Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions
Abstract
1. Introduction
2. Results
2.1. Histopathological Examination and Biochemical Blood Study
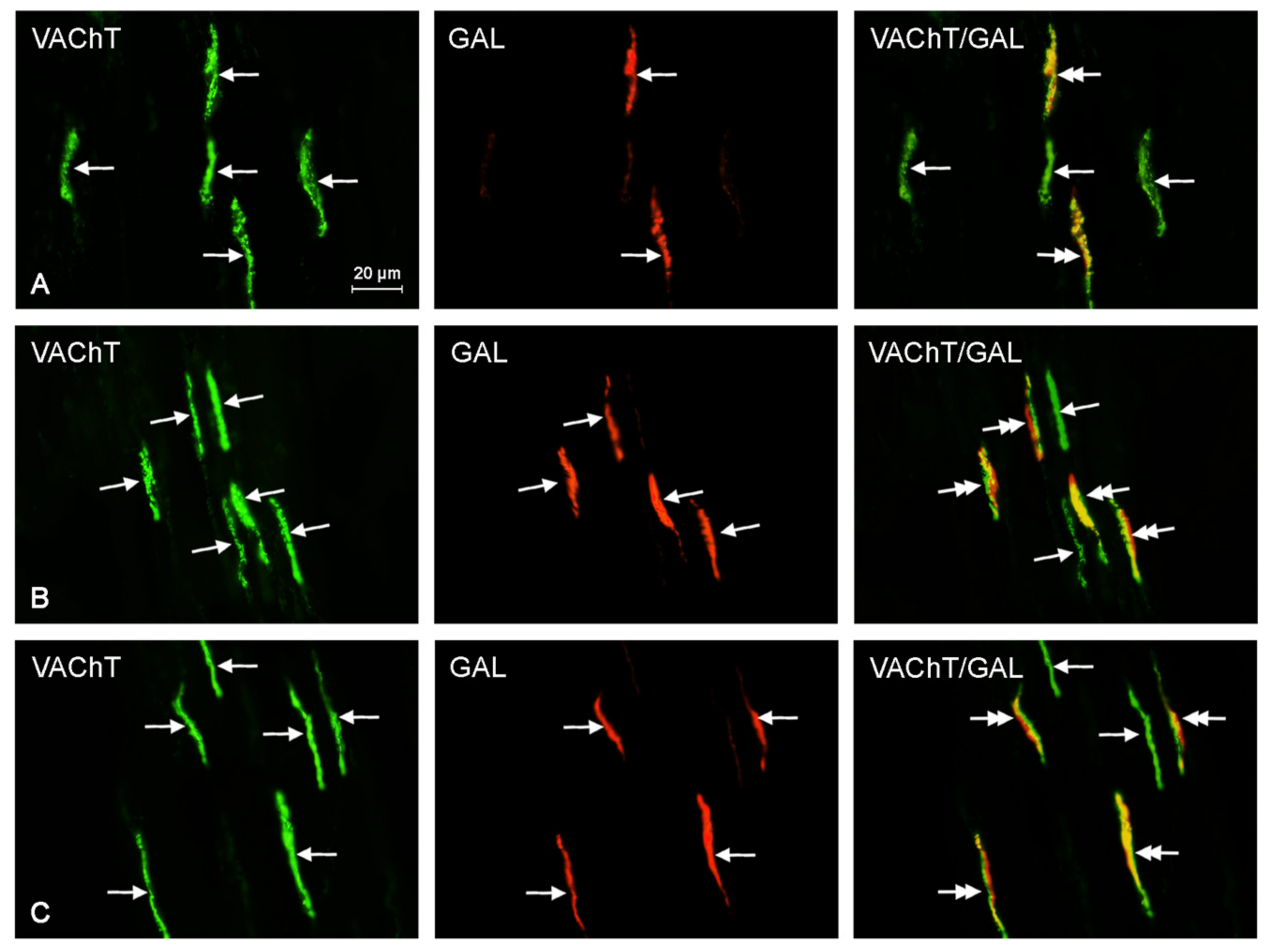
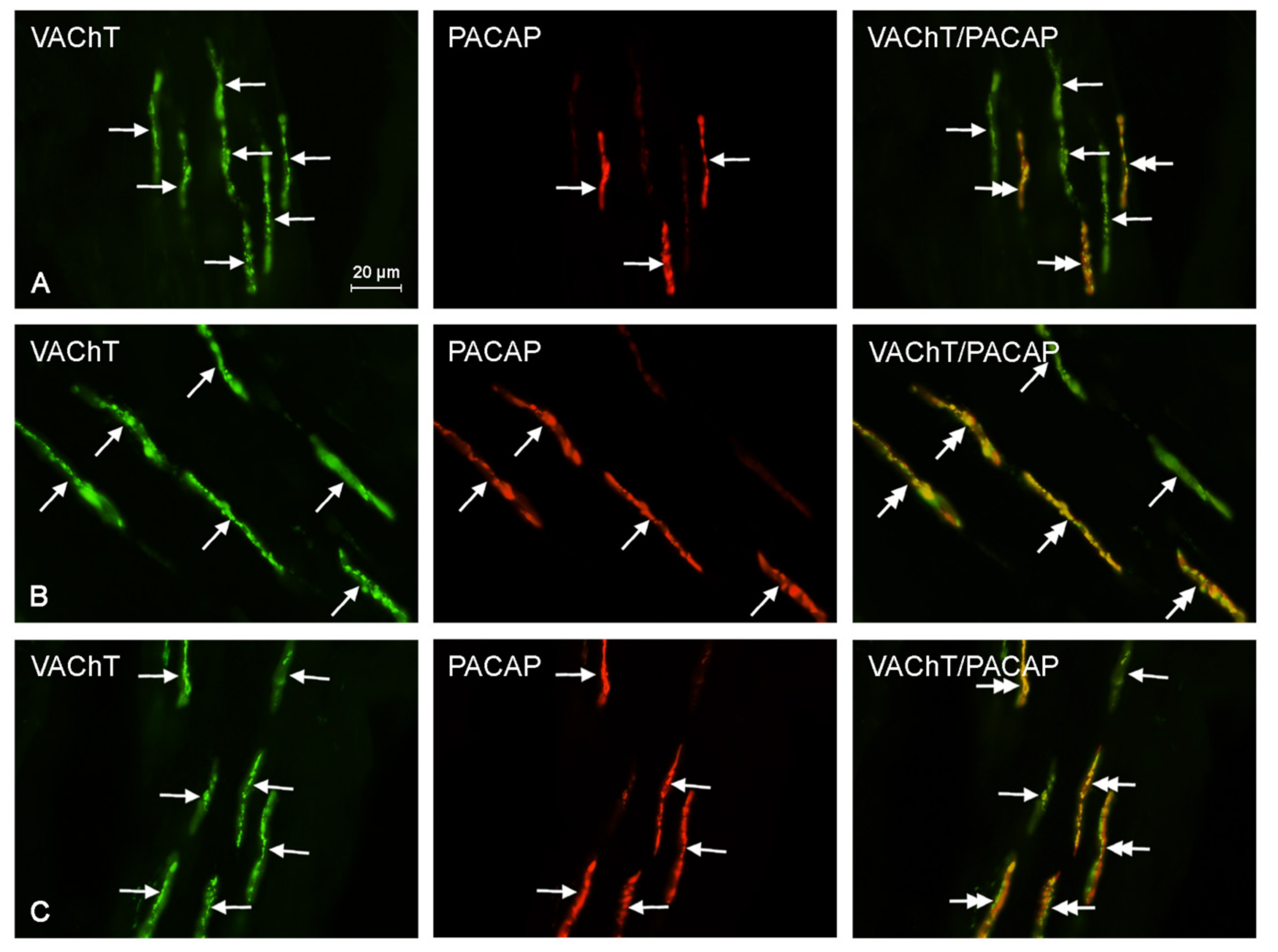
2.2. Neurochemical Characterization of Intrahepatic Nerves
3. Discussion
4. Materials and Methods
4.1. The Animal Models Used in This Study
4.2. Tissue Collection, Fixation, and Immunolabeling
4.3. The Histopathological Investigation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ANS | autonomic nervous system |
| BPA | bisphenol A |
| CART | cocaine and amphetamine regulated transcript |
| CGRP | calcitonin gene regulated peptide |
| EDC | endocrine disrupting compound |
| GAL | galanin |
| PACAP | pituitary adenylate cyclase activating polypeptide |
| PSNS | parasympathetic nervous system |
| SNS | sympathetic nervous system |
| SP | substance P |
| VAChT | vesicular acetylcholine transporter |
References
- Beronius, A.; Ruden, C.; Hakansson, H.; Hanberg, A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of bisphenol A. Reprod. Toxicol. 2010, 29, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Human health risk on environmental exposure to bisphenol A: A review. J. Environ. Sci. Health C 2006, 24, 225–255. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Martin, M.T.; Tan, S.W.; Houck, K.A.; Judson, R.S.; Richard, A.M.; Knudsen, T.B.; Dix, D.J.; Kavlock, R.J. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 2010, 118, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Soliman, A.S.; Colacino, J.A.; Calafat, A.M.; Battige, K.; Hablas, A.; Seifeldin, I.A.; Dolinoy, D.C.; Rozek, L.S. Urinary bisphenol A concentrations in girls from rural and urban Egypt: A pilot study. Environ. Health 2012, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Ehrlich, S.; Belcher, S.M.; Ben-Jonathan, N.; Dolinoy, D.C.; Hugo, E.R.; Hunt, P.A.; Newbold, R.R.; Rubin, B.S.; Saili, K.S.; et al. Low dose effects of bisphenol A. Endocr. Disruptors 2013, 1, e26490. [Google Scholar] [CrossRef]
- Rachoń, D. Endocrine disrupting chemicals (EDCs) and female cancer: Informing the patients. Rev. Endocr. Metab. Disord. 2015, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Fiedor, E.; Ptak, A. Bisphenol A and its derivatives tetrabromobisphenol A and tetrachlorobisphenol A induce apelin expression and secretion in ovarian cancer cells through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Toxicol. Lett. 2017, 269, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hajszan, T.; Leranth, C. Bisphenol A interferes with synaptic remodeling. Front. Neuroendocrinol. 2010, 31, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kinch, C.D.; Ibhazehiebo, K.; Jeong, J.H.; Habibi, H.R.; Kurrasch, D.M. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Zalko, D.; Soto, A.M.; Canlet, C.; Tremblay-Franco, M.; Jourdan, F.; Cabaton, N.J. Bisphenol A exposure disrupts neurotransmitters through modulation of transaminase activity in the brain of rodents. Endocrinology 2016, 157, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Yaoi, T.; Fushiki, S. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology 2012, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Pavlovičová, M.; Karmažínová, M.; Huláková, S.; Ľubica, L. Bisphenol A differently inhibits Cav3.1, Cav3.2 and Cav3.3 calcium channels. Naunyn-Schmiedeberg's Arch. Pharmacol. 2013, 387, 153–163. [Google Scholar]
- Kolšek, K.; Mavri, J.; Dolenc, M.S. Reactivity of bisphenol A-3,4-quinone with DNA. A quantum chemical study. Toxicol. Vitro 2012, 26, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.I.; Eissa, S.M.; El-Ghor, A.A. Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J. Basic Appl. Zool. 2015, 71, 10–19. [Google Scholar] [CrossRef]
- Moustafa, G.G.; Ahmed, A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: Genotoxic and immunohistochemical implications. Toxicol. Rep. 2016, 3, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Jiang, Y.; Li, Y.; Wan, Y.; Liu, J.; Ma, Y.; Mao, Z.; Chang, H.; Li, G.; Xu, B.; et al. Early-life exposure to bisphenol A induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS ONE 2014, 9, e90443. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Mun, G.I.; Choi, E.; Kim, M.; Jeong, J.S.; Kang, K.W.; Jee, S.; Lim, K.M.; Lee, Y.S. Submicromolar bisphenol A induces proliferation and DNA damage in human hepatocyte cell lines in vitro and in juvenile rats in vivo. Food Chem. Toxicol. 2017, 111, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [PubMed]
- Arvidsson, U.; Riedl, M.; Elde, R.; Meister, B. Vesicular acetylcholine transporter (VAChT) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 1997, 378, 454–467. [Google Scholar] [CrossRef]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Litten-Brown, J.C.; Corson, A.M.; Clarke, L. Porcine models for the metabolic syndrome, digestive and bone disorders: A general overview. Animal 2010, 4, 899–920. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, M.U.; Baccino, F.M.; Rita, G.A. The vacuolar degeneration of cells (congestive enlargement of lysosomes). Acta Anat. 1969, 73, 152–181. [Google Scholar] [CrossRef]
- Kakar, S.; Kamath, P.S.; Burgart, L.J. Sinusoidal dilatation and congestion in liver biopsy: Is it always due to venous outflow impairment? Arch. Pathol. Lab. Med. 2004, 128, 901–904. [Google Scholar] [PubMed]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.X.; Lin, Y.X.; Liu, F.P.; Chang, Y.C.; Li, R.; Li, C.W.; Li, Y.; He, J.S.; Ma, X.; Li, Z. Hepatoprotective effects of ethanol extracts from Folium Syringae against acetaminophen-induced hepatotoxicity in vitro and in vivo. J. Chin. Med. Assoc. 2017, 80, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.K.; Kim, J.E.; Song, S.H.; Sung, J.E.; Lee, H.A.; Kim, K.S.; Hong, J.T.; Hwang, D.Y. Ethanol extracts collected from the Styela clava tunic alleviate hepatic injury induced by carbon tetrachloride (CCl4) through inhibition of hepatic apoptosis, inflammation, and fibrosis. J. Toxicol. Pathol. 2017, 30, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, J.L.; Kil, J.H.; Park, K.Y. Bamboo salt attenuates CCl4-induced hepatic damage in Sprague-Dawley rats. Nutr. Res. Pract. 2013, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Losano, N.F.; Condessa, S.S.; de Freitas, R.M.P.; Cardoso, S.A.; Freitas, M.B.; de Oliveira, L.L. Exposure to deltamethrin induces oxidative stress and decreases of energy reserve in tissues of the Neotropical fruit-eating bat Artibeus lituratus. Ecotoxicol. Environ. Saf. 2017, 148, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Punsawad, C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J. Toxicol. Pathol. 2013, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Duru, Q.C.; Njoku, R.C.; Amadi, B.A.; Tamunoibuomie, A.; Keboh, E. Effects of co-exposure to atrazine and ethanol on the oxidative damage of kidney and liver in Wistar rats. Ren. Fail. 2017, 39, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.S.; Kiss, B.; Vlase, L.; Pop, A.; Iepure, R.; Paltinean, R.; Loghin, F. Study of oxidative stress induction after exposure to bisphenol A and methylparaben in rats. Toxicol. Lett. 2011, 59, 539–549. [Google Scholar] [CrossRef]
- Lautt, W.W. Hepatic nerves—A review of their functions and effects. Can. J. Physiol. Pharmacol. 1980, 58, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Lautt, W.W. Hepatic circulation: Physiology and pathophysiology. In Colloquium Series on Integrated Systems Physiology: From Molecule to Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2009; pp. 83–119. [Google Scholar]
- Yi, C.X.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta 2010, 1802, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Jana, B.; Kozłowska, A.; Crayton, R.; Majewski, M.; Zalecki, M.; Baranowski, W.; Radziszewski, P. Innervation pattern of polycystic ovaries in the women. J. Chem. Neuroanat. 2014, 61–62, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Rowniak, M.; Wojtkiewicz, J. Zinc transporter 3 (ZnT3) in the enteric nervous system of the porcine ileum in physiological conditions and during experimental inflammation. Int. J. Mol. Sci. 2017, 18, E338. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Makowska, K.; Bejer-Olenska, E.; Gonkowski, S. Zinc Transporter 3 (Znt3) as an Active Substance in the Enteric Nervous System of the Porcine Esophagus. J. Mol. Neurosci. 2017, 61, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kaminska, B.; Landowski, P.; Całka, J. Immunohistochemical distribution of cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nerve fibers and various degree of co-localization with other neuronal factors in the circular muscle layer of human descending colon. Histol. Histopathol. 2013, 28, 851–858. [Google Scholar] [PubMed]
- Green, T.; Dockray, G.J. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci. Lett. 1987, 76, 151–156. [Google Scholar] [CrossRef]
- McCulloch, J.; Uddman, R.; Kingman, T.A.; Edvinsson, L. Calcitonin gene-related peptide: Functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. USA 1986, 83, 5731–5735. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Kellerth, J.O.; Nilsson, G.; Pernow, B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975, 100, 235–252. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.J.; Ackenheil, M. The role of substance P in depression: Therapeutic implications. Dialogues Clin. Neurosci. 2002, 4, 21–29. [Google Scholar] [PubMed]
- Taborsky, G.J.; Dunning, B.E.; Havel, P.J.; Ahren, B.; Kowalyk, S.; Boyle, M.R.; Verchere, C.B.; Baskin, D.G.; Mundinger, T.O. The canine sympathetic neuropeptide galanin: A neurotransmitter in pancreas, a neuromodulator in liver. Horm. Metab. Res. 1999, 31, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Celi, F.; Bini, V.; Papi, F.; Santilli, E.; Ferretti, A.; Mencacci, M.; Berioli, M.G.; De Giorgi, G.; Falorni, A. Circulating acylated and total ghrelin and galanin in children with insulin-treated type 1 diabetes: Relationship to insulin therapy, metabolic control and pubertal development. Clin. Endocrinol. 2005, 63, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Alzate, H.F.; Agudelo-Zapata, Y.; González-Clavijo, A.M.; Poveda, N.E.; Espinel-Pachón, C.F.; Escamilla-Castro, J.A.; Márquez-Julio, H.L.; Alvarado-Quintero, H.; Rojas-Rodríguez, F.G.; Arteaga-Díaz, J.M.; et al. Serum Galanin levels in young healthy lean and obese non-diabetic men during an oral glucose tolerance test. Sci. Rep. 2016, 6, 31661. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Horowitz, M.; Morley, P.M.; Flood, J.F. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 1992, 13, 1133–1135. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kondo, K.; Terashima, Y.; Arima, H.; Murase, T.; Oiso, Y. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: Lack of evidence for involvement of hypothalamic neuropeptide gene expression. J. Neuroendocrinol. 1998, 10, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Hammack, S.E.; May, V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: Data convergence from animal and human studies. Biol. Psychiatry 2015, 78, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H. Psychiatric implications of PACAP signaling pathway. Jpn. J. Psychopharmacol. 2016, 32, 133–137. [Google Scholar]
- Meisner, H.; Carter, J.R. Regulation of lipolysis in adipose tissue. Horiz. Biochem. Biophys. 1977, 4, 91–129. [Google Scholar] [PubMed]
- Akash, G.; Kaniganti, T.; Tiwari, N.K.; Subhedar, N.K.; Ghose, A. Differential distribution and energy status–dependent regulation of the four CART neuropeptide genes in the zebrafish brain. J. Comp. Neurol. 2014, 522, 2266–2285. [Google Scholar] [CrossRef] [PubMed]
- Bharne, A.P.; Borkar, C.D.; Subhedar, N.K.; Kokare, D.M. Differential expression of CART in feeding and reward circuits in binge eating rat model. Behav. Brain Res. 2015, 291, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Gonkowski, S.; Całka, J. Expression of cocaine and amphetamine regulated transcript (CART) in the porcine intramural neurons of stomach in the course of experimentally induced diabetes mellitus. J. Mol. Neurosci. 2015, 57, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Rytel, L. Co-localisation of cocaine- and amphetamine-regulated transcript peptide and vasoactive intestinal polypeptide in the myenteric plexus of the porcine transverse colon. Folia Morphol. 2013, 72, 328–332. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Gonkowski, S.; Bladowski, M.; Majewski, M. Characterisation of cocaine- and amphetamine- regulated transcript-like immunoreactive (CART-LI) enteric neurons in the porcine small intestine. Acta Vet. Hung. 2012, 60, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Attina, T.M.; Blustein, J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 2012, 308, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhou, Y.; Tang, C.X.; Wu, J.G.; Chen, Y.; Jiang, Q.W. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environ. Health 2012, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Vicentic, A.; Jones, D.C. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. 2007, 320, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in mammals gastrointestinal system—A review. Ann. Anim. Sci. 2017, 17, 3–21. [Google Scholar] [CrossRef][Green Version]
- Surendran, S.; Kondapaka, S.B. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle–myenteric plexus of obesity induced diabetes mouse: Implications on enteric neurodegeneration. Biochem. Biophys. Res. Commun. 2005, 338, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Carolan, E.; Hogan, A.E.; Corrigan, M.; Gaotswe, G.; O’Connell, J.; Foley, N.; O’Neill, L.A.; Cody, D.; O’Shea, D. The impact of childhood obesity on inflammation, innate immune cell frequency, and metabolic microRNA expression. J. Clin. Endocrinol. Metab. 2014, 99, E474–E478. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Sacher, J.; Arelin, K.; Holiga, Š.; Kratzsch, J.; Villringer, A.; Schroeter, M.L. Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults: A combined MRI, serum marker and gene expression study. Transl. Psychiatry 2012, 2, e200. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; de Santis, M.; He, M.; Deng, C. Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: The role of histaminergic and NPY pathways. Pharmacol. Res. 2015, 95–96, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Yolton, K.; Dietrich, K.N.; Hornung, R.; Ye, X.; Calafat, A.M.; Lanphear, B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009, 117, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Maserejian, N.N.; Trachtenberg, F.L.; Hauser, R.; McKinlay, S.; Shrader, P.; Bellinger, D.C. Dental composite restorations and neuropsychological development in children: Treatment level analysis from a randomized clinical trial. Neurotoxicology 2012, 33, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Vishnevetsky, J.; Herbstman, J.B.; Calafat, A.M.; Xiong, W.; Rauh, V.; Wang, S. Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 2012, 120, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, A.; Cacciola, G.; Widmer, D.A.; Viggiano, D. Anxiety as a neurodevelopmental disorder in a neuronal subpopulation: Evidence from gene expression data. Psychiatry Res. 2015, 228, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Gonkowski, S.; Równiak, M.; Crayton, R.; Majewski, M.; Jałyński, M. Neurochemical characterization of zinc transporter 3-like immunoreactive (ZnT3+) neurons in the intramural ganglia of the porcine. J. Mol. Neurosci. 2012, 48, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Równiak, M.; Crayton, R.; Gonkowski, S.; Robak, A.; Zalecki, M.; Majewski, M.; Klimaschewski, L. Axotomy-induced changes in the chemical coding pattern of colon projecting calbindin -positive neurons in the inferior mesenteric ganglia of the pig. J. Mol. Neurosci. 2013, 51, 99–108. [Google Scholar] [CrossRef] [PubMed]
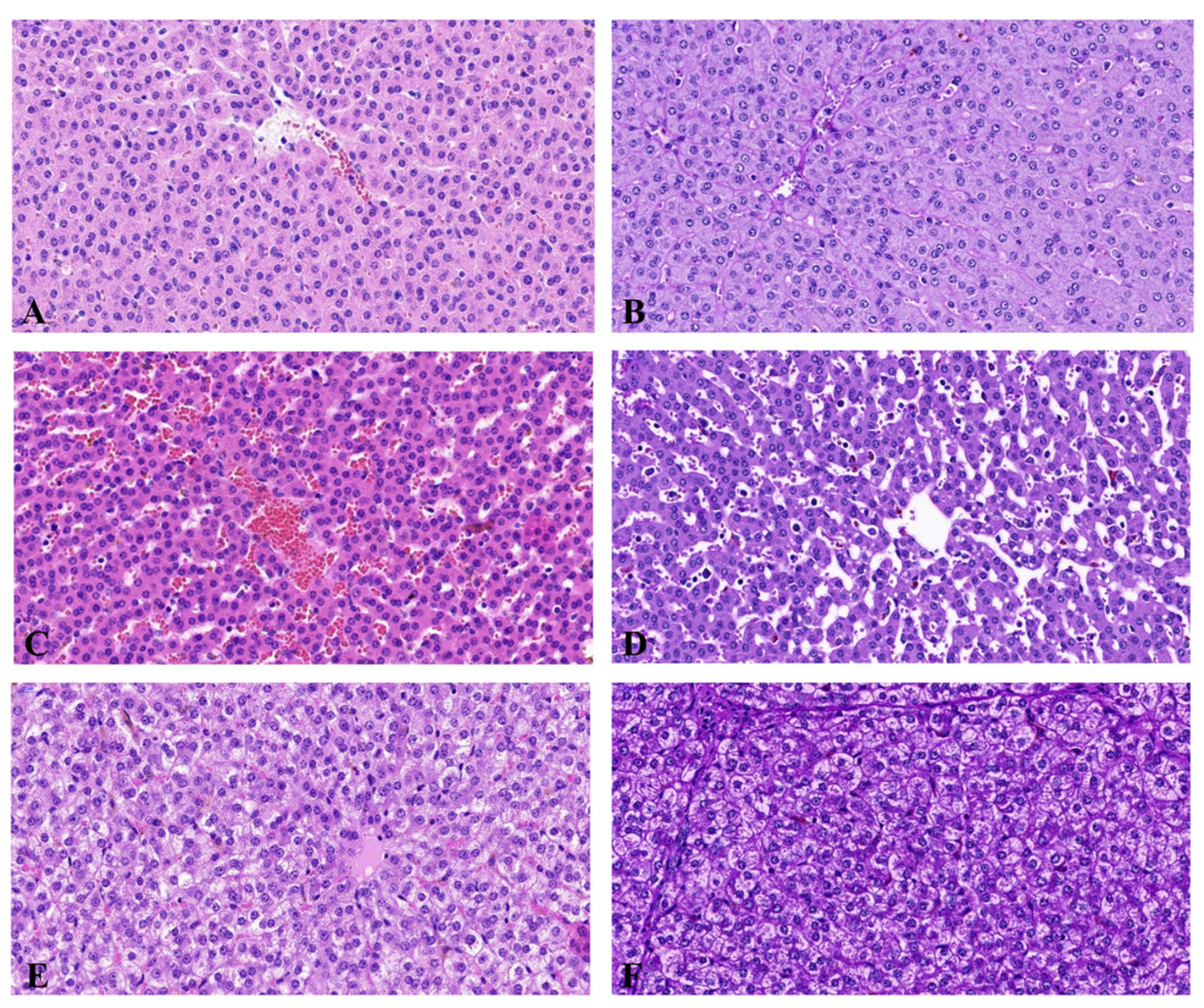
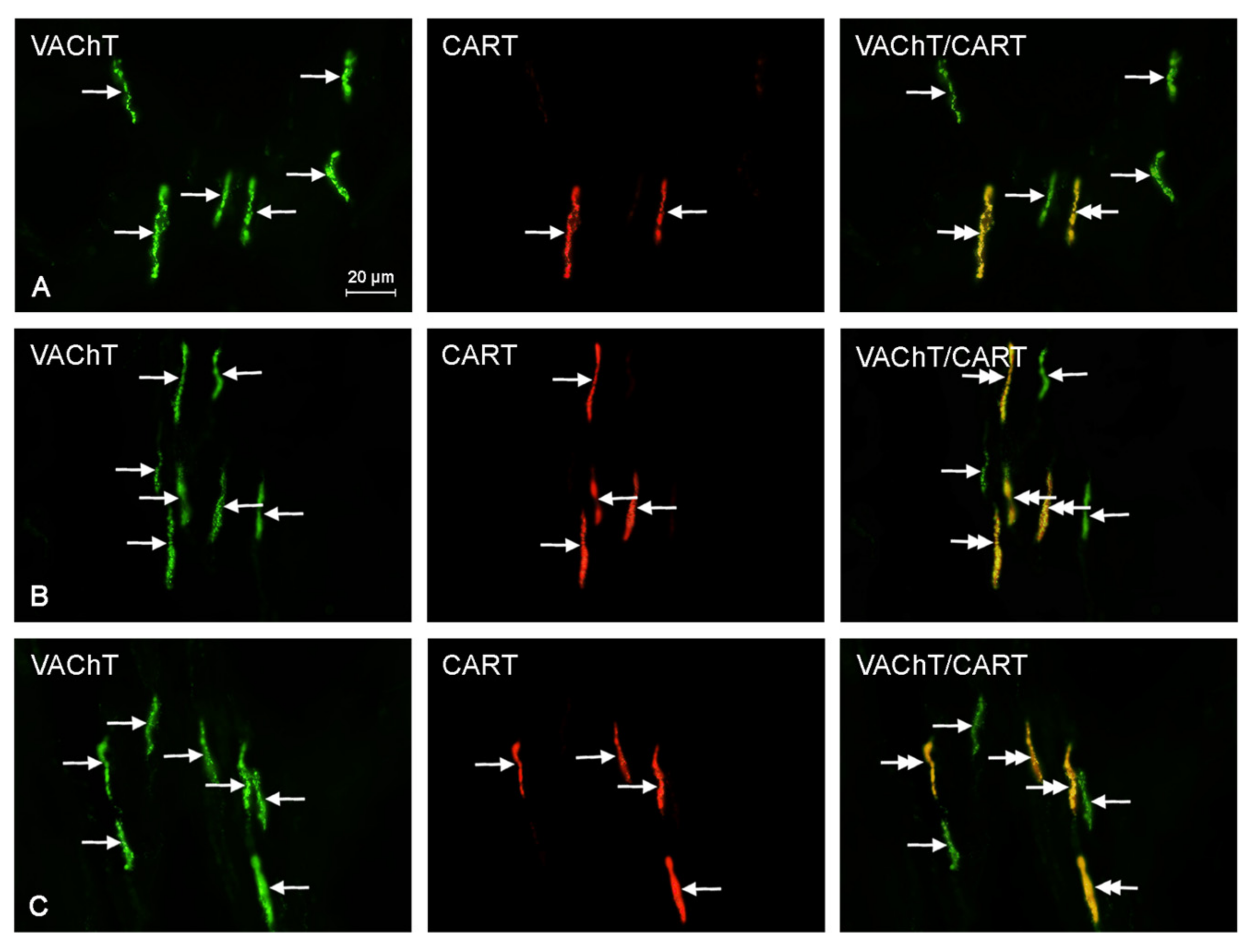
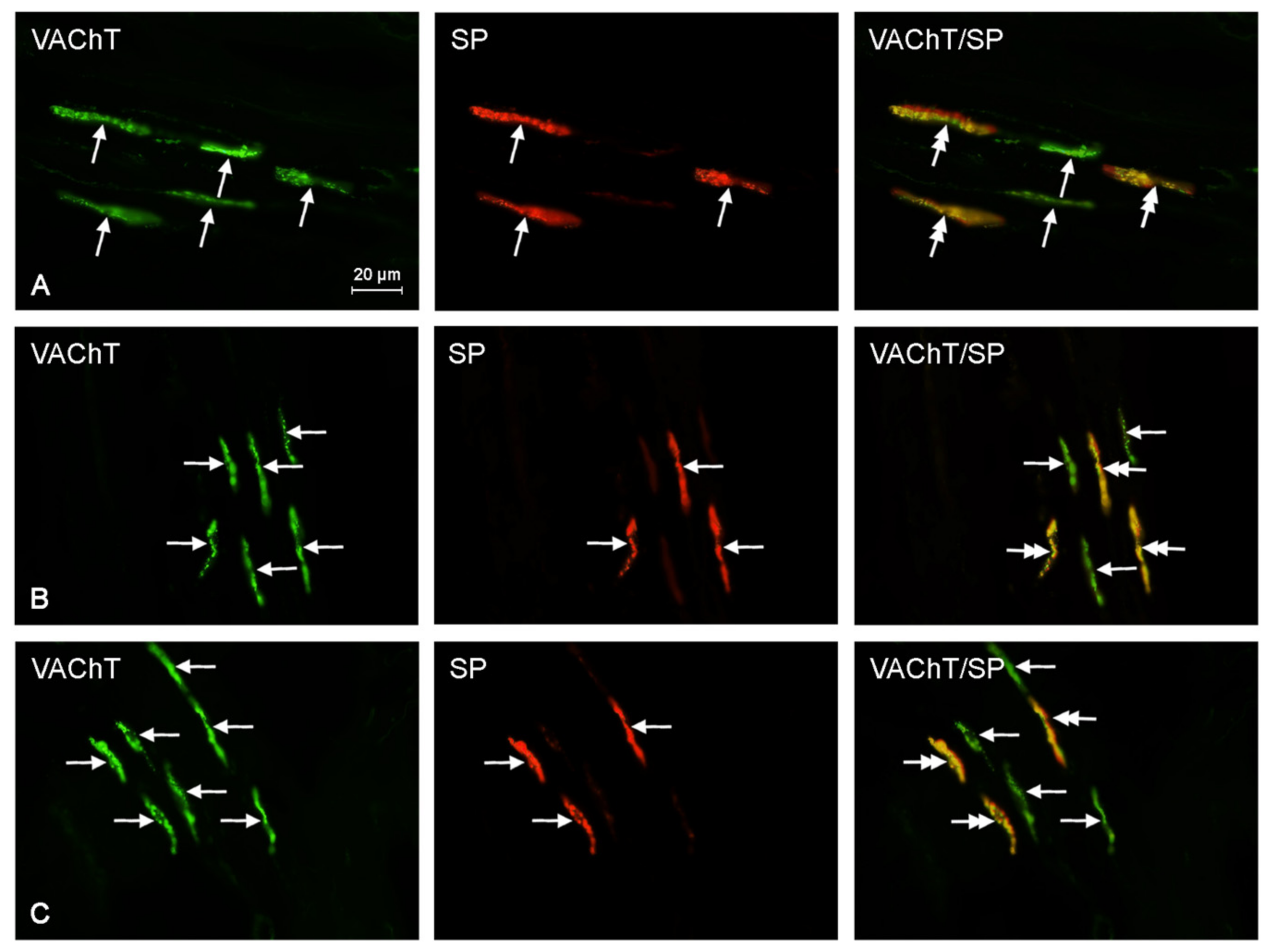
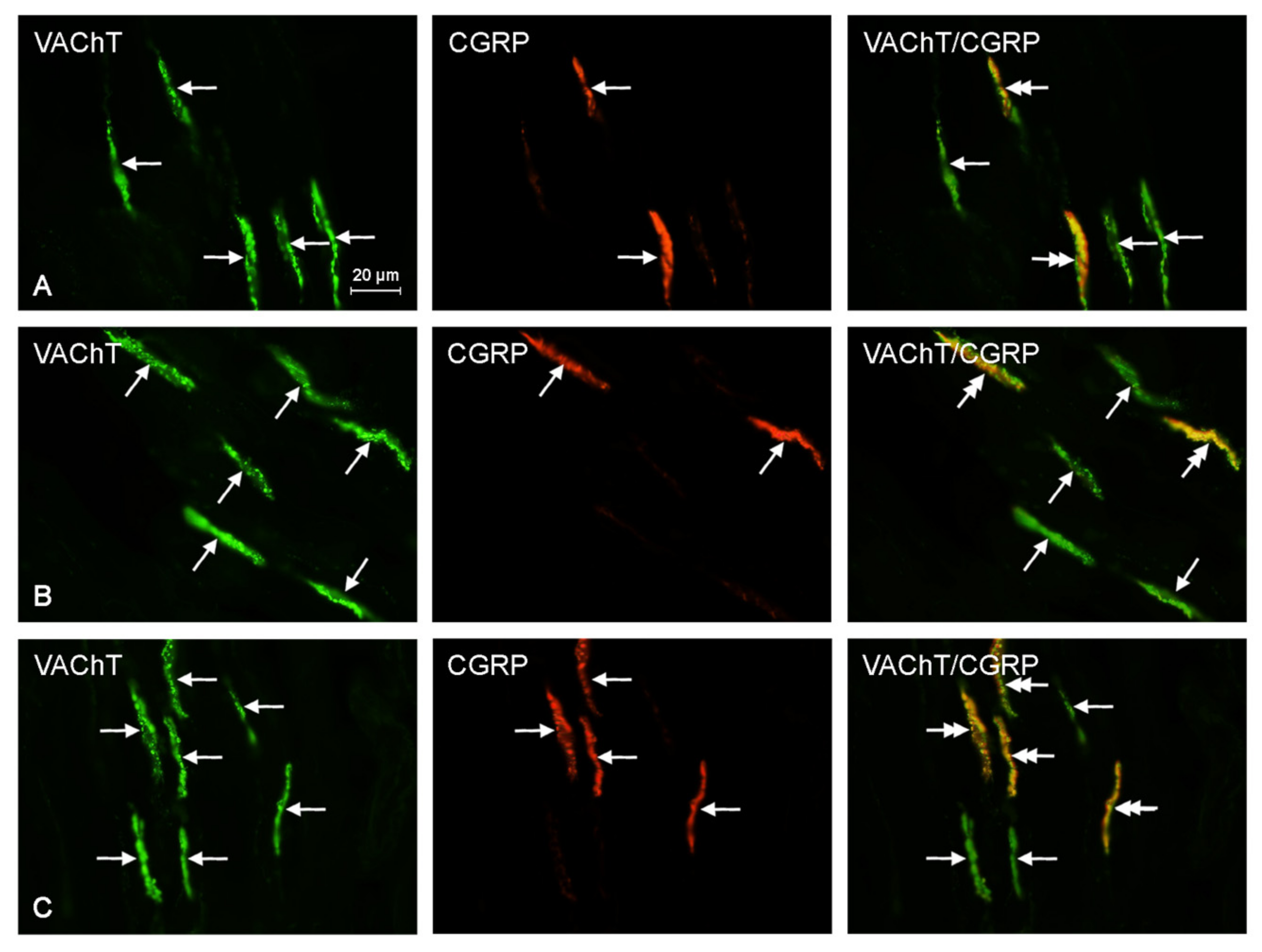
| Neurochemical Characteristic | Groups of Animals | ||
|---|---|---|---|
| CTRL | E1 | E2 | |
| VAChT+ | 27.4 ± 4.41 b | 29.8 ± 3.28 | 34.8 ± 4.25 b |
| VAChT+/CART+ | 10.6 ± 2.80 a,b | 15.4 ± 2.25 a,c | 20.8 ± 2.52 b,c |
| VAChT+/SP+ | 6.2 ± 1.93 b | 8.8 ± 1.77 | 8.9 ± 1.41 |
| VAChT+/CGRP+ | 12.0 ± 3.11 b | 13.6 ± 2.86 c | 20.4 ± 4.43 b,c |
| VAChT+/GAL+ | 3.4 ± 1.33 a,b | 9.1 ± 2.51 a | 12.2 ± 3.02 b |
| VAChT+/PACAP+ | 11.8 ± 3.25 a,b | 17.6 ± 4.23 a | 19.4 ± 5.81 b |
| Primary Antibodies | ||||
|---|---|---|---|---|
| Antisera | Code | Host Species | Dilution | Supplier |
| VAChT | H-V007 | Goat | 1:2000 | Phoenix Europe; www.phoenixpeptide.com |
| CART | H-003-61 | rabbit | 1:22,000 | Phoenix Europe; www.phoenixpeptide.com |
| SP | 8450-0505 | rabbit | 1:10,000 | Biogenesis Inc.; www.biogenesis.co.uk |
| CGRP | 11189 | rabbit | 1:10,000 | MP Biomedicals; www.mpbio.com |
| GAL | RIN7153 | rabbit | 1:10,000 | Peninsula Labs, US; see Bachem AG; www.bachem.com |
| PACAP | IHC 8922 | rabbit | 1:20,000 | Bachem AG; www.bachem.com |
| Secondary Antibodies | ||||
| Reagent | Dilution | Supplier | ||
| Donkey anti-goat IgG (H + L) conjugated with FITC | 1:800 | 715-095-003; Jackson IR Lab, US; www.jacksonimmuno.com | ||
| Biotinylated goat anti-rabbit immunoglobulins | 1:1000 | E0432, DAKO Corporation, US; www.dakousa.com | ||
| Biotin conjugated F(ab)’ fragment of affinity Purified anti-rabbit IgG (H + L) | 1:1000 | 711-1622, BioTrend, Germany; www.biotrend.com | ||
| CY3-conjugated Streptavidin | 1:9000 | 016-160-084, Jackson IR Lab, US; www.jacksonimmuno.com | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoene, M.; Rytel, L.; Dzika, E.; Włodarczyk, A.; Kruminis-Kaszkiel, E.; Konrad, P.; Wojtkiewicz, J. Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions. Int. J. Mol. Sci. 2017, 18, 2726. https://doi.org/10.3390/ijms18122726
Thoene M, Rytel L, Dzika E, Włodarczyk A, Kruminis-Kaszkiel E, Konrad P, Wojtkiewicz J. Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions. International Journal of Molecular Sciences. 2017; 18(12):2726. https://doi.org/10.3390/ijms18122726
Chicago/Turabian StyleThoene, Michael, Liliana Rytel, Ewa Dzika, Andrzej Włodarczyk, Ewa Kruminis-Kaszkiel, Ptaszyński Konrad, and Joanna Wojtkiewicz. 2017. "Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions" International Journal of Molecular Sciences 18, no. 12: 2726. https://doi.org/10.3390/ijms18122726
APA StyleThoene, M., Rytel, L., Dzika, E., Włodarczyk, A., Kruminis-Kaszkiel, E., Konrad, P., & Wojtkiewicz, J. (2017). Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions. International Journal of Molecular Sciences, 18(12), 2726. https://doi.org/10.3390/ijms18122726





