression of Endotoxic Activity in the Limulus Test as Compared to Cytokine Production in Immune Cells. Curr. Med. Chem. 2009, 16, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.; Garidel, P.; Roessle, M.; Richter, W.; Alexander, C.; Fournier, K.; Mach, J.P.; Waelli, T.; Gorczynski, R.M.; Ulmer, A.J.; et al. Structural investigations into the interaction of hemoglobin and part structures with bacterial endotoxins. Innate Immun. 2008, 14, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Tejada, G.; Heinbockel, L.; Ferrer-Espada, R.; Heine, H.; Alexander, C.; Barcena-Varela, S.; Goldmann, T.; Correa, W.; Wiesmuller, K.H.; Gisch, N.; et al. Lipoproteins/peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci. Rep. 2015, 5, 14292. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann, T.; Razquin-Olazaran, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Hornef, M.; Schurholz, T.; Rossle, M.; Sanchez-Gomez, S.; et al. New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Kaconis, Y.; Kowalski, I.; Howe, J.; Brauser, A.; Richter, W.; Razquin-Olazaran, I.; Inigo-Pestana, M.; Garidel, P.; Rossle, M.; Martinez de Tejada, G.; et al. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys. J. 2011, 100, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Funari, S.S.; Koch, M.H.J.; Seydel, U. Investigation into the Acyl Chain Packing of Endotoxins and Phospholipids under Near Physiological Conditions by WAXS and FTIR Spectroscopy. J. Struct. Biol. 1999, 128, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Freudenberg, M.; Galanos, C. Characterization of the lipid A component of genuine smooth-form lipopolysaccharide. Eur. J. Biochem. 1989, 180, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Andrä, J.; Müller, M.; Koch, M.H.J.; Garidel, P. Physicochemical properties of bacterial glycopolymers in relation to bioactivity. Carbohydr. Res. 2003, 338, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Wiese, A. Endotoxins: Relationships between Structure, Function, and Activity. Curr. Top. Med. Chem. 2004, 4, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Mayer, H.; Koch, M.H.J.; Weckesser, J.; Rietschel, E.T.; Seydel, U. Influence of the supramolecular structure of free lipid A on its biological activity. Eur. J. Biochem. 1993, 218, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Heinbockel, L.; Correa, W.; Fukuoka, S.; Gutsmann, T.; Zähringer, U.; Koch, M.H.J. Supramolecular structure of enterobacterial wild-type lipopolysaccharides (LPS), fractions thereof, and their neutralization by Pep19-2.5. J. Struct. Biol. 2016, 194, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Heinbockel, L.; Sánchez-Gómez, S.; Martinez de Tejada, G.; Dömming, S.; Brandenburg, J.; Kaconis, Y.; Hornef, M.; Dupont, A.; Marwitz, S.; Goldmann, T.; et al. Preclinical Investigations Reveal the Broad-Spectrum Neutralizing Activity of Peptide Pep19-2.5 on Bacterial Pathogenicity Factors. Antimicrop. Agents Chemother. 2013, 57, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Komuro, T.; Murai, T.; Kawasaki, H. Effect of sonication on the dispersion state of lipopolysaccharide and its pyrogenicity in rabbits. Chem. Pharm. Bull. 1987, 35, 4946–4952. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Schromm, A.B.; Koch, M.H.; Seydel, U. Conformation and fluidity of endotoxins as determinants of biological activity. Prog. Clin. Biol. Res. 1995, 392, 1671–1682. [Google Scholar]
- Richter, W.; Vogel, V.; Howe, J.; Steiniger, F.; Brauser, A.; Koch, M.H.; Roessle, M.; Gutsmann, T.; Garidel, P.; Mäntele, W.; et al. Morphology, size distribution, and aggregate structure of lipopolysaccharide and lipid A dispersions from enterobacterial origin. Innate Immun. 2011, 17, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiva, M. Possible Mechanism of Low Endotoxin Recovery; American Pharmaceutical Review: Fishers, IN, USA, 2014; pp. 1–5. [Google Scholar]
- Schwarz, H.; Gornicec, J.; Neuper, T.; Parigiani, M.A.; Wallner, M.; Duschl, A.; Horejs-Hoeck, J. Biological Activity of Masked Endotoxin. Sci. Rep. 2017, 7, 44750. [Google Scholar] [CrossRef] [PubMed]
- Gioannini, T.L.; Weiss, J.P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol. Res. 2007, 39, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Galanos, C.; Lüderitz, O.; Westphal, O. A New Method for the Extraction of R Lipopolysaccharides. Eur. J. Biochem. 1969, 9, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Roessle, M.W.; Klaering, R.; Ristau, U.; Robrahn, B.; Jahn, D.; Gehrmann, T.; Konarev, P.; Round, A.; Fiedler, S.; Hermes, C.; et al. Upgrade of the small-angle X-ray scattering beamline X33 at the European Molecular Biology Laboratory, Hamburg. J. Appl. Cryst. 2007, 40, s190–s194. [Google Scholar] [CrossRef]
- Luzzati, V.; Vargas, R.; Mariani, P.; Gulik, A.; Delacroix, H. Cubic Phases of Lipid-containing Systems: Elements of a Theory and Biological Connotations. J. Mol. Biol. 1993, 229, 540–551. [Google Scholar] [CrossRef] [PubMed]
| Thermodyamic Parameters | LPS 055:B5 and Pep19-2.5 Dissolved in the Same Medium | LPS 055:B5 Dissolved in Polysorbates Pep19-2.5 Dissolved in Water | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LPS in HEPES | PS20 10 µg/mL | PS20 200 µg/mL | PS80 10 µg/mL | PS80 200 µg/mL | PS20 10 µg/mL | PS20 200 µg/mL | PS80 10 µg/mL | PS80 200 µg/mL | |
| Mass ratio (Peptide/LPS) | 0.25 | 0.29 | 0.38 | 0.39 | 0.34 | 0.33 | 0.32 | 0.40 | 0.37 |
| Kd (nM) | 862 | 529 | 225 | 104 | 78 | 200 | 218 | 46 | 261 |
| ΔH (kJ/mol) | −67.31 | −59.82 | −45.64 | −58.35 | −56.58 | −48.49 | −49.14 | −43.00 | −48.60 |
| ΔS (kJ/mol·K) | −0.10 | −0.07 | −0.02 | −0.05 | −0.05 | −0.03 | −0.03 | 0.01 | −0.03 |
| Formulation | Polydispersity Index (PDI) | ||
|---|---|---|---|
| LPS R595 | LPS Ra | LPS O55:B5 | |
| HEPES 20 mM pH 7.4 | 0.449 ± 0.012 | 0.983 ± 0.029 | 0.436 ± 0.004 |
| NaCl 0.9% | 0.935 ± 0.112 | 1.000 ± 0.000 | 0.524 ± 0.030 |
| Citrate 30 mM pH 4.0 | 1.000 ± 0.000 | 1.000 ± 0.000 | 0.536 ± 0.018 |
| Polysorbate 20, 10 μg/mL | 1.000 ± 0.000 | 0.934 ± 0.073 | 0.514 ± 0.028 |
| Polysorbate 20, 200 μg/mL | 0.966 ± 0.058 | 0.966 ± 0.058 | 0.506 ± 0.059 |
| Polysorbate 80, 10 μg/mL | 0.911 ± 0.083 | 1.000 ± 0.000 | 0.463 ± 0.002 |
| Polysorbate 80, 200 μg/mL | 0.814 ± 0.050 | 1.000 ± 0.000 | 0.469 ± 0.056 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, W.; Brandenburg, K.; Zähringer, U.; Ravuri, K.; Khan, T.; Von Wintzingerode, F. Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon. Int. J. Mol. Sci. 2017, 18, 2737. https://doi.org/10.3390/ijms18122737
Correa W, Brandenburg K, Zähringer U, Ravuri K, Khan T, Von Wintzingerode F. Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon. International Journal of Molecular Sciences. 2017; 18(12):2737. https://doi.org/10.3390/ijms18122737
Chicago/Turabian StyleCorrea, Wilmar, Klaus Brandenburg, Ulrich Zähringer, Kishore Ravuri, Tarik Khan, and Friedrich Von Wintzingerode. 2017. "Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon" International Journal of Molecular Sciences 18, no. 12: 2737. https://doi.org/10.3390/ijms18122737
APA StyleCorrea, W., Brandenburg, K., Zähringer, U., Ravuri, K., Khan, T., & Von Wintzingerode, F. (2017). Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon. International Journal of Molecular Sciences, 18(12), 2737. https://doi.org/10.3390/ijms18122737





